Abstract
The aim of the present study was to determine concentrations of 15 macro- and micro-elements in 10 commercially available plantation Arabica coffee brands. The elemental concentration was studied in 50 samples of green and roasted coffee beans of various roasting degrees and their infusions. There were four different roasting degrees: City + (C+), Full city (FC), Full city ++ (FC++), and Dark (D). The attention was given to the impact of the roasting process on the elemental composition. Statistically significant differences were found between the green coffee beans and the different roasting degrees in both macro- and micro-elements. The results showed that roasting degrees affected the concentration of elements in resulting infusions. Dietary intakes for macro- and micro-elements based on RDA and PTWI (in the case of Al) were calculated. Based on the RDA values for macro- and micro-elements, it can be concluded that coffee infusions are not an important source of elements in nutrition. In the case of Al, consumption of coffee was considered non-hazardous based on the PTWI value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coffee belongs to the most popular non-alcoholic beverages worldwide [1]. Coffee consumption has increased by 1.90% over the past 3 years to 9.4 million tons per year [2]. This commodity has an enormous economic importance [3]. Its pleasant flavor and aroma together with beneficial influence on health are the main causes of its still increasing consumption [3, 4]. Due to great public interest, numerous researches have focused on its biological and physiological implications [3, 5,6,7,8]. Coffee is suggested to have a role in prevention of chronic diseases such as diabetes mellitus type 2, Parkinson’s disease, and liver disease [9] or may even decrease the risk of colorectal cancer [8, 9].
The genus Coffee (family Rubiaceae) includes around 90 different species with C. arabica and C. canephora (also known as C. robusta) having the strongest economical value [10, 11]. Arabica and Robusta coffees differ in many aspects, such as their ideal growing climates or chemical composition [10]. C. arabica represents approximately 60% of the world’s production [2] and is a superior source of enjoyable flavor and fragrance [4, 12]. C. canephora accounts for around 40% of the global coffee market [2]. As it name suggests, Robusta plants are stronger, more resistant to diseases, and less demanding to climate conditions. Robusta coffee beans contain more caffeine, more soluble solids and elements, and have higher amount of antioxidant substances than C. arabica [4, 12].
Green seeds are raw unprocessed seeds of coffee fruits. They are considerably different in a number of aspects from roasted coffee regularly consumed around the world [4, 12]. The effect of roasting on the chemical changes in the processed product is obvious and alternations of some coffee constituents, such as proteins or dietary fiber, are described in numerous publications [13,14,15]. Particular attention is devoted to the effect of roasting on the concentration of chlorogenic acids and their derivates [16, 17].
Among elements that can be found in the green coffee beans (3.5–4.5%), potassium is the major macro-element accounting for 40% of the total elemental concentration [3, 10, 18]. The remaining concentration is represented by sodium, magnesium, phosphorus, calcium, sulfur, and trace elements including zinc, strontium, silicon, manganese, iron, copper, barium, boron, and aluminum [3, 10, 18,19,20,21]. Differences in elemental concentration of green coffee beans between C. arabica and C. robusta are not significant, although the total mineral content is slightly lower in C. arabica beans (3.5–4.5%) compared to C. robusta (3.9–4.5%) [10]. In a study by Martín et al. [11], concentrations of Cu, Mn, and P were found to be discriminating variables between C. arabica and C. robusta coffee types. However, still, the diverse amounts of elements in coffee beans reflect the character of various factors including physiological characteristics and cultivation condition [3, 4].
Regular consumption of coffee imposes an impact on determination of chemical composition with a great emphasis on elemental composition [22, 23]. To secure food quality and safety, the levels of essential, non-essential, and toxic elements are important to be known and controlled [21, 23]. A number of experimental works have been focused on elemental composition of different coffee samples [19, 21, 23,24,25] and/or coffee brews [19, 23, 25,26,27]. Notable attention is understandably put on hazardous or toxic elements [27, 28], since high concentrations of several heavy metals have been found in some coffee samples [19, 22, 27, 28].
The variability of mineral composition in context of processing and production conditions is discussed by Cruz et al. [12]. However, the knowledge about changes in elemental composition due to roasting is still not very ample [24]. As far as we know, the only investigation of metal concentration alternations in raw and roasted coffee beans was conducted by Van Cuong et al. [24].
Therefore, the aim of the present study was to determine concentrations of 15 macro- and micro-elements in 10 commercially available plantation Arabica coffee and their infusions. Since the applied technological food processing can to certain extent alter the final chemical composition of a product, the attention was predominantly put on the effect of roasting process on the elemental concentration changes. Based on the data on the concentration of the studied elements in the hot water coffee infusions, dietary element intake was defined and whether regular consumption of two cups of coffee per day might pose a potential health risk for a consumer in a long run.
Materials and Methods
Chemicals and Reagents
Nitric acid ≥ 69% (trace purity) and hydrogen peroxide ≥ 30% (trace purity) used for sample mineralization were purchased from Sigma-Aldrich (Sigma-Aldrich Chemie GmbH, Steiheim, Germany). Ultra-pure water (ddH2O) (18.2 MΩ cm−1, 25 °C) was treated in a Simplicity 185 purification system (Millipore SAS, Molsheim, France).
Coffee Samples
The study was focused on 10 green coffee brands, namely: Costa Rica (La Pastora Tarrazú) washed; Brazil Santos (NY 2, Scr. 17/18, strictly soft); Brazil Santos (Scr. 17/18, pulped natural); India Plantation AA (Pampabampara Singly estate, washed); Indonesia Java Blawan (A/WP-1´x´, washed); Indonesia Sumatra Mandheling (pulped natural); Kenya AA+ (Joshua Rehm, AA+, Masai, washed); Ethiopia Harrar (Hand Picked GR. 4, Cert. No. 0298, Long Beary Eeast, unwashed); Ethiopia Sidamo (GR. 2, Cert. No. 0036, washed); Ethiopia Sidamo Honeybird (unwashed) obtained from commercial producer and/or distributor (Barzzuz spol. s r. o., Banská Bystrica, Slovak Republic). Each of the coffee brands was divided into five different categories. First category consisted of green coffee samples (G) and the rest consisted of four different roasting degrees: City + (C+), Full city (FC), Full city ++ (FC++), and Dark (D). The coffee samples were roasted in Toper TKMX-15 (Toper PF, Izmir, Turkey) in the company Barzzuz (Banská Bystrica, Slovakia). The roasting conditions for particular roasting degrees are displayed in Table 1.
Ground Coffee Sample Preparation
Ground coffee beans (green and roasted) were prepared by milling of 10 g of the beans for 30 s in a laboratory grinder IKA A10 basic (IKA Works, Wilmington, USA). The homogenized samples were stored in plastic bags until the next treatment step. For pressure microwave digestion, approximately ⁓ 0.25 g (with a precision to 4 decimal places) of ground coffee was weighed into PTFE digestion tubes. Consequently, 5 mL of HNO3 and 1 mL of H2O2 were added directly to the PTFE vessels. The digestion procedure was carried out using pressure microwave digestion system ETHOS-One (Milestone, Srl., Italy). The mineralized sample solutions were filtered through a quantitative Munktell filter paper No. 390 (Munktell & Filtrak, Bärenstein, Germany) into 50-mL volumetric flasks and filled with ddH2O to the final volume. Sample solutions were stored in polyethylene tubes until ICP-OES analysis. Each sample was prepared in three replicates.
Preparation of Hot Water Coffee Infusions
Seven grams of ground coffee samples were weighed into glass beakers, poured with 120 mL of hot (95 °C) ddH2O, and left under cover. After 5 min of brewing, samples were filtered through a quantitative Munktell filter paper No. 390 (Munktell & Filtrak, Bärenstein, Germany) and transferred into polyethylene tubes until ICP-OES analysis. Infusions were prepared in three replicates from each ground coffee sample and analyzed without any pre-treatment step or dilution. Prior to the ICP analysis, all coffee infusions were filtered through PVDF syringe filter Q-Max (0.22 μm, diameter: 25 mm) (Frisenette ApS, Knebel, Denmark).
ICP-OES Analysis
Elemental analysis was carried out on Agilent ICP-OES spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) with axial plasma configuration and with auto-sampler SPS-3 (Agilent Technologies, Switzerland). Detailed experimental conditions were set as follows: RF power 1.45 kW; plasma gas flow 16.0 L min−1; auxiliary gas flow 1.50 L min−1 and nebulizer gas flow 0.85 L min−1; and CCD detector temperature − 35 °C. Signal accusation time 3 s for three replicates. In total, 150 ground coffee samples (50 samples × 3 replicates) and 150 infusions (50 samples × 3 replicates) were analyzed for concentration of 15 macro- and micro-elements (Al, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, Sr, and Zn). ERM®-CE278k (Mussel tissue; IRMM, Belgium) was used to check the measurement quality. Its recovery values (taking into account the water content) ranged from 85 to 123% for most of the determined elements.
Statistical Analyses and Health Risk Assessment
All the data obtained were analyzed by descriptive statistics for minimum and maximum values, arithmetic average, and standard deviation. The selected variables were tested for normality. In all cases, the variables did not follow the normal distribution according to the Kolmonogov-Smirnov test and to the Shapiro-Wilk test. Therefore, non-parametric procedures were used in the latest analyses. The Kruskal-Wallis test was used to identify significant differences between roasting degrees at significance level 5% using asymptotic p value. Spearman correlation matrix at significance level alpha 0.05, 0.01, and 0.001, respectively, was used to analyze the correlations between the variables. All the statistical evaluation was performed using MS Excel and XLSTAT package program [29].
The recommended daily dose of the studied elements (assuming the consumption of two cups of coffee per day) was evaluated based on RDA (recommended daily allowance). The risk assessment of long-term regular consumption of two cups of coffee per day was performed based on PTWI (provisional tolerable weekly intake) for aluminum (2 mg kg−1 body weight week−1). The RDA and PTWI values are shown in Table 6.
Results and Discussion
Concentration of Macro- and Micro-Elements in Ground Coffee
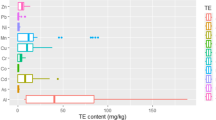
Data of the elements analyzed in coffee bean samples are displayed in Table 2. The element concentrations in the samples according to different roasting degrees are characterized by arithmetic mean value (AVG), the corresponding standard deviation (SD), and ranges. The macro-element concentration in the analyzed samples quite varied with the highest values recorded for potassium. Concentration of K in 1000 g of coffee bean samples ranged as follows: 9913–12,092 mg (green coffee), 11,416–13,159 mg (C+ roasting degree), 11,711–13,226 mg (FC roasting degree), 12,278–14,155 mg (FC++ roasting degree), and 12,322–14,301 mg (D roasting degree). Magnesium concentration ranged from 1315 mg kg−1 in green coffee bean samples to 1950 mg kg−1 in dark roasting degree. The variation in elemental composition could be attributed to the type of soil where the coffee was cultivated. Flowering, harvesting periods, and climatic conditions may also have an impact on the elemental composition [22]. The highest average Ca level was obtained for FC roasting degree (1010 mg kg−1), while the lowest for green coffee beans (895 mg kg−1). The highest average Na level was obtained for FC roasting degree (33.5 mg kg−1), while the lowest for D roasting degree (20.5 mg kg−1). These findings are in agreement with Grembecka et al. [19] and Souci et al. [30]. Much higher levels for K, Mg, and Na were reported by Anderson and Smith [31]. Santos and de Oliveira [22] reported higher levels of all tested macro-elements in Brasilian soluble coffees. The general trend of the macro-element concentration (K > Mg > Ca > Na) is in good agreement with Martin et al. [11] and Grembecka et al. [19]. There were significant differences (p < 0.05) observed in Ca, K, and Mg between green coffee beans and all roasting degrees. However, it is necessary to keep in mind the fact that increasing level of roasting results in the evaporation of water and/or combustible mass in the coffee grain and that leads to concentration of the studied elements. The concentration of Zn in the samples ranged between 4.50 mg kg−1 (green coffee) and 5.68 mg kg−1 (FC++ roasting degree). Similar concentrations as in Zn were recorded in Sr levels. The highest Fe concentration was obtained for C+ roasting degree samples (32.9 mg kg−1). The lowest Fe concentration was obtained in green coffee (23.6 mg kg−1). The concentration of Mn ranged from 21.3 mg kg−1 (green coffee) to 25.9 mg kg−1 (FC++ roasting degree). The Ni concentration ranged between 0.34 mg kg−1 (green coffee) and 0.53 mg kg−1 (FC++ roasting degree). The Cu concentration varied from 10.3 mg kg−1 (green coffee) to 12.9 mg kg−1 (FC++ roasting degree). The highest level of Cd was obtained in C+ and FC roasting degrees (0.02 mg kg−1) and the lowest level of Cd was obtained in green coffee (0.01 mg kg−1). The Cr concentration decreased from 0.12 mg kg−1 (green coffee) to 0.03 mg kg−1 (D roasting degree). The Ba concentration ranged from 3.73 mg kg−1 (green coffee) to 3.14 mg kg−1 (D roasting degree). The Al concentration ranged from 14.3 mg kg−1 (green coffee beans) to 27.3 mg kg−1 (FC roasting degree). The average Co concentration ranged from 0.09 mg kg−1 (FC roasting degree) to 0.14 mg kg−1 (FC++ roasting degree). The results of Zn, Cu, and Mn are comparable to the findings of Grembecka et al. [19], Santos and de Oliveira [22], and Souci et al. [30] who reported higher Fe values in ground and instant coffees. In general, the infusions containing soluble components in hot water had lower concentrations of each elements than the coffee bean samples. There were significant differences (p < 0.05) in Al, Cr, Cu, Fe, and Zn between green coffee beans and all roasting degrees. In the case of Cr, significant differences (p < 0.05) were observed among G-C+-FC-FC++-D; C+-FC++-D; FC-FC++-D roasting degrees. Micro-elemental, similarly to macro-elemental composition of coffee beans, was significantly affected by the roasting process.
Concentration of Macro- and Micro-Elements in Coffee Infusions
The concentration of macro- and micro-elements in hot water infusions is displayed in Table 3. The concentration of K ranged between 284 mg L−1 (green coffee infusion) and 341 mg L−1 (C+ coffee infusion). The highest concentration of Mg was detected in C+ coffee infusion 68.7 mg L−1, while the lowest concentration of Mg was detected in the infusion prepared from green beans (36.6 mg L−1). The concentration of Na ranged from 0.72 mg L−1 (green coffee infusion) to 1.14 mg L−1 (FC++ coffee infusion). The highest level of Ca was observed in C+ coffee infusion (21.6 mg L−1) while the lowest level of Ca was observed in the infusion prepared from green beans (12.7 mg L−1). The concentrations of Cd, Co, Cr, and Ni were under the limits of detection. There were very low concentrations of micro-elements leached in the infusions in all tested groups. The percentages of all macro- and micro-elements leaching to infusions estimated in this study were much lower than those reported by Grembecka et al. [19] and Ashu and Chandravanshi [28]. The highest leaching percentages could be caused by using acidification with HNO3. Stelmach et al. [25] investigated the concentration of Ca, Cu, Fe, Mg, and Mn in green coffee brews. They used no treatment or acidification with HNO3 at the extraction from coffee brew step. The results of the presented study are comparable to the work of Stelmach et al. [25].
Correlation Analyses
The Spearman correlation coefficients are presented in Table 4 (ground coffee) and Table 5 (coffee infusions). Most of the elements exhibited significant positive and negative correlations. Table 4 shows that a strong significant positive relationship was observed between concentrations of Co-Ni, Co-Mn, Ba-Mn, and Mg-Cu. A moderate positive significant relationship was observed between Co-Ba, Co-Cu, Co-Zn, Ca-K, Ca-Fe, K-Mg, K-Cu, K-Fe, Mg-Mn, Mg-Zn, Ba-Cu, Ba-Ni, Ba-Sr, Ba-Zn, Cu-Mn, Cu-Zn, Mn-Ni, Mn-Sr, Ni-Zn, and Sr-Zn. There were no strong negative relationships observed between the investigated elements. Moderate negative significant relationships were found between K-Cr and Mg-Cr. Table 5 shows strong significant positive relationships between Ca-Mg, Ba-Mn, Ba-Sr, Cu-Fe, and Mn-Sr. There were also moderate significant positive relationships observed between Ca-Ba, Ca-Mn, Ca-Sr, Ca-Zn, Mg-Ba, Mg-Mn, Mg-Sr, Ba-Zn, and Mn-Zn. There were moderate significant negative relationships between Ca-Cu, Ca-Fe, K-Cu, K-Fe, Mg-Cu, and Mg-Mn. Grembecka et al. [19] found significant positive relationships between concentrations of Mg-Na, Ca-P, Ca-Zn, Ni-Co, P-Cr, P-Zn, and Fe-Ni in ground coffee samples. They found significant negative correlations between the concentrations of Mg-P, Mg-Cr, P-Na, Zn-Na, Zn-Mg, and Mn-Na in ground coffee samples. Growing process of coffee plants, including the use of inorganic fertilizers, organic manures, pesticides, and also environmental pollutions, may be the cause of positive correlations between P-Zn, K-Cu, Mn-Ni, B-Cu, Mg-Cu, and As-Hg pairs in the coffee beans [22, 23].
Estimation of Dietary Element Intake
The dietary intakes of elements were estimated for consumption of two cups of coffee (prepared from 2 × 7 g of coffee) per day according to Şemen et al. [23]. Daily mineral intakes (DMI, %) were calculated as follows:
where: C = element concentration (in mg) in 2 × 120 mL coffee infusion, RDA = recommended daily allowance [32]. Estimated dietary intakes (%) according to the reference values are presented in detail in Table 6. For Sr and Ba as trace elements, no RDA values have been established yet [32]. Provisional tolerable weekly intake (PTWI) was calculated for Al [33]. The values ranged from 0.01 to 0.02% (Table 6). Therefore, the amounts of Al consumed via coffee infusions of different roasting degrees were considered non-hazardous. Based on RDA, consumption of two cups of coffee supplies to human body 0.43–0.62% Ca, 4.11–4.19% K, 3.40–4.43% Mg, 0.56–0.82% Cu, 0.17–0.20% Fe, 2.57–6.98% Mn, 0.01–0.15% Zn (Table 6). Estimated dietary intakes of Sr and Ba through consumption of two cups of coffee were 0.01–0.03 mg day−1 and 0.01 mg day−1 of Sr and Ba, respectively. The exposure amounts are low and no element can be referred as significant since none reaches 15% [24, 34].
Şemen et al. [24] reported that consumption of two cups of ground coffee (6 × 2 g of green coffee) per day contribute on average to 5.94 μg day−1 of Sr, 2.70 μg day−1 of Ba in green coffee, 15.2 μg day−1 of Sr, 7.41 μg day−1 of Ba in mud coffee, 15.2 μg day−1 of Sr, and 7.41 μg day−1 of Ba for Turkish coffee. PTWI for Al was 0.005–0.01%. Few studies showed that the mineral intake related to roasted and grounded coffee consumption [24, 25]. Based on the RDA, Grembecka et al. [19] reported that consumption of two cups of ground coffee (300 mL prepared from 6 × 2 g coffee) contributes, on average, to 4.72% of RDA for Mg, 0.14% for Ca, 3.52% for K, 1.95% for P, 0.13% for Zn, 0.32% for Cu, 3.41% for Mn, 38.9% for Co, and 15.6% for Ni. Şemen et al. [24] have noticed that regular consumption (12 g) supplies mean intakes less than 1% for most of the elements, except for Mg, P, K, Mn, Co, and Cu that represent 2.62%, 1.10%, 2.80%, 1.78%, 54.3%, and 7.28% of the RDA values, respectively. According to the presented dietary intakes based on the RDA values for macro- and micro-elements, it can be concluded that coffee infusions prepared from ground coffee in different roasting degrees are not an important source of elements in nutrition.
Conclusion
Coffee is very popular and widely consumed non-alcoholic beverage in the world. Several research studies were analyzed in the literature in order to determine the characteristics of the coffees and positive or negative effects of its consumption. In this study, 15 macro- and micro-elements were determined in coffee samples, and correlation between the elements was compared for coffee bean samples as well as coffee infusions. There were significant differences (p < 0.05) observed in Ca, K, and Mg between green coffee beans and all roasting degrees. Similarly, significant differences (p < 0.05) were found in Al, Cr, Cu, Fe, and Zn between green coffee beans and all roasting degrees. In the case of Cr, significant differences (p < 0.05) were observed among G-C+-FC-FC++-D; C+-FC++-D; FC-FC++-D roasting degrees. Micro-elemental composition, similarly to macro-elemental composition of coffee beans, was significantly affected by the roasting process. The results showed that roasting degrees affected the concentration of elements in resulting infusions. Dietary intakes for macro- and micro-elements based on RDA and Al based on the PTWI were calculated. Based on the RDA values for macro- and micro-elements, it can be concluded that coffee infusions are not an important source of elements in nutrition. In the case of Al, the amounts determined in coffee infusions were considered non-hazardous based on the PTWI value.
References
Parras P, Martineztome M, Jimenez A, Murcia M (2007) Antioxidant capacity of coffees of several origins brewed following three different procedures. Food Chem 102(3):582–592. https://doi.org/10.1016/j.foodchem.2006.05.037
International Coffee Organization (ICO). Statistics. Total production by exporting countries. Available from: http://www.ico.org/new_historical.asp?section=Statistics. Accessed on February, 2018
Esquivel P, Jiménez V (2012) Functional properties of coffee and coffee by-products. Food Res Int 46(2):488–495. https://doi.org/10.1016/j.foodres.2011.05.028
Farah A (2012) Coffee constituents. In: Chu YF (ed) Coffee: emerging health effects and disease prevention, 1st edn. John Wiley & Blackwell Publishing Ltd., New York, pp 21–58
Tverdal A, Stensvold I, Solvoll K, Foss O, Lund-Larsen P, Bjartveit K (1990) Coffee consumption and death from coronary heart disease in middle aged Norwegian men and women. BMJ 300(6724):566–569. https://doi.org/10.1136/bmj.300.6724.566
Revuelta-Iniesta R, Al-Dujaili E (2014) Consumption of green coffee reduces blood pressure and body composition by influencing 11β-HSD1 enzyme activity in healthy individuals: a pilot crossover study using green and black coffee. Biomed Res Int 2014:1–9. https://doi.org/10.1155/2014/482704
Papakonstantinou E, Kechribari I, Sotirakoglou Κ, Tarantilis P, Gourdomichali T, Michas G (2015) Acute effects of coffee consumption on self-reported gastrointestinal symptoms, blood pressure and stress indices in healthy individuals. Nutr J 15(1):26–36. https://doi.org/10.1186/s12937-016-0146-0
Schmit S, Rennert H, Rennert G, Gruber S (2016) Coffee consumption and the risk of colorectal Cancer. Cancer Epidemiol Biomark Prev 25(4):634–639. https://doi.org/10.1158/1055-9965.epi-15-0924
Higdon J, Frei B (2006) Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr 46(2):101–123. https://doi.org/10.1080/10408390500400009
Clarke RJ (1985) Water and mineral contents. In: Clarke RJ, Macrae R (Eds.), In: Coffee, vol. 1: Chemistry, Springer Dordrecht, pp 42–82. https://doi.org/10.1007/978-94-009-4948-5
Martı́n M, Pablos F, González A (1999) Characterization of arabica and robusta roasted coffee varieties and mixture resolution according to their metal content. Food Chem 66(3):365–370. https://doi.org/10.1016/s0308-8146(99)00092-8
Cruz R, Morais S, Casal S (2015) Mineral composition variability of coffees: a result of processing and production. In: Preedy V (ed) Processing and impact on active components in food. Elsevier Inc., USA, pp 549–558
Montavon P, Mauron A, Duruz E (2003) Changes in green coffee protein profiles during roasting. J Agric Food Chem 51(8):2335–2343. https://doi.org/10.1021/jf020832b
Gniechwitz D, Brueckel B, Reichardt N, Blaut M, Steinhart H, Bunzel M (2007) Coffee dietary Fiber contents and structural characteristics as influenced by coffee type and technological and brewing procedures. J Agric Food Chem 55(26):11027–11034. https://doi.org/10.1021/jf072389g
Silván J, Morales F, Saura-Calixto F (2010) Conceptual study on Maillardized dietary Fiber in coffee. J Agric Food Chem 58(23):12244–12249. https://doi.org/10.1021/jf102489u
Moon J, Yoo H, Shibamoto T (2009) Role of roasting conditions in the level of Chlorogenic acid content in coffee beans: correlation with coffee acidity. J Agric Food Chem 57(12):5365–5369. https://doi.org/10.1021/jf900012b
Kučera L, Papoušek R, Kurka O, Barták P, Bednář P (2016) Study of composition of espresso coffee prepared from various roast degrees of Coffea arabica L. coffee beans. Food Chem 199:727–735. https://doi.org/10.1016/j.foodchem.2015.12.080
Clarke RJ (2003) Coffee: green coffee/roast and ground. In: Caballero B, Trugo LC, Finglas P (eds) Encyclopedia of food science and nutrition, vol 3, 2nd edn. Academic Press, Oxford, pp 1487–1493
Grembecka M, Malinowska E, Szefer P (2007) Differentiation of market coffee and its infusions in view of their mineral composition. Sci Total Environ 383(1–3):59–69. https://doi.org/10.1016/j.scitotenv.2007.04.031
Fraňková A, Drábek O, Havlík J, Száková J, Vaněk A (2009) The effect of beverage preparation method on aluminium content in coffee infusions. J Inorg Biochem 103(11):1480–1485. https://doi.org/10.1016/j.jinorgbio.2009.06.012
Tesfay Gebretsadik A (2015) Levels of selected essential and nonessential metals in roasted coffee beans of Yirgacheffe and Sidama, Ethiopia. Am J Environ Prot 4(4):188. https://doi.org/10.11648/j.ajep.20150404.13
Santos É, de Oliveira E (2001) Determination of mineral nutrients and toxic elements in Brazilian soluble coffee by ICP-AES. J Food Compos Anal 14(5):523–531. https://doi.org/10.1006/jfca.2001.1012
Şemen S, Mercan S, Yayla M, Açıkkol M (2017) Elemental composition of green coffee and its contribution to dietary intake. Food Chem 215:92–100. https://doi.org/10.1016/j.foodchem.2016.07.176
Van Cuong T, Hong Ling L, Kang Quan G, Jin S, Shu Jie S, Le Linh T, Duc Tiep T (2014) Effect of roasting conditions on concentration in elements of Vietnam Robusta coffee. Acta Universitatis Cibiniensis Series E: Food Technol 18(2):19–34. https://doi.org/10.2478/aucft-2014-0011
Stelmach E, Pohl P, Szymczycha-Madeja A (2015) The content of Ca, cu, Fe, mg and Mn and antioxidant activity of green coffee brews. Food Chem 182:302–308. https://doi.org/10.1016/j.foodchem.2015.02.105
Szymczycha-Madeja A, Pohl P, Welna M, Stelmach E, Jedryczko D (2016) The evaluation of the suitability of different alternative sample preparation procedures prior to the multi-elemental analysis of brews of ground roasted and instant coffees by FAAS and ICP OES. Food Res Int 89:958–966. https://doi.org/10.1016/j.foodres.2015.09.031
Sabrina A, Fabrícia Q, Marcelo R, Flavia R, Andre M, Katia R, Frederico G (2017) Determination of heavy metals in the roasted and ground coffee beans and brew. Afr J Agric Res 12(4):221–228. https://doi.org/10.5897/ajar2016.11832
Ashu R, Chandravanshi BS (2011) Concentration levels of metals in commercially available Ethiopia roasted coffee powders and their infusions. Bull Chem Soc Ethiop 25(1):11–24 ISSN: 1011-3924
Addinsoft (2014) XLSTAT, Analyse de données et statistique avec MS Excel. Addinsoft, New York
Souci SW, Fachmann H, Kraut H (2008) Food composition and nutrition tables, 6th edn. Medpharm Scientific Publishers CRC Press, Stuttgart, p 1182
Anderson K, Smith B (2002) Chemical profiling to differentiate geographic growing origins of coffee. J Agric Food Chem 50(7):2068–2075. https://doi.org/10.1021/jf011056v
EEC (2008) Amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowances, energy conversion factors and definitions. Official Journal of the European Union, Commission Directive 2008/100/EC. http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1169. Accessed on February, 2018
CAC (Codex Alimentarius Commission (2011) Joint FAO/WHO Food Standards Programme, Codex Committee on Food Additives and Contaminants, Fifth Session, The Hague, The Netherlands, 21–25 March 2011, Document Reference CF/5 INF/1. Accessed on February, 2018
Oliveira M, Casal S, Morais S, Alves C, Dias F, Ramos S (2012) Intra- and interspecific mineral composition variability of commercial instant coffees and coffee substitutes: contribution to mineral intake. Food Chem 130(3):702–709. https://doi.org/10.1016/j.foodchem.2011.07.113
Funding
This work was supported by the Ministry of Education, Science, Research and Sport of the Slovak Republic projects VEGA 1/0591/18 (0.70) and by Research Center AgroBioTech built in accordance with the project Building Research Center “Agro-BioTech” ITMS 26220220180 (0.30).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Árvay, J., Šnirc, M., Hauptvogl, M. et al. Concentration of Micro- and Macro-Elements in Green and Roasted Coffee: Influence of Roasting Degree and Risk Assessment for the Consumers. Biol Trace Elem Res 190, 226–233 (2019). https://doi.org/10.1007/s12011-018-1519-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1519-3