Abstract
We investigated lead (Pb)-induced oxidative stress and immune damage in the chicken bursa of Fabricius (BF) and the ameliorative effect of selenium (Se). Seven-day-old male chickens were randomly divided into four groups and were provided standard diet and drinking water, Na2SeO3 added to the standard diet and drinking water, standard diet and (CH3COO)2Pb added to drinking water, and Na2SeO3 added to the standard diet and (CH3COO)2Pb added to drinking water for 30, 60, and 90 days. The presence of Pb inhibited total antioxidant capacity (T-AOC), glutathione peroxidase (GPx), glutathione S-transferase (GST), superoxide dismutase (SOD), and catalase (CAT) activities; decreased glutathione (GSH) content; increased malondialdehyde (MDA) and hydrogen peroxide (H2O2) contents; inhibited interleukin (IL)-2 and interferon-γ (IFN-γ) messenger RNA (mRNA) expression; and increased IL-4, IL-6, IL-10, IL-12β, and IL-17 mRNA expression. The presence of Se relieved all of the above Pb-induced changes. There were close correlations among GSH, CAT, T-AOC, SOD, GPx, MDA, and H2O2 and among IL-2, IL-4, IL-6, IL-12β, IL-17, and IFN-γ. Our data showed that Pb caused oxidative stress and immune damage in the chicken BF. Se alleviated Pb-induced oxidative stress and immune damage in the chicken BF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Widespread usage of lead (Pb) in industrial and agricultural production, such as acid battery and pigments (Mao et al. 2008), has caused environmental pollution and is a matter of international concern (Kong et al. 2015). Moreover, Pb is a toxic heavy metal that is hazardous to humans and animals. Pb impaired intelligence in children (Canfield et al. 2003), increased mortality in white sturgeon over time (Vardy et al. 2014), and induced oxidative damage in rat livers (Mohammadi et al. 2014; Mabrouk et al. 2016). Birds are also sensitive to Pb exposure. High level of Pb has been linked to the death of egret and heron chicks from Pyeongtaek, Korea (Kim and Oh 2015), and 59 terrestrial bird species are known to have ingested Pb or suffered Pb poisoning from ammunition sources (Fisher et al. 2006). Pb can inhibit the normal function of mouse splenic macrophages (Bishayi and Sengupta 2006) and impair immune system functioning (Dietert and Piepenbrink 2006). The bursa of Fabricius (BF) is the central humoral immune organ and is unique to birds (Ribatti et al. 2006). However, little is known about the effect of excess Pb on chicken BF. Therefore, we investigated the effect of Pb toxicity on the chicken BF.
Oxidative stress is an important phenomenon in Pb-induced toxicity (Wang et al. 2009). Excess Pb increased malondialdehyde (MDA) content; decreased total antioxidant capacity (T-AOC), glutathione peroxidase (GPx), and superoxide dismutase (SOD) activities; decreased glutathione (GSH) content; and caused oxidative stress in tilapia spleens (Dai et al. 2012). In rat livers and erythrocytes, excess Pb decreased GPx, glutathione S-transferase (GST), SOD, and catalase (CAT) activities; decreased GSH content; increased MDA and hydrogen peroxide (H2O2) contents; and caused oxidative stress (Omobowale et al. 2014). Cytokines are biomarkers of heavy metal-induced immune toxicity. Cadmium (Cd) decreased interleukin (IL)-2 and interferon-γ (IFN-γ) messenger RNA (mRNA) expression and suppressed immune function in chicken splenic lymphocytes (Xu et al. 2015). In oral mucosal epithelial cells, mercury induced IL-4 mRNA expression and lupus-like lesions (Seno et al. 2013). Cd induced IL-6 and inflammatory response in rat plasma (Kataranovski et al. 2009). IL-2, IL-4, IL-6, IL-12β, IL-17, and IFN-γ involved in manganese (Mn)-induced toxicity in chicken splenic lymphocytes (Lu et al. 2015). We speculated that Pb poisoning would alter oxidative stress indicators and cytokines in the chicken BF. Therefore, we examined indicators of oxidative stress (T-AOC, SOD, CAT, GPx, GST, GSH, MDA, and H2O2) and cytokines (IL-2, IL-4, IL-6, IL-12β, IL-17, and IFN-γ).
Selenium (Se), an essential trace element in human beings and animals, can alleviate the toxicity of heavy metal ions such as Pb. Se alleviated Pb-induced toxicity in the livers and brains of Cyprinus carpio (Ozkan-Yilmaz et al. 2014) and in the kidney of Nile tilapia (Hashish et al. 2015). Se also alleviated Pb-induced toxicity in chicken livers (Xu et al. 2016) and sword cartilage (Gao et al. 2016). Previous research from our group demonstrated that Se alleviated Pb-induced toxicity in chicken livers (Wang et al. 2015) and muscles (Jin et al. 2016). However, Pb toxicity and the alleviative effect of Se on Pb toxicity in chicken BF remain unclear. In this study, we established a combined model of Se and Pb to investigate the effect of Pb on indicators of oxidative stress (T-AOC, SOD, CAT, GPx, GST, GSH, MDA, and H2O2) and cytokines (IL-2, IL-4, IL-6, IL-12β, IL-17, and IFN-γ) and to determine whether Se can prevent oxidative stress and immune injury induced by Pb in the chicken BF.
Materials and methods
Animal model and tissue samples
All procedures used in this study were approved by the Northeast Agricultural University Institutional Animal Care and Use Committee. One hundred eighty 1-day-old healthy Hyline male chickens were fed a standard commercial diet (containing 0.49 mg/kg Se) and drinking water for 7 days prior to experiments. Chickens were randomly divided into four groups: the control group, the Se group, the Pb group, and the Se/Pb group. The control group was fed a standard commercial diet and drinking water. The Se group was fed Na2SeO3 (analytical reagent grade, Tianjinzhiyuan Chemical Reagent Co. Ltd., Tianjin, China) added to the standard commercial diet at 1 mg/kg Se and drinking water. The Pb group was fed a standard commercial diet and (CH3COO)2Pb (analytical reagent grade, Tianjinzhiyuan Chemical Reagent Co. Ltd., Tianjin, China) was added to the drinking water at 350 mg/L Pb, according to median lethal dose (LD50) for Pb in chickens (Vengris and Mare 1974) and the need of the chicken experiment in toxicology (Klaassen 2013). The Se/Pb group was fed Na2SeO3, which was added to a standard commercial diet at 1 mg/kg Se, and (CH3COO)2Pb, which was added to the drinking water at 350 mg/L Pb. All animals were housed in the Laboratory Animal Center, College of Veterinary Medicine, Northeast Agricultural University (Harbin, China), and given feed and water ad libitum, in accordance with chicken feeding operation procedures.
On the 30th, 60th, and 90th days of the experiment, 15 chickens in each group were randomly selected for euthanasia. BF samples were immediately collected, washed with ice-cold saline, and divided into three parts. The first part was fixed with 2.5% glutaraldehyde phosphate buffered saline (v/v, pH 7.2) for ultrastructure observation. The second part was immediately homogenized to determine GSH, MDA, and H2O2 contents and the activities of T-AOC, GPx, GST, SOD, and CAT. The third part was immediately frozen in liquid nitrogen and stored at −80 °C for later determination of IL-2, IL-4, IL-6, IL-12β, IL-17, and IFN-γ mRNA expression.
Ultrastructure
Tissue from the BF (1.0 mm × 1.0 mm × 1.0 mm) was immediately put into 2.5% glutaraldehyde phosphate buffer saline (v/v, pH 7.2) for 3 h at 4 °C followed by immersion in 1% (v/v) osmium tetroxide for 1 h at 4 °C. Samples were washed in phosphate buffer saline then impregnated with epoxy resin. The ultrathin sections were stained with uranyl acetate and lead citrate. Ultrastructure was observed under a transmission electron microscope.
T-AOC, GPx, GST, SOD, and CAT activities and GSH, MDA, and H2O2 contents
T-AOC, GPx, GST, SOD, and CAT activities and GSH, MDA, and H2O2 contents were determined using diagnostic kits produced by NanJing JianCheng Bioengineering Institute (NanJing, China) following the manufacturer’s instructions.
IL-2, IL-4, IL-6, IL-12β, IL-17, and IFN-γ mRNA expression
Primer synthesis
Primer sequences for β-actin (NM_205518), IL-2 (NM_204153), IL-4 (NM_001007079), IL-6 (NM _204628), IL-12β (NM_213571), IL-17 (NM_204460), and IFN-γ (NM_205149) published in GenBank were selected and synthesized by the Invitrogen Biotechnology Co. Ltd. (Shanghai, China) using the following sequences: β-actin forward sense: CTCTCGGCTGTGGTGGTGAA; β-actin reverse sense: CCGCTCTATGAAGGCTACGC; IL-2 forward sense: GAACCTCAAGAGTCTTACGGGTCTA; IL-2 reverse sense: ACAAAGTTGGTCAGTTCATGGAGA; IL-4 forward sense: GTGCCCACGCTGTGCTTAC; IL-4 reverse sense: AGGAAACCTCTCCCTGGATGTC; IL-6 forward sense: AAATCCCTCCTCGCCAATCT; IL-6 reverse sense: CCCTCACGGTCTTCTCCATAAA; IL-12β forward sense: TGTCTCACCTGCTATTTGCCTTAC; IL-12β reverse sense: CATACACATTCTCTCTAAGTTTCCACTGT; IL-17 forward sense: CATGTTGTCAGCCAGCATTTCT; IL-17 reverse sense: CATCTTTTTGGGTTAGGCATCC; IFN-γ forward sense: AAGTCATAGCGGCACATCAAAC; and IFN-γ reverse sense: CTGGAATCTCATGTCGTTCATCG. β-actin was used as an internal reference gene.
Total RNA extraction and reverse transcription
Total RNA was extracted from frozen BF samples using RNAiso Plus (Takara, Japan) according to the manufacturer’s protocol. The ratios of the optical densities at 260 and 280 nm (OD260/OD280) were measured using a GeneQuant 1300/100 spectrophotometer (Healthcare Bio-Sciences AB, Sweden) to determine RNA purity. The total RNA was immediately used to synthesize complementary DNA (cDNA). The cDNA was synthesized using a reverse transcriptase (RT) mix (HiGene, Harbin, China) containing 2.5 μL of golden MLV reverse transcriptase, 6 μL of 10× RT buffer, 3 μL of dNTP mixture (10 mM each), 6 μL of total RNA, 3 μL of 20× oligo (dT) (25) and random primer, 1.5 μL of RNase inhibitor (40 U/μL), and 38 μL of RNase-free H2O. The RT program was 30 °C for 15 min, 55 °C for 30 min, and 80 °C for 10 min. The cDNA product generated by RT was diluted fivefold with sterile water and stored at −20 °C for real-time quantitative PCR.
Real-time quantitative PCR
Real-time quantitative PCR was performed using a LightCycler®96 (Roche, Switzerland) according to the manufacturer’s instructions. Reactions were performed in a 10-μL mixture including 1 μL of diluted cDNA, 0.3 μL of forward primer (10 μM), 0.3 μL of reverse primer (10 μM), 5 μL of SYBR green master, and 3.4 μL of PCR-grade water. The PCR program was 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s, 95 °C for 10 s, 65 °C for 60 s, and 97 °C for 1 s. The melting curve showed a single peak for each PCR product. The experiment was repeated three times for each sample. The relative abundance of mRNA was calculated according to the Pfaffl method (Pfaffl 2001).
Statistical analysis
All data analyses were performed using SPSS (version 17; SPSS Inc., Chicago, IL, USA) for Windows. Statistical comparisons were performed using one-way ANOVA and verified by non-parametric Kruskal-Wallis and Mann-Whitney U tests. Different lowercase letters indicate significance among all groups at the same time point, with P < 0.05. Data are expressed as the mean ± standard deviation. Pearson’s r was used to measure the linear correlation among multiple variables. Principal component analysis (PCA) was used to define the most important parameters, which were also used as key factors for individual variations using the Statistics 6.0 program (version 19, SPSS Inc., Chicago, IL, USA). All variables were standardized using z-scores and then calculated using the squared Euclidean distance. Finally, hierarchical clustering was performed with a standardized dataset using Ward’s method.
Results
Ultrastructure
Observation of the ultrastructure of chicken BF on the 90th day revealed that cells in the control group (Fig. 1a) and in the Se group (Fig. 1b) were normal, with smooth rounded nucleolus (NU), normal chromatin, and intact mitochondria (MI) with normal cristae. Cells in the Se/Pb group had swollen mitochondria with degenerated and disordered cristae (Fig. 1c1, 1c2). Cells in the Pb group had swollen mitochondria with even more pronounced cristae degeneration and vacuoles (Fig. 1d1, 1d2).
T-AOC, GPx, GST, SOD, and CAT activities and GSH, MDA, and H2O2 contents
Measuring T-AOC, GPx, GST, SOD, and CAT activities and GSH, MDA, and H2O2 contents revealed no significant differences (P > 0.05) in T-AOC (Fig. 2a), GST (Fig. 2c), SOD (Fig. 2d), and CAT (Fig. 2e) activities and the contents of GSH (Fig. 2f), MDA (Fig. 2g), and H2O2 (Fig. 2h) between the control group and the Se group on the 30th, 60th, and 90th days of the study. However, GPx (Fig. 2b) activity in the Se group was significantly higher (P < 0.05) than that in the control group. T-AOC, GPx, GST, SOD, and CAT activities and GSH content in the Pb group were significantly lower (P < 0.05) than those in the control, Se, and Se/Pb groups on the 30th, 60th, and 90th days. T-AOC, GPx, GST, SOD, and CAT activities and GSH content in the Se/Pb group were significantly lower (P < 0.05) than those in the control and Se groups on the 30th, 60th, and 90th days. MDA and H2O2 contents in the Pb group were significantly higher (P < 0.05) than those in the control, Se, and Se/Pb groups on the 30th, 60th, and 90th days. MDA and H2O2 contents in the Se/Pb group were significantly higher (P < 0.05) than those in the control and Se groups on the 30th, 60th, and 90th days.
The effect of Pb on T-AOC, SOD, CAT, GPx, and GST activities; GSH, MDA, and H2O2 contents; and antagonistic effect of Se on Pb in chicken BF. Forty-five chickens consisted of 5 replicate pens, with each pen containing 9 chickens. Three chickens were randomly selected from each pen on the 30th, 60th, and 90th days. One chicken each pen was measured. Bars represent mean ± SD (n = 5). Bars with different lowercase letters in different groups at the same time point are significantly different (P < 0.05)
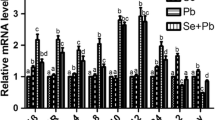
IL-2, IL-4, IL-6, IL-12β, IL-17, and IFN-γ mRNA expression
As shown in Fig. 3, there were no significant differences (P > 0.05) of IL-2 (Fig. 3a), IL-4 (Fig. 3b), IL-6 (Fig. 3c), IL-12β (Fig. 3d), IL-17 (Fig. 3e), and IFN-γ (Fig. 3f) mRNA expression between the control group and the Se group on the 30th, 60th, and 90th days in the experiment. IL-2 and IFN-γ mRNA expression in the Pb group was significantly lower (P < 0.05) than that in the control, Se, and Se/Pb groups on the 30th, 60th, and 90th days. IL-2 and IFN-γ mRNA expression in the Se/Pb group was significantly lower (P < 0.05) than that in the control and Se groups on the 30th, 60th, and 90th days. IL-4, IL-6, IL-12β, and IL-17 mRNA expression in the Pb group was significantly higher (P < 0.05) than that in the control, Se, and Se/Pb groups on the 30th, 60th, and 90th days. IL-4, IL-6, IL-12β, and IL-17 mRNA expression in the Se/Pb group was significantly higher (P < 0.05) than that in the control and Se groups on the 30th, 60th, and 90th days.
The effect of Pb on IL-2, IL-4, IL-6, IL-12β, IL-17, and IFN-γ mRNA expression and antagonistic effect of Se on Pb in chicken BF. Forty-five chickens consisted of five replicate pens, with each pen containing nine chickens. Three chickens were randomly selected from each pen on the 30th, 60th, and 90th days. One chicken each pen was measured. Bars represent mean ± SD (n = 5). Bars with different lowercase letters in different groups at the same time point are significantly different (P < 0.05)
Chemometrics
Correlation coefficient analysis
Pearson’s r correlation coefficient analysis (Table 1) indicated significant positive correlations among CAT, GPx, GSH, SOD, and T-AOC (at the 0.01 level); among IL-4, IL-6, IL-12β, and IL-17 (at the 0.01 level); between H2O2 and MDA (at the 0.01 level); between IL-2 and IFN-γ (at the 0.01 level); and between GSH and IL-12β (at the 0.05 level). There were significant negative correlations between MDA and IL-12β (at the 0.05 level); between H2O2 and CAT, GPx, GSH, SOD, and T-AOC (at the 0.01 level); between MDA and CAT, GPx, GSH, SOD, and T-AOC (at the 0.01 level); between IL-2 and IL-4, IL-6, IL-12β, and IL-17 (at the 0.01 level); and between IFN-γ and IL-4, IL-6, IL-12β, and IL-17 (at the 0.01 level). The results in Table 1 described the relationships among these factors and clearly revealed significant correlations among antioxidant factors and among cytokines.
Principal component analysis
The results of the PCA are shown in Tables 2 and 3 and Fig. 4. Parameter determination was based on ordination plots, corresponding to the first and second principal components as 56.067 and 33.637%, respectively (Tables 2 and 3). In addition, Fig. 4 clearly indicated that GSH, CAT, T-AOC, SOD, GPx, MDA, and H2O2 were closest to each other in the principal component (PC) 1 matrix, meaning that their relationships were closer based on PC 1. Among these antioxidant factors, GSH, CAT, T-AOC, SOD, and GPx had clearly opposite relationships with MDA and H2O2 in PC 1 (Table 3 and Fig. 4). Moreover, Table 3 and Fig. 4 show that IL-4, IL-6, IL-12β, and IL-17 were positively correlated with PC 2, contrary to IL-2 and IFN-γ.
Discussion
Organisms maintain their homeostasis through antioxidant enzyme system, such as T-AOC, GPx, GST, SOD, and CAT, and non-enzymatic antioxidant system, such as GSH. Pb can damage mitochondria and the antioxidant enzyme system and reduce the antioxidant capacity of organisms (Venkareddy and Muralidhara 2015; Mabrouk et al. 2016). In rats, excess Pb reduced the levels of GPx and GSH in livers (Liu et al. 2011) and kidneys (Liu et al. 2011; Lakshmi et al. 2013); increased MDA content in livers and kidneys (Lakshmi et al. 2013); and caused oxidative stress. Ni et al. (2004) found that Pb increased H2O2 content and caused oxidative stress in human coronary artery endothelial cells and vascular smooth muscle cells. However, little is known about the effect of excess Pb on T-AOC, GPx, GST, SOD, and CAT activities and the contents of GSH, MDA, and H2O2 in chicken BF. Therefore, we studied the effect of excess Pb on oxidative stress factors in the chicken BF. We found that Pb decreased T-AOC, GPx, GST, SOD, and CAT activities; decreased GSH content; and increased MDA and H2O2 contents. Our results suggested that excess Pb caused oxidative stress in the chicken BF. Previous studies have shown similar mechanisms. Excess Mn decreased GPx activity, increased MDA content, and caused oxidative stress in chicken splenic lymphocytes (Zhu et al. 2016). Pb increased MDA content and reduced GPx, SOD, and CAT activities in worker erythrocytes (Chinde et al. 2014). Liu et al. (2013) showed that excess Mn reduced T-AOC, GPx, and SOD activities; increased MDA content; and caused oxidative stress in chicken BF.
Oxidative stress has been shown to impair the immune function of chickens (Zhang et al. 2012). Pb is toxic to immune function in humans and other species (Dietert et al. 2004). Heavy metals can change cytokines and cause immune toxicity in organisms. Excess Mn decreased IL-2 mRNA expression and induced immune suppression in chicken BF (Liu et al. 2012b) and splenic lymphocytes (Lu et al. 2015). Hemdan et al. (2005) reported that Pb induced IL-4, inhibited IFN-γ, and caused immune toxicity in human peripheral mononuclear blood cells. Excess Pb increased IL-6 and caused inflammation in rat kidneys (Liu et al. 2012a). In rats, Pb poisoning during late pregnancy increased IL-12 and caused immune toxicity in the splenic cells of their offspring (Bunn et al. 2001). Cd increased IL-17 mRNA expression and induced immune suppression in rat spleens (Demenesku et al. 2014). Pb reduced IFN-γ and caused immune suppression in rat T cells (Fang et al. 2012). Similar results were obtained in our study. We found that Pb increased IL-4, IL-6, IL-12β, and IL-17 mRNA expression and decreased IL-2 and INF-γ mRNA expression in the chicken BF. Our results suggested that excess Pb caused immune toxicity in the chicken BF. Moreover, our morphological examination revealed that excess Pb caused ultrastructural changes and immune damage in the chicken BF. Liu et al. (2013) also found that excess Mn damaged the structure of chicken BF.
Se is involved in a variety of physiological processes in the form of selenoproteins (Yao et al. 2013). In our study, Se increased GPx activity in the chicken BF. Se can counteract Cd-induced oxidative stress and enhance immunity (Zwolak and Zaporowska 2012). Se intake improved immune function in adults (Broome et al. 2004) and antagonized the toxic effect of Cd and arsenic. Se ameliorated Cd-induced SOD and GPx activities and oxidative stress in chicken testes (Li et al. 2010) and livers (Li et al. 2013). In rats, Se ameliorated changes in MDA and IL-6 levels, oxidative stress, and hepatotoxicity induced by arsenic (Shafik and El Batsh 2016). Se also ameliorated the Cd-induced decrease in GPx, SOD, and CAT activities; GSH and IFN-γ concentrations; increase in MDA and IL-6 concentration; oxidative stress; and immune suppression in rat livers and kidneys (El-Boshy et al. 2015). Se alleviated Cd-induced reticulum damage (Zhao et al. 2014); changes in IL-2, IL-4, IL-17, and IFN-γ mRNA expression; and immune toxicity in chicken splenic lymphocytes (Xu et al. 2015). Se improved fish immune response induced by copper toxicity (Abdel-Tawwab et al. 2007). However, little is known about whether Se can alleviate Pb-induced immune toxicity. Our study showed that Se alleviated Pb-induced changes in indicators of oxidative stress (T-AOC, SOD, CAT, GPx, GST, GSH, MDA, and H2O2) and cytokines (IL-2, IFN-γ, IL-4, IL-6, IL-12β, and IL-17), suggesting that Se alleviated Pb-induced oxidative stress and immune toxicity in the chicken BF.
In summary, our data showed that Pb caused mitochondrial swelling with cristae degeneration and vacuoles; inhibited T-AOC, GPx, GST, SOD, and CAT activities; decreased GSH content; increased MDA and H2O2 contents; inhibited IL-2 and IFN-γ mRNA expression; and induced IL-4, IL-6, IL-10, IL-12β, and IL-17 mRNA expression. Se alleviated Pb-induced changes to ultrastructure and to all of the above factors. Our data demonstrated that Pb caused oxidative stress and immune damage in the chicken BF. Se alleviated Pb-induced oxidative stress and immune damage in the chicken BF. Our research suggested that the mechanism for Pb-induced injury to the BF of chickens is as follows: excess Pb induces oxidative stress, then alters biomacromolecule functions of immune cells and the structure of BF, and finally influences cytokine mRNA expression in the chicken BF.
References
Abdel-Tawwab M, Mousa MAA, Abbass FE (2007) Growth performance and physiological response of African catfish, Clarias gariepinus (B.) fed organic selenium prior to the exposure to environmental copper toxicity. Aquaculture. doi:10.1016/j.aquaculture.2007.09.004
Bishayi B, Sengupta M (2006) Synergism in immunotoxicological effects due to repeated combined administration of arsenic and lead in mice. Int Immunopharmacol. doi:10.1016/j.intimp.2005.09.011
Bunn TL, Parsons PJ, Kao E, Dietert RR (2001) Exposure to lead during critical windows of embryonic development: differential immunotoxic outcome based on stage of exposure and gender. Toxicol Sci. doi:10.1093/toxsci/64.1.57
Broome CS, McArdle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ (2004) An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 80(1):154–162
Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP (2003) Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. doi:10.1056/NEJMoa022848348
Chinde S, Kumari M, Devi KR, Murty US, Rahman MF, Kumari SI, Mahboob M, Grover P (2014) Assessment of genotoxic effects of lead in occupationally exposed workers. Environ Sci Pollut Res. doi:10.1007/s11356-014-3128-9
Dai W, Liu S, Fu L, Du H, Xu Z (2012) Lead (Pb) accumulation, oxidative stress and DNA damage induced by dietary Pb in tilapia (Oreochromis niloticus). Aquac Res. doi:10.1111/j.1365-2109.2011.02817.x
Demenesku J, Mirkov I, Ninkov M, Popov Aleksandrov A, Zolotarevski L, Kataranovski D, Kataranovski M (2014) Acute cadmium administration to rats exerts both immunosuppressive and proinflammatory effects in spleen. Toxicology. doi:10.1016/j.tox.2014.10.012
Dietert RR, Lee JE, Hussain I, Piepenbrink M (2004) Developmental immunotoxicology of lead. Toxicol Appl Pharmacol. doi:10.1016/j.taap.2003.08.020
Dietert RR, Piepenbrink MS (2006) Lead and immune function. Crit Rev Toxicol. doi:10.1080/10408440500534297
El-Boshy ME, Risha EF, Abdelhamid FM, Mubarak MS, Hadda TB (2015) Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol. doi:10.1016/j.jtemb.2014.05.009
Fang L, Zhao F, Shen X, Ouyang W, Liu X, Xu Y, Yu T, Jin B, Chen J, Luo W (2012) Pb exposure attenuates hypersensitivity in vivo by increasing regulatory T cells. Toxicol Appl Pharmacol. doi:10.1016/j.taap.2012.10.001
Fisher IJ, Pain DJ, Thomas VG (2006) A review of lead poisoning from ammunition sources in terrestrial birds. Biol Conserv. doi:10.1016/j.biocon.2006.02.018
Gao H, Liu CP, Song SQ, Fu J (2016) Effects of dietary selenium against lead toxicity on mRNA levels of 25 selenoprotein genes in the cartilage tissue of broiler chicken. Biol Trace Elem Res. doi:10.1007/s12011-015-0579-x
Hashish EA, Elgaml SA, El-Murr A, Khalil R (2015) Nephroprotective and antioxidant significance of selenium and alpha-tocopherol on lead acetate-induced toxicity of Nile tilapia (Oreochromis niloticus). Fish Physiol Biochem. doi:10.1007/s10695-015-0035-z
Hemdan NY, Emmrich F, Adham K, Wichmann G, Lehmann I, El-Massry A, Ghoneim H, Lehmann J, Sack U (2005) Dose-dependent modulation of the in vitro cytokine production of human immune competent cells by lead salts. Toxicol Sci. doi:10.1093/toxsci/kfi177
Jin X, Liu CP, Teng XH, Fu J (2016) Effects of dietary selenium against lead toxicity are related to the ion profile in chicken muscle. Biol Trace Elem Res. doi:10.1007/s12011-015-0585-z
Kataranovski M, Jankovic S, Kataranovski D, Stosic J, Bogojevic D (2009) Gender differences in acute cadmium-induced systemic inflammation in rats. Biomed Environ Sci. doi:10.1016/S0895-3988(09)60014-3
Kim J, Oh JM (2015) Tissue distribution of heavy metals in heron and egret chicks from Pyeongtaek, Korea. Arch Environ Contam Toxicol. doi:10.1007/s00244-014-0110-y
Klaassen C (2013) Casarett & Doull's toxicology: the basic science of poisons. McGraw-Hill, New York
Kong P, Luo W, Lu Y, Wang T, Jiao W, Hu W, Naile JE, Khim JS, Giesy JP (2015) Distribution and bioaccumulation of lead in the coastal watersheds of the northern Bohai and yellow seas in China. Environ Geochem Health. doi:10.1007/s10653-014-9664-x
Lakshmi BV, Sudhakar M, Aparna M (2013) Protective potential of black grapes against lead induced oxidative stress in rats. Environ Toxicol Pharmacol. doi:10.1016/j.etap.2013.01.008
Li JL, Gao R, Li S, Wang JT, Tang ZX, Xu SW (2010) Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals. doi:10.1007/s10534-010-9334-0
Li JL, Jiang CY, Li S, Xu SW (2013) Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotox Environ Safe. doi:10.1016/j.ecoenv.2013.07.007
Liu CM, Ma JQ, Sun YZ (2011) Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem Toxicol. doi:10.1016/j.fct.2011.09.007
Liu CM, Sun YZ, Sun JM, Ma JQ, Cheng C (2012a) Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-kappaB pathway. Biochim Biophys Acta. doi:10.1016/j.bbagen.2012.06.011
Liu XF, Li ZP, Tie F, Liu N, Zhang ZW, Xu SW (2013) Effects of manganese-toxicity on immune-related organs of cocks. Chemosphere. doi:10.1016/j.chemosphere.2012.10.072
Liu X, Li Z, Han C, Zhang Z, Xu S (2012b) Effects of dietary manganese on Cu, Fe, Zn, Ca, Se, IL-1β, and IL-2 changes of immune organs in cocks. Biol Trace Elem Res. doi:10.1007/s12011-012-9377-x
Lu X, Zhu Y, Bai R, Li S, Teng X (2015) The effect of manganese-induced toxicity on the cytokine mRNA expression of chicken spleen lymphocytes in vitro. Res Vet Sci. doi:10.1016/j.rvsc.2015.05.009
Mabrouk A, Bel HSI, Chaieb W, Ben CH (2016) Protective effect of thymoquinone against lead-induced hepatic toxicity in rats. Environ Sci Pollut Res. doi:10.1007/s11356-016-6419-5
Mao JS, Dong J, Graedel TE (2008) The multilevel cycle of anthropogenic lead: II. Results and discussion. Resour Conserv Recy. doi:10.1016/j.resconrec.2008.04.005
Mohammadi M, Ghaznavi R, Keyhanmanesh R, Sadeghipour HR, Naderi R, Mohammadi H (2014) Caloric restriction prevents lead-induced oxidative stress and inflammation in rat liver. Thescientificwordjo. doi:10.1155/2014/821524
Ni Z, Hou S, Barton CH, Vaziri ND (2004) Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells. Kidney Int. doi:10.1111/j.1523-1755.2004.66032.x
Omobowale TO, Oyagbemi AA, Akinrinde AS, Saba AB, Daramola OT, Ogunpolu BS, Olopade JO (2014) Failure of recovery from lead induced hepatoxicity and disruption of erythrocyte antioxidant defence system in Wistar rats. Environ Toxicol Pharmacol. doi:10.1016/j.etap.2014.03.002
Ozkan-Yilmaz F, Ozluer-Hunt A, Gunduz SG, Berkoz M, Yalin S (2014) Effects of dietary selenium of organic form against lead toxicity on the antioxidant system in Cyprinus carpio. Fish Physiol Biochem. doi:10.1007/s10695-013-9848-9
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. doi:10.1093/nar/29.9.e45
Ribatti D, Crivellato E, Vacca A (2006) The contribution of Bruce Glick to the definition of the role played by the bursa of Fabricius in the development of the B cell lineage. Clin Exp Immunol. doi:10.1111/j.1365-2249.2006.03131.x
Seno K, Ohno J, Ota N, Hirofuji T, Taniguchi K (2013) Lupus-like oral mucosal lesions in mercury-induced autoimmune response in Brown Norway rats. BMC Immunol. doi:10.1186/1471-2172-14-47
Shafik NM, El Batsh MM (2016) Protective effects of combined selenium and Punica granatum treatment on some inflammatory and oxidative stress markers in arsenic-induced hepatotoxicity in rats. Biol Trace Elem Res. doi:10.1007/s12011-015-0397-1
Vardy DW, Santore R, Ryan A, Giesy JP, Hecker M (2014) Acute toxicity of copper, lead, cadmium, and zinc to early life stages of white sturgeon (Acipenser transmontanus) in laboratory and Columbia River water. Environ Sci Pollut Res. doi:10.1007/s11356-014-2754-6
Vengris VE, Mare CJ (1974) Lead poisoning in chickens and the effect of lead on interferon and antibody production. Can J Comp Med 38(3):328–335
Venkareddy LK, Muralidhara (2015) Potential of casein as a nutrient intervention to alleviate lead (Pb) acetate-mediated oxidative stress and neurotoxicity: first evidence in Drosophila melanogaster. Neurotoxicology. doi:10.1016/j.neuro.2015.03.014
Wang H, Li S, Teng X (2015) The antagonistic effect of selenium on lead-induced inflammatory factors and heat shock proteins mRNA expression in chicken livers. Biol Trace Elem Res. doi:10.1007/s12011-015-0532-z
Wang L, Wang H, Hu M, Cao J, Chen D, Liu Z (2009) Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol. doi:10.1007/s00204-009-0425-z
Xu F, Liu S, Li S (2015) Effects of selenium and cadmium on changes in the gene expression of immune cytokines in chicken splenic lymphocytes. Biol Trace Elem Res. doi:10.1007/s12011-015-0254-2
Xu T, Gao X, Liu G (2016) The antagonistic effect of selenium on lead toxicity is related to the ion profile in chicken liver. Biol Trace Elem Res. doi:10.1007/s12011-015-0422-4
Yao HD, Wu Q, Zhang ZW, Li S, Wang XL, Lei XG, Xu SW (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta. doi:10.1016/j.bbagen.2013.01.007
Zhang ZW, Wang QH, Zhang JL, Li S, Wang XL, Xu SW (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res. doi:10.1007/s12011-012-9439-0
Zhao W, Liu W, Chen X, Zhu Y, Zhang Z, Yao H, Xu S (2014) Four endoplasmic reticulum resident selenoproteins may be related to the protection of selenium against cadmium toxicity in chicken lymphocytes. Biol Trace Elem Res. doi:10.1007/s12011-014-0135-0
Zhu Y, Li S, Teng X (2016) The involvement of the mitochondrial pathway in manganese-induced apoptosis of chicken splenic lymphocytes. Chemosphere. doi:10.1016/j.chemosphere.2016.03.081
Zwolak I, Zaporowska H (2012) Selenium interactions and toxicity: a review. Cell Biol Toxicol. doi:10.1007/s10565-011-9203-9
Acknowledgements
This study was funded by the Scientific Research Projects of Education Department of Heilongjiang Province (No. 12541006) and the Heilongjiang Province on Natural Fund Project (No. 41400172-4-14089).
All authors have read the manuscript and agreed to submit it for consideration in its current form for publication in Environmental Science and Pollution Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Xiaoyan Jiao and Kai Yang contributed equally and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Jiao, X., Yang, K., An, Y. et al. Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ Sci Pollut Res 24, 7555–7564 (2017). https://doi.org/10.1007/s11356-016-8329-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8329-y