Abstract
Aflatoxin B1 (AFB1) is the most toxic among the mycotoxins and causes detrimental health effects on the liver of human and animals. Selenium (Se) plays an important role in protection of various animal species against numerous notorious toxic agents. The present study is designed to explore the protective effects of Se against AFB1-induced liver pathogenesis by the methods of histopathology, flow cytometry, quantitative real-time polymerase chain reaction (qRT-PCR), and biochemical analysis. A total of 312, 1-day-old healthy Cobb-500 broilers were randomly divided into four groups and fed with basal diet (control group), 0.6 mg/kg AFB1 (AFB1 group), 0.4 mg/kg Se (+ Se group), and 0.6 mg/kg AFB1 + 0.4 mg/kg Se (AFB1 + Se group) for 21 days, respectively. Our results showed that 0.4 mg/kg Se supplement in broiler’s diets could alleviate the AFB1-induced histological lesions in the liver. The apoptosis analysis by flow cytometry showed that 0.4 mg/kg Se ameliorated the AFB1-induced apoptosis in the liver. Moreover, the mRNA expression levels of Fas, TNF-α, FAS-associated death domain, TNF receptor-associated death domain, TNF receptor-associated factor 2, caspase 10, caspase 8, B cell lymphoma 2, IκB kinase, X-linked inhibitor of apoptosis protein, caspase 9, and caspase 3 analyzed by qRT-PCR demonstrated that 0.4 mg/kg Se could relieve the impact caused by AFB1 to these parameters. The biochemical analyses of activities of CAT, GSH-Px and SOD, hydroxyl ion scavenging and contents of MDA and GSH in liver cells also indicated that 0.4 mg/kg Se has positive effect on AFB1-induced oxidative stress in the liver. In conclusion, Se could relieve AFB1-induced apoptosis by the molecular regulation of death receptors pathway in the liver of broilers. The outcomes from the present study may lead to a better understanding of the nature of selenium’s essentiality and its protective roles against AFB1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aflatoxins belong to group of approximately 20 related fungal metabolites mainly produced by Aspergillus flavus and Aspergillus parasiticus. Aflatoxins can affect more than one organ system simultaneously, thereby producing a multitude of responses in the affected organisms [1]. This malicious nature of aflatoxins made them comprehensively researched mycotoxin. The most prevalent aflatoxins are B1, B2, G1, G2, M1, and M2, which are naturally found in many food and dairy products [2]. Among these, aflatoxin B1 (AFB1) is the widely known aflatoxin [3] due to its proven carcinogenicity in human, lab animal, pets, and domestic animals [4,5,6]. AFB1 has been reported to provoke hepatotoxic, genotoxic, and immunotoxic effects in several animal species [7]. The toxicity of AFB1 depends on its dose, duration of intake, and affected animal species [8]. Poultry birds are considered highly susceptible to AFB1 due to their high metabolic rate and AFB1 prevalence in poultry feed, along with other toxins. Since AFB1 is naturally present in poultry feed, it poses hepatotoxic, immune toxic, and other high detrimental threats to the poultry production which can range from lower egg/meat production to mortality [7]. The liver is considered most crucial organ in human and animals performing many vital functions, along with serving as the incinerator for toxins and harmful substances. It is also the main affected organ following the ingestion of the AFB1 [9]. Studies have proved that aflatoxins react negatively with different cell proteins, leading to inhibition of carbohydrate and lipid metabolism and protein synthesis, and ultimately induce apoptosis [10]. Our previous work has also revealed that AFB1 negatively affect the liver, spleen, thymus, jejunum, and ileum, when fed to broiler [11, 12].
Selenium (Se) is an essential nutrient for normal physiological processes [13]. It has also been shown to protect various animal species against numerous notorious toxic agents [14]. For instance, Se protected cadmium-induced liver toxicity in rat by improving oxidative stress status [15] and ameliorated DNA damage caused by mercury [16]. Se and alpha-tocopherol amended the lead-induced toxicity by improving serum biochemical parameters, lipid peroxidation, and antioxidant enzyme activities in fish [17]. Another recent study in chicken has shown to improve the lead-induced oxidative stress and immune damage, when selenium was fed to the lead intoxicated birds [18] .
Our previous study has revealed that AFB1 negatively affects broiler’s liver, and we also elucidated the mechanisms by which AFB1 induces apoptosis at cellular and molecular level [19]. Looking at our previous results, it would be enthralling to find out how Se can amend the toxic effects of AFB1 on the broiler liver as Se has been shown to provide protective effects against several toxic agents. Therefore, the present study is designed to explore the protective effects of Se against AFB1-induced liver pathogenesis by analyzing histological, ultra-structural, biochemical, flow cytometry, and relative gene expression changes in chicken hepatocytes.
Materials and Methods
Birds and Diet
A total of 312, 1-day-old healthy Cobb-500 broilers were purchased from Chia Tai Group of Wenjiang, Sichuan, China. The broilers were weighed and randomly divided into four groups which are the control group (0 mgAFB1/kg of basal diet), AFB1 group (0.6 mg AFB1/kg of basal diet), Se group (0.4 mg Se/kg of basal diet), and AFB1 + Se group (0.6 mg AFB1/kg + 0.4 mg Se/kg of basal diet). Each group was in three replicates, each with 26 birds. Chicks were provided the corresponding diets and water ad libitum throughout the 21 days of experimentation. Room lights were set on a 24-h continuous schedule, temperature was initially maintained at 33 °C and gradually lowered by 2 °C each week, and relative humidity was maintained between 65 and 67%. All procedures performed in studies involving animals were in accordance with the international guidelines, and the laws and the ethical standards of China West Normal University Animal Care and Use Committee.
The control diet (basal diet) was formulated according to National Research Council (NRC, 1994) [20] and Chinese Feeding Standard of Chicken (NY/T33-2004) recommendations.
AFB1-contaminated diet was made by following method: 27 mg AFB1 (A6636, Sigma-Aldrich, USA) was dissolved into 30 ml methanol, and then this 30 ml mixture was mixed into 45 kg corn–soybean basal diet to formulate AFB1-contaminated diet. The equivalent methanol was mixed into 45 kg corn–soybean basal diet to produce the control diet. The methanol of diets was evaporated at 98 °F (37 °C). After preparing the diet, the control and the AFB1-contaminated diets were analyzed by high-performance liquid chromatography (HPLC) and fluorescence detection (Waters Model 2475) to ensure the AFB1 concentration in the experimental diets. AFB1 content was 0.601 mg/kg in the contaminated diet and less than 0.001 mg/kg in the control diet. Basal diet composition is mentioned in Table 1.
Gross Observation of the Liver
At days 7, 14, and 21, six chickens in each group were randomly selected and weighed. After euthanization, gross pathological condition of each organ was observed with a special emphasis on livers. After the liver from each chicken was dissected, gross pathological changes were observed, and weight was detected and liver index was calculated using following formula.
Histopathological and Ultrastructural Examination of the Liver
At the age of 7, 14, and 21 days, six chickens in each group were euthanized and the livers were fixed in 4% paraformaldehyde (PFA) and routinely processed in paraffin. Thin sections (5 μm) of tissue were sliced, mounted on glass slides, and stained with hematoxylin and eosin Y. The histological organization of the tissues was contemplated and snapped with a digital camera (Nikon, eclipse 50i, Japan).
One chick per replicate in each group was euthanized and then immediately necropsied at the end of the trial. Small pieces of liver tissues were immediately fixed with 2.5% glutaraldehyde and post-fixed in 2% Veronal acetate-buffered OsO4. The tissues were embedded in Araldite after dehydrating in alcohol gradient. The blocks were sectioned in 65–75-nm-thick sections in a microtome with a glass knife and placed in uncoated copper grids. The sections were stained with uranyl acetate and post-stained with 0.2% lead citrate. The subcellular architecture of liver was examined with a Hitachi H-600 transmission electron microscope (Japan).
Annexin-V Apoptosis Detection of the Liver by Flow Cytometry
At 7, 14, and 21 days of the experiment, six chickens in each group were euthanized, and the livers were sampled from each chick to determine the percentage of apoptotic cells by flow cytometer, using the method by Del Bino (2010) [21]. Briefly, the dissected livers were thereupon homogenized to form a cell suspension. After filtered and washed, these cells were resuspended in phosphate buffer at a concentration of 1 × 106 cells/mL. Five microliters of Annexin-V-Fluorescein isothiocyanate (V-FITC) and 5 μL propidium iodide (PI) were added into 100 μL cell suspension, and then the mixture was incubated at 25 °C for 15 min in the dark. Four hundred microliters of 1× Annexin binding buffer was added to the mixture, and then the apoptotic cells were assayed by flow cytometer (BD FACSCalibur) within 1 h. The Annexin-V-FITC Kit was obtained from BD Pharmingen (USA, 556547).
Detection of Death Receptors of the Liver by qRT-PCR
The livers from six chickens in each group were removed at 7, 14, and 21 days of age and instantly stored in liquid nitrogen. The liver samples were homogenized in liquid nitrogen, by crushing with a mortar and pestle and the powdered tissues were collected into eppendorf tubes and stored at − 80 °C. Total RNA was extracted using TriPure Isolation Reagent (Cat No. 11667165001, Roche Applied Science, Germany) following manufacturer’s protocol. The quality and quantity of total RNA were measured spectrophotometrically. Extracted RNA was forthwith reverse-transcribed into cDNA using Transcriptor First Strand cDNA Synthesis Kit (Cat No: 04897030001, Roche Applied Science, Germany). qRT-PCR reactions were performed in a total volume of 20 μL using FastStart Universal SYBR Green Master mix (Cat No: 04913914001, Roche Applied Science, Germany), at the following thermocycler program: initial denaturation at 95 °C for 10 min, followed by 44 cycles of “10 s at 95 °C and 30 s at melting temperature (Tm) of a specific primer pair”, and melt curve analysis by 10 s at 95 °C, and 72 °C for 10 s, using Thermal Cycler (Step One Plus, Applied BioSystems, USA). β-Actin was used as an internal control [22, 23]. Primer information is provided in Table 2. The qRT-PCR data were analyzed, and fold changes in expressions were calculated using 2−∆∆Ct calculation method described by reference [24]. The forward and reverse primers are listed at Table 2.
Biochemical Analysis of the Liver
Six chickens in each group were euthanized and immediately dissected at 7, 14, and 21 days of age. Then, the livers were immediately taken out and chilled to 0 °C in 0.85% NaCl solution and then dried, weighed, and homogenized in nine times volume of ice cold 0.85% NaCl in a chilled homogenizer and centrifuged at 3500×g at 4 °C for 10 min. Total protein was measured by the method of Bradford. The commercial kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) and used to detect total protein (Total protein quantification kit No.A045), activities of SOD (superoxide dismutase detection kit no. A001-1), CAT (catalase detection kit no. A007), GSH-Px (glutathione peroxidase detection kit no. A005) and contents of GSH (glutathione detection kit no. A006) and MDA (malonaldehyde detection kit no. A003-1), and ─OH radical (hydroxyl free radical detection kit no. A018) in the supernatant, according to the manufacturer’s instructions.
Statistical Analysis
The results were expressed as mean ± standard deviation (X ± SD). Statistical analyses were performed using one-way analysis of variance and Tukey was employed for multiple comparisons. Letters A, B, C, and D represent the significant difference (p < 0.01) between the control group, AFB1 group, Se group, and AFB1 + Se group respectively, and letters a, b, c, and d represent the difference (p < 0.05) between the control group, AFB1 group, Se group, and AFB1 + Se group respectively.
Results
Body Weight and Clinical Observation
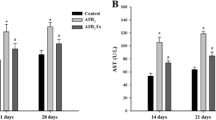
At day 7, no significant differences (p < 0.05 or p < 0.01) among the control and treatment groups were found. There was no significant reduction in the body weight of chickens at day 7, whereas at days 14 and 21, a marked reduction in the body weight of AFB1 group was seen (p < 0.05 or p < 0.01). Furthermore, an increase in the body weight was seen in the Se group in comparison to the control group (p < 0.05). What is more, the body weight of the AFB1 + Se group was comparable to the control group (p > 0.05). The results are shown in Fig. 1a.
Body weights of experimental chickens (a) and relative liver weight of experimental chicken (b). Letters A, B, C, and D represent the significant difference (p < 0.01) between the control group, AFB1 group, Se group, and AFB1 + Se group, respectively, and letters a, b, c, and d represent difference (p < 0.05) between the control group, AFB1 group, Se group, and AFB1+Se group, respectively, the same as followings
Grossly, no major differences were observed in the liver in all four groups at day 7. A mild yellowish discoloration and enlargement of the liver was seen at days 14 and 21 in AFB1 exposed chickens as compared to the control, whereas in the Se group, there were no such gross pathological changes observed when compared to the control group. In the AFB1 + Se group, these gross characteristics were found to be comparable to the control.
There was no significant change (p > 0.05) in the relative weight of livers at day 7 in all four groups, whereas at days 14 and 21, the relative weight of livers were seen increasing (p < 0.05 or p < 0.01) in the AFB1 group as compare to other groups. Furthermore, in the AFB1 + Se group, the relative weight of the liver was equivalent to the control group as shown in Fig. 1b.
Histological and Ultrastructural Changes of the Liver
Compared to the control group, microscopically degenerative reversible lesions were observed in a varying manner in AFB1 group (Fig. 2a), such as congestion, slight to moderate vacuolar degeneration, and proliferation of bile duct (Fig. 2b), while no such histological differences were seen in the control, Se group and AFB1 + Se group except slight hydropic degeneration (insignificant) in the AFB1 + Se group (Fig. 2a, c, d).
Histopathological and ultrastructural changes displayed by liver cells exposed to AFB1. a–d The histological examination of H & E stained liver tissues of control and treatment groups from 21 days old chickens (Bar = 50 μm). e–h The ultrastructural examination of uranyl acetate and lead citrate stained liver tissue from the control group and treatment groups (control group, AFB1 group, Se group, AFB1 + Se group)
Results of ultrastructural observation revealed irregular, fragmented, and condensed nucleus, swollen mitochondria with reduced number of cristae, and swollen endoplasmic reticulum in the AFB1 group (Fig. 2f) when compared to the control (Fig. 2e). Slightly swollen mitochondria and fatty degeneration were also seen in the AFB1 + Se group (Fig. 2h) in comparison to the control group, while no obvious ultrastructural changes were observed in the Se (Fig. 2g) and control group.
Apoptotic Percentage by Flow Cytometer
Annexin-V FITC was used to quantitatively determine the percentage of apoptotic cells. Apoptotic cell counts were determined by examining the total percentage of early (Annexin-V positive and PI negative) and late (both Annexin-V and PI positive) apoptotic cells. Figure 3a–d shows scattered analysis of apoptosis at 21 days in the control, AFB1 group, Se group, and AFB1 + Se group respectively. At days 7, 14, and 21, a significant increase (p < 0.01) in apoptotic cell percentage was observed in the AFB1 group, while a significant decrease (p < 0.01) in apoptotic cell percentage was seen in the AFB1 + Se group (Fig. 3e) when compared with the AFB1 group.
Apoptotic percentage and scattered analysis of early and late apoptosis in chicken hepatocytes between control, AFB1, Se group, and AFB1 + Se. Figure a, b, c, and d represent scattered analysis of early (Annexin-V positive and PI negative) and late (Annexin-V and PI positive) hepatocytes apoptosis in the control, AFB1, Se group and AFB1 + Se at 21 days respectively, while e is a bar graph showing apoptotic percentage rate in the same groups at days 7, 14, and 21, measured by flow cytometer when compared to the control
qRT-PCR Analysis of Relative Expression of Genes Involved in Death Receptor–Induced Apoptosis
The death receptor genes FAS (fatty acid synthesize receptor) and TNF-R1 (tumor necrosis factor receptor 1) and the genes involved in DISC (death-inducing signaling complex) formation i–e FADD (FAS-associated death domain), TRADD (TNF receptor-associated death domain), and TRAF2 (TNF receptor-associated factor 2) were seen significantly downregulated (p < 0.05 or p < 0.01) at 7, 14, and 21 days with an exception of TRAF2 which was not significantly downregulated (p > 0.05) at day 7 and 14 in the AFB1 + Se group when compared with the AFB1 group. The mRNA levels of CASPASE (cysteine-aspartic protease) family genes, i.e., caspase 3, caspase 8, caspase 9, and caspase 10 were seen significantly decreased at 7, 14, and 21 days (p < 0.05 or p < 0.01) in AFB1 + Se group compared to the AFB1 group. BCL-2 (B cell lymphoma 2), XIAP (X-linked inhibitor of apoptosis protein) and IKK (IκB kinase) genes displayed notable upregulation (p < 0.05 or p < 0.01) at days 7, 14, and 21 in the AFB1 + Se group when compared with the AFB1 group (Fig. 4).
Relative expression of genes involved in death receptor–induced apoptosis in the liver of chickens exposed to AFB1. Figure represents the mRNA levels of Fas, TNF-α, FADD, TRADD, TRAF2, caspase 10, caspase 8, BCL-2, IKK, XIAP, caspase 9, and caspase 3 in the liver of the AFB1-fed chickens and expressed as fold change relative to the control group (n = 6)
A time bound significant increase (p < 0.05 or p < 0.01) was observed in the expression of TNF-R, FAS, FADD, TRADD, caspase 8, caspase 9, and caspase 3 at days 7, 14, and 21 in the AFB1 group when compared to the control group, whereas no significant difference (p > 0.05) was observed in the AFB1 group at days 7 and 14. However, the mRNA expressions of TRAF2 and caspase 10 displayed maximum expression at day 14 in the AFB1 group. Also, BCL-2 and XIAP displayed the time bound decrease in the expression at days 7, 14, and 21 in the AFB1 group when compared to the control group. Se group exhibited the similar expression of the genes involved in death receptor–induced apoptosis, when compared to the control group at all the time points.
Biochemical Analysis
Compared to the control group, the activities of CAT (Catalase) and GSH-Px (glutathione peroxidase) in the AFB1 + Se group were observed significantly upregulated (p < 0.05 or p < 0.01) throughout the experiment at different time points when compared to the AFB1 group. Activity of SOD (superoxide dismutase) and hydroxyl free radical scavenging were also seen remarkably increase (p < 0.05 or p < 0.01) in the AFB1+ Se group compared to the AFB1 group at 7, 14, and 21 days. A significantly downregulated contents of MDA (Malondialdehyde) were observed at 7, 14, and 21 days (p < 0.01), while GSH (glutathione) contents were seen increased (p < 0.05 or p < 0.01) at 7, 14, and 21 days in the AFB1 + Se group when compared to the AFB1 group as represented in Fig. 5. The activities and contents of the same enzymes were not seen significantly different among control, Se group, and AFB1 + Se groups.
Biochemical analyses of activities of CAT, GSH-Px, SOD, hydroxyl ion scavenging, and contents of MDA and GSH in liver cells in different groups. a–c Activities of CAT, GSH-Px, and SOD among groups respectively. Bar graph d shows hydroxyl free radical scavenging while e and f bar graphs display contents of MDA and GSH in chicken hepatocytes when compared to the control
Discussion
Many studies have documented that AFB1 has hepatotoxic effects in human beings and animals [25,26,27]. Se, as an essential micronutrient, plays a key role in antioxidant and detoxification functions [28,29,30]. Previous studies showed that Se could ameliorate AFB1-induced hepatotoxicity [31, 32], and our studies have proven that diet with supplementation of 0.4 mg/kg Se has negative effects on immune organs [12, 33, 34]. In this study, we also observed AFB1-induced hepatotoxicity, including the cellular degeneration, and subcellular lesions including swollen mitochondria with reduced number of cristae and swollen endoplasmic reticulum. However, no obvious lesions were found in the AFB1 + Se group, + Se group, and control group. Looking at our previous results, it would be enthralling to find out how Se can amend the toxic effects of AFB1 on the broiler liver as Se has been shown to provide protective effects against several toxic agents.
Biochemical analysis suggested that Se supplementation could protect hepatocytes from AFB1-induced oxidative damage by upregulation of GSH content, SOD, CAT, GSH-Px activities, and downregulation of MDA level in the AFB1 + Se groups. It has been reported that excessive production of ROS or deactivation of antioxidant enzymes will cause oxidative stress [35] and then induce apoptosis. In addition, these enzymatic antioxidants have been recognized to play an important role in the anti-oxidant mechanism of the body [36]. Therefore, we speculated that supplemented 0.4 mg/kg Se could effectively protect the chicken’s liver against the AFB1-induced histopathological lesions by reducing oxidative stress.
It is reported that excessive apoptosis is actively involved in liver injury in various circumstances [37, 38]. In our study, a significant increase in apoptotic cell percentage was observed in AFB1 group by flow cytometry assay, but it was alleviated in the AFB1 + Se group. Why can Se alleviate AFB1-induced cell apoptosis? In this study, we detected the relative expression of genes involved in death receptors pathway, including FAS, TNF-R1, TRADD, FADD, TRAF2, Casp-10, Casp-9, Casp-8, Casp-3, IKK (IKBIP), XIAP, and BCL-2. The results showed that when compared with those of the AFB1 group, the death receptor genes FAS and TNF R1 and the genes involved in death-inducing signaling complex formation such as FADD, TRADD, and TRAF2 were significantly downregulated in Se + AFB1 group. The mRNA levels of caspase family genes, caspase 3, caspase 8, and caspase 9 were seen significantly decreased, while BCL-2 and XIAP genes were increased in Se + AFB1 group.
TNF is a major mediator of apoptosis, the initial step in TNF signaling involves in the binding of the TNF trimer to the extracellular domain of TNF-R1 and the release of the inhibitory protein silencer of death domains (SODD) from TNF-R1’s intracellular domain (ICD) [39]. The resulting aggregated TNF-R1 ICD is recognized by the adaptor protein TRADD, which recruits additional adaptor proteins receptor-interacting protein (RIP), TRAF2, and FADD. FADD is a pivotal component of death receptor-mediated extrinsic apoptosis and necroptosis [40]. Fas plays an initial role for extrinsic pathway of apoptosis and it acts as a death-receptor for triggering apoptosis [41]. The Fas death inducing signaling complex (DISC) also contains the adaptor protein FADD and caspases 8 and 10, which can initiate the process of apoptosis [42]. Fas-mediated apoptosis is controlled by a plethora of regulators of the mitochondrial pathway of cell death, for example, by BCL-2 family members [43]. In this study, the above-mentioned factors were decreased in the AFB1 + Se group, which indicated that the AFB1-induced apoptosis is inhibited by appropriate dietary Se.
It is well known that XIAP is the prominent member of the inhibitor of apoptosis proteins (IAPs) [44]. IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis [45]; IKK inhibits TNFα-induced apoptosis [46]. IKK appears to directly interfere with BCL-2 activity through phosphorylation in HNE-mediated apoptosis independent of NFκB signaling [47]. BCL-2 is known to target the protein kinase Raf-1 to mitochondria, allowing the kinase to phosphorylate and thereby inactivate proapoptotic Bad [48], and then causes caspase activation by forming a complex with Apaf-1 and procaspase-9, leading to activation of caspase-9, which in turn activates procaspase-3 [49, 50] and then induced apoptosis. It has been reported that selenium can lead to significant reduction in caspase-3 level [32], which consists with our present findings. It is suggested that Se could inhibit the activation of death receptors pathway in the liver caused by AFB1.
In conclusion, 0.4 mg/kg Se supplied in diet could protect the liver from AFB1-induced histopathological lesions by diminishing oxidative damage and relieve AFB1-induced apoptosis by the molecular regulation of death receptors pathway in the liver of broilers. The outcomes from the present study may lead to a better understanding of the nature of selenium’s essentiality and its protective roles against AFB1.
References
Ferenčík M, Ebringer L (2003) Modulatory effects of selenium and zinc on the immune system. Folia Microbiol 48:417–426
Oliveira CAF, Kobashigawa E, Reis TA, Mestieri L, Albuquerque R, Correa LMB (2000) Aflatoxin B1 residues in eggs of laying hens fed a diet containing different levels of the mycotoxin. Food Addit Contam 17:459–462
Saini SS, Kaur A (2012) Aflatoxin B1: toxicity, characteristics and analysis: mini review. Glob Adv Res J Chem Mater Sci 1:63–70
Hussain I, Anwar J (2008) A study on contamination of aflatoxin M1 in raw milk in the Punjab province of Pakistan. Food Control 19:393–395
Newberne PM, Butler WH (1969) Acute and chronic effects of aflatoxin on the liver of domestic and laboratory animals: a review. Cancer Res 29:236–250
Richard JL (2007) Some major mycotoxins and their mycotoxicoses—an overview. Int J Food Microbio 119:3–10
Kalpana S, Aggarwal M, Rao GS, Malik JK (2012) Effects of aflatoxin B1 on tissue residues of enrofloxacin and its metabolite ciprofloxacin in broiler chickens. Environ Toxicol Phar 33:121–126
Dhanasekaran D, Shanmugapriya S, Thajuddin N, Thajuddin N, Panneerselvam A (2011) Aflatoxins and aflatoxicosis in human and animals[M] Aflatoxins - Biochemistry and Molecular Biology. In Tech, p 121–123
Rawal S, Kim JE, Coulombe R Jr (2010) Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res Vet Sci 89:325–331
Bbosa GS, Kitya D, Odda J, Ogwal-Okeng J (2013) Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health 5:14–34
Peng X, Zhang S, Fang J, Cui H, Zuo Z, Deng J (2014) Protective roles of sodium selenite against aflatoxin B1-induced apoptosis of jejunum in broilers. Int J Environ Res Pub Health 11:13130–13143
Wang F, Shu G, Peng X, Fang J, Chen K, Cui H, Chen Z, Zuo Z, Deng J, Geng Y, Lai W (2013) Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Int J Environ Res Pub Health 10:2834–2844
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Mughal MJ, Peng X, Kamboh AA, Zhou Y, Fang J (2017) Aflatoxin b1 induced systemic toxicity in poultry and rescue effects of selenium and zinc. Biol Trace Elem Res 178:292–300
Imed M, Fatima H, Abdelhamid K (2009) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver of the rat: effects on the oxidative stress. Ecotoxicol Environ Saf 72:1559–1564
Tran D, Moody AJ, Fisher AS, Foulkes ME, Jha AN (2007) Protective effects of selenium on mercury-induced DNA damage in mussel haemocytes. Aquat Toxicol 84:11–18
Elgaml SA, Khalil R, Hashish EA, El-Murr A (2015) Protective effects of selenium and alpha-tocopherol against lead-induced hepatic and renal toxicity in oreochromis niloticus. J Aquac Res Dev 6:1
Jiao X, Yang K, An Y, Teng X, Teng X (2017) Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ Sci Pollut Res 24:7555–7564
Mughal MJ, Peng X, Zhou Y, Jing F (2017) Aflatoxin B1 invokes apoptosis via death receptor pathway in hepatocytes. Oncotarget 8:8239–8249
Council NR (1994) National research council nutrient requirements of poultry: ninth revised edition. National Academies Press, Washington, DC
Del Bino G (2010) Features of apoptotic cells measured by flow cytometry. Cytometry A 13:795–808
Hong YH, Lillehoj HS, Lillehoj EP, Lee SH (2006) Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet Immunol Immunopathol 114:259–272
Shini S, Kaiser P (2009) Effects of stress, mimicked by administration of corticosterone in drinking water, on the expression of chicken cytokine and chemokine genes in lymphocytes. Stress 12:388–399
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔ CT method. Methods 25:402–408
Saadhussein A, Abdalla MS, Shousha WG, Moubarz G, Mohamed AH (2014) Oxidative role of aflatoxin B1 on the liver of wheat milling workers. Maced J Med Sci 7:141–146
Knipstein B, Huang J, Barr E, Sossenheimer P, Dietzen D, Egner PA, Groopman JD, Rudnick DA (2015) Dietary aflatoxin-induced stunting in a novel rat model: evidence for toxin-induced liver injury and hepatic growth hormone resistance. Pediatr Res 78:120–127
Rastogi R, Srivastava AK, Rastogi AK (2010) Long term effect of aflatoxin b(1) on lipid peroxidation in rat liver and kidney: effect of picroliv and silymarin. Phytother Res 15:307–310
Rieswijk L, Claessen SMH, Bekers O, Herwijnen MV, Theunissen DHJ, Jennen DGJ, de Kok TMCM, Kleinjans JCS, van Breda, SGJ (2016) Aflatoxin b1 induces persistent epigenomic effects in primary human hepatocytes associated with hepatocellular carcinoma. Toxicology 350-352:31–39
Doğan S, Yazici H, Yalçinkaya E, Erdoğdu HI, Tokgöz SA, Sarici F, Namuslu M, Sarikaya Y (2016) Protective effect of selenium against cisplatin-induced ototoxicity in an experimental model. J Craniofac Surg 27:e610–e614
Sweins A (2010) Protective effect of selenium against arsenic-induced chromosomal damage in cultured human lymphocytes. Hereditas 98:249–252
Limaye A, Yu RC, Chou CC, Liu JR, Cheng KC (2018) Protective and detoxifying effects conferred by dietary selenium and curcumin against afb1-mediated toxicity in livestock: a review. Toxins 10:25–43
Liao S, Shi D, Clemonschevis CL, Guo S, Su R, Qiang P, Tang ZX (2014) Protective role of selenium on aflatoxin b1-induced hepatic dysfunction and apoptosis of liver in ducklings. Biol Trace Elem Res 162:296–301
Chen K, Shu G, Peng X, Fang J, Cui H, Chen J, Wang F, Chen Z, Zuo Z, Deng J, Geng Y, Lai W (2013) Protective role of sodium selenite on histopathological lesions, decreased T-cell subsets and increased apoptosis of thymus in broilers intoxicated with aflatoxin B1. Food Chem Toxicol 59:446–454
Chen K, Jing F, Xi P, Cui H, Chen J, Wang F, Chen Z, Zuo Z, Deng J, Lai W, Zhou Y (2014) Effect of selenium supplementation on aflatoxin B1 -induced histopathological lesions and apoptosis in bursa of Fabricius in broilers. Food Chem Toxicol 74:91–97
Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD (2004) Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India 52:794–804
Liu J, Li N, Ma L, Duan Y, Wang J, Zhao X, Wang S, Wang H, Hong F (2010) Oxidative injury in the mouse spleen caused by lanthanides. J Alloys Compd 489:708–713
Guicciardi ME, Gores GJ (2005) Apoptosis: a mechanism of acute and chronic liver injury. Gut 54:1024–1033
Taher A, Siregar NC, Sjamsuhidayat R, Lalisang TJ (2011) Inhibition of bile acid accumulation decreased the excessive hepatocyte apoptosis and improved the liver secretion functions on obstructive jaundice patients. Makara Seri Kesehatan 15:1–5
Chen G, Goeddel DV (2002) TNF-R1 signaling: a beautiful pathway. Science 296:1634–1635
Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M, Kim SJ, Ro JY, Park KM, Lee HW, Park EJ, Chun KH, Song J (2012) Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nat Commun 3:978–990
Ziegler DS, Kung AL (2008) Therapeutic targeting of apoptosis pathways in cancer. Curr Opin Oncol 20:97–103
Wajant H (2002) The Fas signaling pathway: more than a paradigm. Science 296:1635–1636
Krammer PH (2000) CD95's deadly mission in the immune system. Nature 407:789–795
Prager G, Brunner P, Mihaly J, Koschelnick Y, Binder BR (2007) Urokinase (uPA) induces endothelial cell survival via the X-linked inhibitor of apoptosis protein (XIAP). Cancer Res 68:1089–1089
Li ZW, Chu WM, Hu YL, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M (1999) The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J Exp Med 189:1839–1845
Yan J, Xiang J, Lin Y, Ma J, Zhang J, Zhang H, Sun J, Danial NN, Liu J, Lin A (2013) Inactivation of BAD by IKK inhibits TNFα-induced apoptosis independently of NF-κB activation. Cell 152:304–315
Bodur C, Kutuk O, Tezil T, Basaga H (2012) Inactivation of Bcl-2 through IκB kinase (IKK)-dependent phosphorylation mediates apoptosis upon exposure to 4-hydroxynonenal (HNE). J Cell Physiol 227:3556–3565
Wang HG, Rapp UR, Reed JC (1996) Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell 87:629–638
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic cascade. Cell 91:479–489
Funding
This work was supported by the program for Changjiang scholars, the University Innovative Research Team (IRT 0848), and the Education Department of Sichuan Province (2012FZ0066 and 2013FZ0072).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures performed in studies involving animals were in accordance with the international guidelines, and the laws and the ethical standards of China West Normal University Animal Care and Use Committee.
Conflict of Interest
The authors declare that there is no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, B., Mughal, M.J., Fang, J. et al. The Protective Role of Selenium Against AFB1-Induced Liver Apoptosis by Death Receptor Pathway in Broilers. Biol Trace Elem Res 191, 453–463 (2019). https://doi.org/10.1007/s12011-018-1623-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1623-4