Abstract
In this study, 7 Arabica (A) and 3 Robusto (R) green coffee beans (GCB) grown in different countries were used. These beans were roasted (RCB), and their coffee silverskin (CSS) produced during roasting was separated. The objective of the study was to determine the total phenolic contents, antioxidant capacities and bioaccessibility of green coffee beans and roasted coffee beans and their coffee silverskin. Three different antioxidant capacity determination methods (ABTS, CUPRAC, FRAP) were used for the determination of the antioxidant capacity; the Folin–Ciocalteau method was employed for the determination of the total phenol content (TPC); and the in-vitro enzymatic extraction method was applied for the determination of the bioaccessibility. The average bioaccessibility values of the TPCs of the GCB and RCB and their coffee silverskin were determined to be 50.05%, 41.45%, and 39.18%, respectively. The highest % bioaccessibiliy results for CSS were determined in the Ethiopia coffee for Arabica species and in the Indonesia-grown coffee for Robusta species. The highest % bioaccessibiliy in the green and roasted coffee beans of Arabica species was determined in the Kenya and Costa Rica coffees, while in Robusta species, it was determined in the India coffee. In general, although the total phenolic content and antioxidant capacity of the coffee beans were found to be higher in the Robusta, the Arabica was found to be higher in terms of % bioaccessibility results. As a result, it is recommended to use GCB and RCB and their CSS in the development of foods and beverages with functional properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coffee is one of the most consumed beverages worldwide and the second most commercially-produced product after petroleum. Coffee is estimated to be consumed by 80% of the world's adult population [1]. According to the International Coffee Organization (ICO), a total of 170,561,000 sacks (60 kg) of coffee, including approximately 100,290,000 sacks (60 kg) of Arabica species and 70,271,000 sacks (60 kg) of Robusta species, were globally produced in 2018. Brazil is the biggest coffee producer, followed by Vietnam, Columbia, Indonesia, Ethiopia, Honduras, and India [2].
Coffee (Coffea spp.) belongs to the Rubiaceae family and to the Cinchonoideae subfamily and has 124 known species [3]. Among these species, Coffea arabica (Arabica) and Coffea canephora (Robusta) are two prominent species with commercial importance [4]. Arabica coffee species, which grows at high altitudes, has a fragrant, aromatic, delicious, and softer coffee flavor, and provides 70–80% of the world coffee production, whereas Robusta, which gives a strong flavor to coffee containing more caffeine, is less favored [5]. The quality and preferability of coffee are affected by genetic and environmental factors, process conditions, and differences in chemical composition [6].
The coffee fruit is coated by a smooth and hard pericarp. Its color is initially green and turns to red and purple during ripening. The pericarp surrounds the soft, yellowish, fibrous, and sweet pulp, followed by a mucilage layer and a thin and yellowish endocarp layer, respectively. Finally, the seed membrane, “silverskin”, covers the two hemispheres of coffee seeds [7]. Coffee silverskin (CSS) is removed from green coffee beans as the byproduct of the coffee roasting process [8, 9].
The positive effects of coffee on health are usually attributed to its components with antioxidant capacity such as chlorogenic acids and caffeine [10, 11]. Moreover, coffee seeds are rich in polyphenols [12]. In addition, coffee silverskin has high levels of soluble dietary fibers, and functions as a good source of prebiotics [13]. Various studies have shown the high antioxidant capacities of these compounds [14, 15]. However, there is no study that shows the in vitro bioaccessibility properties of coffee seeds and by-products together.
In the present study, the antioxidant properties and bioaccessibility of the green and roasted coffee seeds and CSS of Arabica and Robusta coffee species grown in different countries were examined. Thus, the differences between the coffee species were determined in addition to the determination of the effects of the roasting of coffee beans.
Materials and methods
Materials
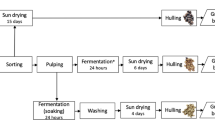
Green coffee beans (GCB) and roasted coffee beans (RCB) grown in different countries were obtained from Bayramefendi Osmanlı Kahvecisi A.Ş. In the study, seven Arabica (A)—(Colombia, Ethiopia, Costa Rica, Kenya, Guatemala, Brazil-Santos, Brazil-Rio Minas) and three Robusto (R) (Indonesia, India, Central African Republic) species grown in different countries were used. The coffee beans were roasted in an industrial roaster at 180–200 °C for 15–20 min and immediately cooled. They were, then, placed in polyethylene bags and brought to the laboratory. During these roasting processes, the coffee silverskin was obtained as a by-product, and put into separate polyethylene bags and separated. All the samples were stored in a dry, dark, and non-humid environment. Before the coffee beans and coffee silverskin were analyzed, they were ground using an electric coffee grinder (Tefal, Turkey) and made homogeneous by passing through a 60-mesh-sieve.
Methods
Two different extraction methods (extracted and hydrolyzed fraction) were used in order to determine the free and bound antioxidant compounds of the coffee beans and their coffee silverskin, whereas an in vitro enzymatic extraction method, which mimics digestive system conditions, was applied in order to determine the bioaccessibility of the coffee beans and their coffee silverskin [16, 17]. The prepared extracts were used to determine TPC and antioxidant capacity (ABTS, CUPRAC, FRAP).
Chemicals
All the reagents and chemicals used in the experiments were of analytical grade. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), neocuproine, gallic acid, potassium chloride, ABTS (2,2´-azobis(2-methylpropionamidina)-dihydrochloride), sodium hydroxide, pepsin, bile extract, pancreatin, concentrated hydrochloric acid (37% w/v), and concentrated sulfuric acid (95–98%) were purchased from Sigma (St. Louis, MO, USA). Folin–Ciocalteau phenol reagent, HPLC-grade methanol, ethanol, sodium chloride, sodium carbonate, sodium acetate, ammonium acetate, and copper (II) chloride were purchased from Merck (Darmstadt, Germany). High-quality water was used, obtained by the Milli-Q system (Millipore, Bedford, MA, USA).
Sample preparation
The extractable, hydrolyzable, and bioaccessible fractions of the coffee beans and their CSS samples were performed according to the method developed by Vitali et al. [16] and Naczk and Shahidi [17] with slight modifications. The extractions of each type of fraction were carried out in triplicate samples for each coffee bean and CSS. For extractable fraction, the samples (2.0 g) were mixed with 20 mL of HCl conc/methanol/water (1:80:10, v/v) and shaken using a rotary shaker (JB50-D; China) at 250 rpm for 2 h at 20 °C. The mixture was, then, centrifuged at 3500 × g for 10 min at 4 °C (Sigma 3 K 30). The residue was separated, and the supernatants were stored at − 16 °C until the time of use. The residue was combined with 20 mL of methanol/ H2SO4 conc (10:1), and they were kept at 85 °C for 20 h and then cooled at room temperature. The mixtures were centrifuged at 3500 × g for 10 min at 4 °C (Sigma 3 K 30). The supernatants were separated as a hydrolyzable fraction and stored at − 16 °C until the time of use. An artificial stomach and intestinal environment were prepared in a laboratory for the extraction of bioaccessible fractions with slight modifications [18]. Briefly, 2 g of samples were mixed with simulated gastric fluid (containing pepsin) and the pH was adjusted to 2.0; the samples were then incubated for 2 h at 37 °C with an orbital shaker. After gastric digestion, the intestinal phase was performed by incorporating 10 ml of simulated intestinal fluid (containing pancreatin and bile salts); the pH was adjusted to 7.0 and incubated for 2.5 h with an orbital shaker. At the end of the period, the samples were centrifuged at 3500 rpm for 10 min (Sigma 3K30, Germany). The supernatant was separated as a bioaccessible fraction and stored at − 16 °C until the time of analysis.
Determination of total phenolic contents (TPC)
The total phenolic contents of all the fractions were determined based on the Folin–Ciocalteau colorimetric method as described by Nack and Shahidi (2004). They were expressed as gallic acid equivalents (mg GAE/100 g). The total phenolic content was calculated as the sum of extractable and hydrolyzable fractions. Analyses were carried out three times for each extract.
Antioxidant capacity
In order to determine antioxidant capacities, considering the mechanisms, selectivity, sensitivity, and applicability of the methods used, it is recommended to compare the antioxidant capacities of foods through the utilization of more than one method [19, 20]. Therefore, the ABTS (Radical Scavenging Assay), CUPRAC (Cupric ion reducing antioxidant capacity assay), and FRAP (Ferric reducing antioxidant power) methods were used to determine the antioxidant capacity of the extractable, hydrolyzable, and bioaccessible fractions of the samples. All assays were repeated three times for each extract and analyzed by using a spectrophotometer (Shimadzu UV-1280). A calibration curve was prepared, using Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), and the results were expressed as µmol TE/g for each method.
ABTS assay
The determination of the ABTS radical cation decolorization assay was conducted according to the method of Apak [20]. For ABTS*+ radical solution, 7 mM ABTS in water and 2.45 mM potassium persulfate, stored in the dark at room temperature for 12–16 h before use to obtain an absorbance at 734 nm. ABTS*+ radical solution of blue-green color was diluted with ethanol (96%) at a ratio of 1:10. The procedure was used adding 1 mL of the ABTS solution to (x) mL of extract and (4.0—x) mL of ethanol, and the absorbance was measured at 734 nm after 6 min by using UV/Vis spectrometer (Optizen 3220 UV, Mecasys).
CUPRAC assay
The antioxidant capacity of the extracts was determined spectrophotometrically following the procedure by Apak [19.] Briefly, 1 mL of 1 × 10–2 M CuCl2, 1 mL 7.5 × 10−3 M neocuproine, 1 mL ammonium acetate buffer solutions, x mL extract of the samples, and (4-x) mL of water were added and mixed. The final mixture at 4.0 mL total volume was allowed to stand at room temperature, and after 30 min, the absorbance at 450 nm (Optizen 3220 UV, Mecasys) was recorded against a reagent blank.
FRAP assay
The antioxidant capacity of the coffee beans and their coffee silverskin was estimated spectrophotometrically following the procedure by Benzie and Strain (1996), with slight modifications. To prepare the Ferric reducing antioxidant power (FRAP) reagent, TPTZ solution, FeCl3 solution, and acetate buffer solutions were prepared, taking 250 mL, 250 mL, and 62.5 mL, respectively, mixed and heated to 37 °C. Then, 100 μL of the sample, 300 μL of water, and 3 mL of the FRAP solution were put into the analysis tubes. After 15 min in a hot water bath at 37 °C, the absorbance values were measured at 595 nm in a spectrophotometer (Optizen 3220 UV-Mecasys). The waiting period was determined as a result of the preliminary orders.
Statistics
The results obtained from the analyses were evaluated statistically using the SPSS 22.0 program. All results were expressed as the mean ± standard deviation (SD). The significant difference was calculated by the LSD test; values p < 0.05. the analyzes were performed in three replicates. To determine similar groups of different coffee beans belonging to different countries, the total phenolic content and total antioxidant capacity results of the green and roasted coffee beans and their coffee silverskin were determined using the “Hierarchical Clustering Method” (Cluster) – Ward's technique – in the JMP software. Dendrogram graphics were created to identify the groups close to each other. In the color map in the graphs, the analysis results were shown from the smallest to the largest in blue, gray, and red color tones from light to dark.
Results
Total phenolic content
The different fractions of the TPC of the green and roasted coffee beans and their coffee silverskin are presented in Fig. 1. The TPC of the extractable fractions of the green and roasted coffee beans varied between 114.71–172.49 mg GAE/100 g and 92.03–134.70 mg GAE/100 g, respectively. The highest results in GCB were found in Ethiopia, the lowest in India, the highest in RCB in Ethiopia and the lowest in Guatemala depending on the growing location (p < 0.05). The TPC of the hydrolyzable fractions of the coffee beans were determined to be higher than the extractable fractions (p < 0.05). In the hydrolyzable fractions, the highest TPC was determined in the green (228.44 mg GAE/100 g) and roasted (190.55 mg GAE/100 g) coffee beans of Robusto species grown in Indonesia (p < 0.05) followed by results from the green and roasted coffee beans of Arabica species grown in the Rio-Minas and Santos region of Brazil, Costa Rica, Ethiopia and Kenya (Fig. 1a).
Evaluating the TPC of the green coffee beans in terms of coffee species, Robusto coffees showed higher results with 332.31–400.93 mg GAE/100 g compared to those of Arabica (293.37–363.31 mg GAE/100 g). Among all the coffee beans analyzed, it was determined that the Arabica coffee beans grown in Costa-Rica had the highest total phenol content (363.31 mg GAE/100 g), while the lowest result was in those grown in Guatemala (Fig. 1a). The highest TPC was determined in the Arabica coffee beans grown in Ethiopia (314.34 mg GAE/100 g), while the lowest result was found in the roasted coffee beans grown in Costa Rica (247.82 mg GAE / 100 g). No statistically significant differences were found between the TPC of the Ethiopia-A, Indonesia-R, India-R, and Central African Republic-R roasted coffee bean samples (p > 0.05). Priftis et al. [21] reported that green coffee beans had a higher TPC compared to roasted ones. Jeszka-Skowron et al. [22] stated that coffee phenolic acids, which contribute to high TPC activity, had losses during the roasting process and that roasting temperature and time were, therefore, quite effective.
Examining the TPC of the coffee silverskin, it was determined that the Robusto coffee beans grown in India had the highest total phenol content (168.27 mg GAE/100 g), followed by the Arabica species grown in Brazil's Rio-Minas (160.07 mg GAE/100 g) and Kenya (164.50 mg GAE/100 g) (p < 0.05) (Fig. 1b). The total phenol contents of the coffee silverskin of the hydrolyzable fractions (70.28–110.90 mg GAE /100 g) were determined significantly higher than those of the extractable fractions (51.72–67.71 mg GAE / 100 g). Gemechu (2020) reported that the TPC content of the coffee silverskin varied between 10.75 and 17.3 g GAE/100 g [23]. Costa et al. [24] stated that the TPC value changed in different extractions of the coffee silverskin used with water (173 mg GAE/L) and ethanol (85.1 mg GAE/L). Similar results were also reported by Panusa et al. [25]. Our study results were similar to those in the literature.
Based on the average results, the total phenol content of the samples can be listed as GCB > RCB > CSS. It was reported that this was due to the higher amount of some phenol compounds in green and roasted coffees [26].It was also stated that the storage conditions (storage temperature, humidity, and storage equipment) of green coffee beans affected the shelf life and coffee quality. In the literature, it was showed that the effect of antioxidants, such as chlorogenic acids, other phenolic acids, polyphenols and alkaloids in green coffee beans, varied according to coffee bean species (Arabica, Robusta) used [27].
Antioxidant capacity
The antioxidant capacities of the extractable and hydrolyzable fractions and the total antioxidant capacity of the samples were determined by the ABTS, CUPRAC, and FRAP methods. The antioxidant capacity of the green and roasted coffee beans and their coffee silverskin measured with different methods is given in Fig. 2. In the comparison of the levels of all methods for antioxidant capacities among the coffee beans and their coffee silverskin, differences were observed (p < 0.05).
Antioxidant capacity (μmol TE/g) of green and roasted coffee beans and their coffee silverskin measured with different methods. *Antioxidant capacity methods: ABTS radical scavenging assay, CUPRAC cupric ion reducing power assay, FRAP ferric reducing antioxidant power assay. 1Coffee type: A-Arabica, R-Robusta
ABTS assay
The antioxidant capacity results obtained using the ABTS method are given in Fig. 2a for coffee beans and in Fig. 2b for coffee silverskin. According to the antioxidant capacity results of the green coffee beans, the Robusta coffee beans yielded higher results than the Arabica coffee beans with 135.18–162.01 µmol TE/g. Among all the coffee beans analyzed, it was determined that the Arabica coffee beans grown in the Santos region of Brazil had the highest antioxidant capacity (134.82 µmol TE / g), whereas those grown in Costa Rica yielded the lowest results (105.71 µmol TE / g) (p < 0.05). The antioxidant capacity of the extractable fractions of the coffee beans ranged between 33.82 and 56.28 µmol TE/g, and the highest was detected in Colombia and the lowest in Costa-Rica (Fig. 2a). It was determined that the antioxidant capacity of the hydrolyzable fractions of the green coffee beans was higher than the extractable fractions (p < 0.05). In the hydrolyzable fractions, the Robusto coffee beans yielded the highest value with 108.57 µmol TE/g, followed by the coffee beans grown in the Central African Republic with 103.89 µmol TE/g. In the Arabica coffee beans, the highest antioxidant capacity (95.54 µmol TE / g) was determined in the coffee beans grown in the Santos region of Brazil, followed by those grown in Kenya, Brazil's Rio-Minas region, and Ethiopia (Fig. 2a).
When the antioxidant capacities of the roasted coffee beans were evaluated in terms of coffee species, the Robusta coffee beans showed significantly higher results (p < 0.05) with 111.07- 189.60 µmol TE/g compared to the Arabica coffee beans. It was reported that this was due to the higher content of chlorogenic acid, which was produced by the decomposition of various phenolic compounds during the roasting of Robusta coffees, and this resulted in bitterness, compared to the Arabica coffee beans [11]. Among the Arabica coffee beans, it was determined that those grown in Colombia had the highest antioxidant capacity (184.16 µmol TE / g), whereas those grown in Costa-Rica (113.21 µmol TE / g) yielded the lowest result (Fig. 2a). The antioxidant capacity of the extractable fractions of the roasted coffee beans ranged from 36.50 to 78.84 µmol TE / g, whereas the highest was found in Colombia and the lowest in Kenya (p < 0.05). In the hydrolyzable fractions, the Robusto coffee beans produced the highest result in Indonesia with 108.57 µmol TE/g, followed by the coffee beans grown in the Central African Republic with 103.89 µmol TE/g. In Arabica, the highest antioxidant capacity (105.32 µmol TE / g) was determined in the coffee beans grown in Colombia, followed by those grown in Guatemala, and the Santos and Rio-Heritage regions of Brazil (Fig. 2a). In a study in which Cammerer and Kroh (2006) roasted coffee samples (80% Arabica and 20% Robusta) at three different roasting temperatures (light, medium and dark), the antioxidant capacity of the coffee samples was determined by the DPPH and ABTS method results [28]. It was stated by the researchers that antioxidant capacity decreased from medium roasted coffee to dark roasted coffee and that this result was associated with the amount of phenolic compounds in the coffee decreasing with increasing roasting temperature.
The antioxidant capacities (AC) of the coffee silverskin according to the ABTS method were significantly higher (p < 0.05) in the Robusta (84.20–91.29 µmol TE / g) coffee beans than those of Arabica (71.25–112.94 µmol TE / g). The Kenya-A, Brazil Santos-A, and India-R CSS samples showed the highest AC results (Fig. 2b).
CUPRAC assay
The antioxidant capacity results obtained using the CUPRAC method are given in Fig. 2c for coffee beans and Fig. 2d for coffee silverskin. According to the antioxidant capacity results of the green coffee beans, the Robusta coffee beans, with 489.45–676.18 µmol TE/g, yielded higher results than those of Arabica species. Among all the green coffee beans analyzed, it was determined that those grown in Indonesia had the highest antioxidant capacity with 676.18 µmol TE/g, followed by those grown in Guatemala with 547.53 µmol TE/g and Colombia, with 522.58 µmol TE/g. The lowest antioxidant capacity was found to be 438.02 µmol TE/g in the coffee beans grown in Ethiopia (p < 0.05) (Fig. 2c).
The antioxidant capacity of the extractable fractions of the green coffee beans varied between 171.24 and 277.56 µmol TE/g for Arabica species. The highest was determined in those grown in Colombia, whereas the lowest was found in those grown in Ethiopia. Among the Robusto coffee beans, it was observed that those grown in Indonesia and those grown in the Central African Republic had the lowest antioxidant capacities with 286.52 µmol TE / g and 163.92 µmol TE / g (p < 0.05), respectively. The antioxidant capacity of the green coffee beans yielded the highest in the hydrolyzable fractions of Robusta species, followed by those grown in Indonesia with 389.67 µmol TE/g and those grown in the Central African Republic with 326.53 µmol TE/g. In the Arabica coffee beans, the highest antioxidant capacity was determined in the coffee beans grown in the Rio-Miras region of Brazil, followed by those grown in the Guatemala region, Kenya, and Ethiopia.
According to the CUPRAC method, the antioxidant capacity of the roasted coffee beans varied between 413.61 and 515.98 µmol TE/g for Arabica species, and 401.10 and 500.93 µmol TE/g for Robusta species. The most important factor affecting the quality of roasted coffee beans is temperature and time, and the chlorogenic acid values among these compounds decreases with increasing roasting temperature [11]. Researchers reported that medium-dark roasted coffee had the highest antioxidant potential and that the roasting process played a key role in the antioxidant capacity of coffee species and origin (Arabica or Robusta) [28]. In the present study, since the green coffee beans were medium-roasted, the antioxidant capacity of the roasted coffee beans was also positively affected. Among all the analyzed coffee beans, it was determined that those grown in Ethiopia (515.98 µmol TE / g) had the highest antioxidant capacity, whereas those grown in India (401.10 µmol TE / g) had the lowest result. The antioxidant capacity of the extractable fractions of the roasted coffee beans varied between 140.11 and 224.10 µmol TE/g, and the highest was found in Indonesia whereas the lowest was determined in the Central African Republic (p < 0.05). In the hydrolyzable fractions of the roasted Robusto coffee beans, those grown in the Central African Republic yielded the highest values with 281.25 µmol TE g, followed by the coffee beans grown in Indonesia with 276.83 µmol TE / g. As for Arabica, the coffee beans grown in Colombia had the highest antioxidant capacity, followed by those grown in the Rio-Minas region of Ethiopia and Brazil. Among the hydrolyzable fractions in Arabica species, the lowest antioxidant capacity was found in the coffee beans grown in Guatemala (Fig. 2c).
According to the CUPRAC method, the antioxidant capacities (AC) of the coffee silverskin (CSS) were determined in the range of 208.89–283.22 µmol TE/g in the Robusta coffee beans and 205.22–314.02 µmol TE/g in the Arabica coffee beans. The coffee silverskin of the Kenya-A and Indonesia-R coffee beans yielded significantly higher results (p < 0.05) in the region compared to the other coffee species (Fig. 2d). Examining the antioxidant capacity results of all coffee sliverskin in different fractions, it was found that the Kenya-A coffee sample yielded significantly (p < 0.05) higher results. It was reported that CSS was one of the phenolic compounds and that especially its high chlorogenic acid content resulted in good bioaccessibility and antioxidant properties [7, 29].
FRAP Assay
The antioxidant capacity results obtained using the FRAP method are given in Fig. 2e for coffee beans and Fig. 2f for the coffee silverskin. According to the antioxidant capacity results of the green coffee beans, the Robusta coffee beans (187.16–231.42 µmol TE/g) yielded higher results than the Arabica coffee beans (157.58–225.84 µmol TE / g). Of all the coffee beans analyzed, those of the Robusta coffee beans grown in Indonesia had the highest antioxidant capacity (231.42 µmol TE / g), whereas the Arabica coffee beans grown in Brazil's Rio-Minas region had the lowest antioxidant capacity (157.58 µmol TE / g) (p < 0.05) (Fig. 2e). The antioxidant capacity of the hydrolyzable fractions of the green coffee beans (mean 121.25 µmol TE / g) was determined to be higher than the extractable fractions (average 83.19 µmol TE/g). The antioxidant capacity of the extractable fractions varied between 73.19 and 92.38 µmol TE/g, with the highest result determined in the coffee beans grown in India, whereas the lowest was found in those grown in Indonesia. In the hydrolyzable fractions, among the Robusto coffee beans, those grown in Indonesia had the highest antioxidant capacity with 158.78 µmol TE/g, followed by Kenya with 135.05 µmol TE/g.
The antioxidant capacity of the roasted coffee beans varied between 197.24 and 278.39 µmol TE/g for Robusta species and 152.90- 263.76 µmol TE/g for Arabica species. Comparing the coffee species in terms of antioxidant capacity, a statistically significant difference was found at p < 0.05 level in the Robusta coffee beans (average 241.30 µmol TE/g) compared to the Arabica coffee beans (average 217.45 µmol TE/g). Among all the coffee beans analyzed, it was determined that the Robusta coffee beans grown in India had the highest antioxidant capacity (278.39 µmol TE/g), while the lowest antioxidant capacity was found in the Arabica coffee beans (152.90 µmol TE/g) grown in Ethiopia. The antioxidant capacity of the extractable fractions of the roasted coffee beans varied between 66.25 and 113.89 µmol TE/g, and the highest was detected in Kenya whereas the lowest was determined in Ethiopia (Fig. 2e). The antioxidant capacity averages of the Arabica coffee beans were 130.20 µmol TE/g in the hydrolyzable fractions, while it was 139.70 µmol TE/g for the Robusto coffee beans. Pokorna et al. [30] investigated the antioxidant potential of raw and roasted Arabica and Robusta coffee beans with four different test methods and reported that FRAP and TPC methods were in a good correlation and that coffee species was a more effective variable than the roasting degree.
According to the antioxidant capacity results of the coffee silverskin, the Robusta coffee beans (314.77–365.32 µmol TE / g) yielded higher results than the Arabica coffee beans (294.04–370.94 µmol TE / g). Of all the coffee beans analyzed, those of the Robusta species grown in Indonesia had the highest antioxidant capacity (365.32 µmol TE / g) and the Arabica coffee beans grown in Ethiopia had the highest antioxidant capacity (370.94 µmol TE / g). Those grown in the Rio-Minas region of Brazil had the lowest antioxidant capacity (294.04 µmol TE / g) (p < 0.05) (Fig. 2f). The antioxidant capacity of the extractable fractions of the coffee silverskin varied between 102.91 and 131.81 µmol TE/g, and the antioxidant capacities of Guatemala-A, India-R, Colombia-A, which showed the highest values, were statistically similar (p < 0.05). The Costa Rica-A, Kenya-A, Ethiopia-A, and Indonesia-R samples, respectively, showed the highest results in terms of the antioxidant capacity of the hydrolyzable fractions of the coffee silverskin (Fig. 2f). It was reported that, compared using the FRAP method, CSS showed similar results to foods containing valuable antioxidant sources such as dark chocolate, herbs, and spices [7].
Evaluating all the analysis results, it was found that different species of coffee beans (Arabica, Robusta) and different geographic regions had significant (p < 0.05) effects on the antioxidant properties of the green (GCB) and roasted (RCB) coffee beans and their coffee silverskin (CSS). Similar results were obtained in recent studies on the determination of the antioxidant properties of coffee beans [31]. Robusta coffee bean species showed higher results in terms of antioxidant capacity in the DPPH method compared to Arabica. Coffee beans from Uganda and Vietnam, among Robusta coffee beans, showed the highest antioxidant capacity whereas among Arabica species, the lowest antioxidant capacity was determined in those grown in Ethiopia and Guatemala [32]. The results of the present study were in agreement with the literature data. It was reported that Robusta species showed high antioxidant capacity due to their higher chlorogenic acid and caffeine content [32, 33]. Ciaramelli et al. [14] examined green, medium roasted and ground coffee beans of different country-originated Arabica and Robusta species in terms of bioactive components and antioxidant capacity, and in parallel with the high contents of chlorogenic acid and caffeine, the antioxidant capacity of Robusta green coffee beans was higher than that of Arabica coffee beans. However, it was reported that similar antioxidant capacity values were obtained in both species of roasted coffee beans, depending on the formation of melanoidin. Song et al. [34] reported that the roasted coffee beans of the C. robusta species grown in India contained higher levels of bioactive components and showed higher antioxidant capacity than that of Arabica grown in Guatemal. It was reported that new phenolic compounds were formed during the roasting of coffee beans and that roasted coffee beans showed higher antioxidant capacity than green coffee beans. It was stated that medium-roasting at 220 °C for 12 min was the best for coffee beans, above which the antioxidant capacity decreased. In similar studies using different antioxidant capacity methods, Arabica and Robusta coffee beans showed different antioxidant capacity results according to the region where they were grown [35, 36]. In the present study, in which similar results were obtained with the literature results, significant differences were found between the antioxidant capacity analysis results according to the coffee species and growing region.
Studies on the antioxidative properties of coffee siverskin are more limited compared to those on coffee beans. It was reported that the high dietary fiber and polyphenol content of CSS, a by-product of roasted coffee beans, positively increased the antioxidant capacity of melanoidins formed as a Maillard reaction product during roasting. [25]. In other studies showing results parallel to those of the present study, it was reported that the coffee silverskin showed high antioxidant capacity in the results of different antioxidant capacity methods applied with different extraction methods [8, 31]. Studies stated that the use of CSS increased the antioxidant capacity and sensory properties in beverages and some cereal products due to the increase of bioactive components [37,38,39]. In grouping different coffee beans belonging to the countries in the study, according to the results of the dendrogram graph given in Fig. 3, four different groups were formed according to the TPC results. Indonesia-R yielded the best results, and the RCB and CS groups were found to be close to each other (Fig. 3a). According to the results of three different methods used to determine the total antioxidant capacity, Kenya-A in the ABTS method (Fig. 3b), Indonesia-R and Kenya-A in the CUPRAC method (Fig. 3c), and Brazil-Rio Minas-A in the FRAP method (Fig. 3d) showed different and better properties compared to all the groups. According to the coffee bean groups of different countries, the general properties of the RCB and CSS samples were close to each other, and the GCB samples yielded higher results (Fig. 3).
Hierarchical clustering analysis-dendrogram for all cracker samples concerning the content of TPC (a), TAC-ABTS (b), TAC-CUPRAC (c), and TAC-FRAP (d). *TPC: Total Phenolic content, TAC Total Antioxidant Capacity, ABTS radical scavenging assay, CUPRAC cupric ion reducing power assay; FRAP: ferric reducing antioxidant power assay. 1Coffee type: A-Arabica, R-Robusta
In vitro bioaccessibility of total phenolic compounds and antioxidants
A very small fraction of nutrients taken from food can be absorbed, stored and then used by the body [40].Therefore, it is more important to know the amount of biologically accessible bioactive components in foods than the amount of bioactive components in food [41]. Determination of bioaccessibility, defined as the amount of nutrient absorbed from the intestines (Barba et al. [42]), was carried out under in vitro conditions by determining the components potentially absorbable from the intestines after passing through the artificially created digestive system (GI) under laboratory conditions. Bioaccessibility varies depending on many reasons such as food matrix, chemical composition, processing and storage conditions, and individual digestion capacity [43]. There are no studies in the literature investigating the bioaaccessibility properties of green and roasted coffee beans and their silverskin.
The bioaccessibility of the antioxidant properties of the green and roasted coffee beans are given in Table 1 and Table 2. Considering the evaluation of the bioaccessibility of the total phenol content according to the coffee species, the average value for the Robusta species was 189.78 mg GAE/100 g, while the average for the Arabica species was 161.54 mg GAE/100 g, and the difference between them was statistically significant (p < 0.05).
When the bioaccessibility of antioxidant capacity according to the ABTS, CUPRAC, and FRAP methods was evaluated, the average of the Arabica coffee beans was 60.43 µmol TE/g, 314.72 µmol TE/g and 88.19 µmol TE/g, and the average of the Robusta coffee beans was 76.55 µmol TE/g, 321.04 µmol TE/g and 110.29 µmol TE/g, respectively. Evaluating the bioaccessible fractions of the green coffee beans in terms of antioxidative properties, it was found that the total phenol content and antioxidant capacity of the Robusta coffee beans grown in Indonesia were high, whereas those grown in the Rio-Minas region of Brazil were low (Table 1). When the bioaccessible fractions of the roasted coffee beans were evaluated in terms of total phenol content and antioxidant capacity, the total phenol content of the Indonesia-R and Ethiopia-A coffee beans and the antioxidant capacity of the Ethiopia-A, Kenya-A coffee samples were found to be high (p < 0.05) (Table 2).
When evaluated in terms of the total phenol content and antioxidant capacity of the bioaccessible fractions of the coffee silverskin, Ethiopia-A and India-R had the highest TPC values (Fig. 1b), whereas AC was the highest in Brazil-Rio-Minas-A and the lowest in the Central African Republic-R (Fig. 2b) according to the ABTS method. According to the CUPRAC method, the highest AC value was determined in Kenya-A whereas the lowest was determined in Colombia-A (Fig. 2d). The highest AC value according to the FRAP method was determined in Indonesia-R, whereas the lowest was determined in Colombia-A (Fig. 2f) (p < 0.05).
The % bioaccessibility values of the green and roasted coffee beans and their coffee silverskin according to their total phenol content and antioxidant capacity (ABTS, CUPRAC, and FRAP) are given in Fig. 4a, b, and c, respectively. The % bioaccessibility of the total phenol content of the green coffee beans ranged from 43.76% (Brazil—Rio Minas region) to 54.74% (India), and the average was determined to be 50.05%. The average bioaccessibility of the total phenol content of the Arabica coffee beans was 48.5%, while that of the Robusta coffee beans was 53.67%. The difference between the two species of coffee was statistically significant (p < 0.05). The highest bioaccessibility of the total phenol content was determined to be Ethiopia with 54.57%, whereas the lowest was found in those grown in the Brazil-Rio Minas region with 43.76%. Evaluating according to the Robusta species, the highest was determined in the coffee beans grown in India (54.74%) whereas the lowest in those grown in the Central African Republic (52.87%) (Fig. 4). Evaluating the % bioaccessibility of the antioxidant capacity of the green coffee beans, significant differences were found between all three methods. The highest average % bioaccessibility value was determined in the CUPRAC method (62.80%), followed by the FRAP (47.22%) and ABTS (33.52%) methods. According to the CUPRAC method, the highest % bioaccessibility was determined in those grown in Costa Rica with 85.03%, whereas the lowest % bioaccessibility was found in the coffee beans grown in the Rio-Minas region of Brazil with 39.50% (Fig. 4). The average bioaccessibility of the Arabica coffee beans was 64.48%, while the average bioaccessibility of the Robusta coffee beans was 58.89%. According to the FRAP method, the coffee beans grown in Colombia had the highest bioaccessibility (61.23%), followed by those in India (56.17%) and Costa Rica (52.73%). The lowest bioaccessibility was obtained in those grown in Guatemala with 36.19%, followed by Kenya with 39.65% and the Rio-Minas region of Brazil with 39.92%. According to this method, the antioxidant capacity average values were determined to be 45.31% in the Arabica coffee beans and 51.68% in the Robusta coffee beans. The ABTS method yielded the lowest results in terms of determining the antioxidant capacity of the green coffee beans, and the % bioaccessibility ranged between 26.37% (Central African Republic) and 45.18% (Colombia). In general, the Arabica coffee beans (average 34.7%) were observed to have higher % bioaccessibility values than the Robusta coffee beans (average 30.77%).
Bioaccessibility (%) of antioxidant properties of green and roasted coffee beans and their coffee silverskin. *Total phenol content (TPC) was calculated as the sum of extractable and hydrolyzable phenols. Bioaccessibility was calculated as the percentage of total phenolic content. 1Antioxidant capacity methods: ABTS radical scavenging assay; CUPRAC cupric ion reducing power assay; FRAP ferric reducing antioxidant power assay. 2Coffee type: A-Arabica, R-Robusta
The average bioaccessibility of the roasted coffee beans and their total phenol content and antioxidant capacity according to the ABTS, CUPRAC, FRAP methods were determined to be 41.44%, 62.56%, 44.76%, and 31.81%, respectively. The bioaccessibility of the total phenol content of the roasted coffee beans ranged from 35.76% (Costa Rica) to 47.92% (Colombia). The average bioaccessibility of the total phenol content of the Arabica coffee beans was 41.86%, while that of the Robusta coffee beans was found to be 40.47%. Among the Arabica species, the % bioaccessibility of the total phenol content was determined to be the highest in those grown in Colombia (47.92%), followed by those grown in Brazil’s Rio Minas region (47.72%) and those grown in Ethiopia (43.02%). The lowest % bioaccessibility of the total phenol content was found in the coffee beans grown in Costa Rica with 35.76%. When evaluated according to the Robusta species, the bioaccessibility of the total phenol content was ranked from the highest to the lowest as Indonesia (41.89%), Central African Republic (41.37%), and India (38.17%), respectively. The difference between the two species of coffee was statistically significant (p < 0.05).
The % bioaccessibility of the antioxidant capacities of the roasted coffee beans ranked as ABTS > CUPRAC > FRAP from high to low in terms of applied methods. According to the % bioaccessibility results determined by using the ABTS method, it was determined that the highest % bioaccessibility was in those grown in Kenya (83.81%), whereas the lowest was in those grown in the Central African Republic (45.83%). In general, the Arabica coffee beans (average 63.48%) were found to have higher % bioaccessibility values than the Robusta coffee beans (average 60.42%). According to the CUPRAC method, the highest % bioaccessibility was determined in those grown in the Santos region of Brazil with 51.79%, whereas the lowest was found in the coffee beans grown in Costa Rica with 36.07% (Fig. 3b). The average bioaccessibility of the Arabica coffee beans was 44.37%, while it was 45.66% for Robusta. According to the % bioaccessibility results determined using the FRAP method, the Robusta species varied between 27.39 and 34.39% (average 30.83%), and the Arabica species varied between 21.22 and 37.51% (average 32.22%).
In general, considering the green and roasted coffees in terms of antioxidative properties, it was determined that the % bioaccessibility values of the antioxidative properties of the green coffee beans were higher in all other methods except the results obtained by the ABTS method and that there was a statistically significant difference at (p < 0.05) level. When evaluated in terms of coffee species, although the antioxidative contents of both green and roasted coffees were found to be high in the Robusta coffee beans in general, a reverse situation was seen in terms of bioaccessibility. In other words, the % bioaccessibility of the Arabica coffee beans was determined to be higher. Among the antioxidant capacity methods, it was determined that the CUPRAC method yielded the highest results in general, followed by the ABTS method.
The average bioaccessibility of the total phenol content of the coffee silverskin and its antioxidant capacities according to the ABTS, CUPRAC, FRAP methods were determined to be 39.18%, 57.09%, 21.60%, and 37.28%, respectively (Fig. 3c). The total phenol content of the roasted coffee beans ranged from 33.12% (Kenya-A) to 45.74% (Ethiopia-A). The average bioaccessibility of the total phenol content of the Arabica coffee beans was 38.92%, while that of the Robusta coffee beans was 39.78%. The highest TPC in the Robusta coffee beans with 43.24% was detected in the silverskin of the coffee beans grown in the India region. According to the % bioaccessibility results determined using the ABTS method, the Robusta species varied between 43.85 and 63.71% (average 52.68%) and the Arabica species between 47.73 and 69.15% (average 58.98%). According to the % bioaccessibility results determined using the CUPRAC method, the Robusta species varied between 18.65 and 26.91% (average 22.42%) and the Arabica species between 18.12 and 27.66% (average 21.25%). According to the % bioaccessibility results determined using the FRAP method, the Robusta species varied between 36.45 and 39.30% (average 37.92%) and the Arabica species between 31.09 and 44.32% (average 37.01%). According to the CUPRAC and FRAP methods, the difference between the average results of the coffee silverskin of both coffee species was statistically not significant (p ≥ 0.05). When an evaluation was made in terms of coffee species, the antioxidative content and % bioaccessibility values of the coffee silverskin of the Arabica coffee beans grown in different places were found to be higher than those of the Robusta coffee beans. For coffee silverskin, it was determined that the ABTS method yielded the highest results in general among the antioxidant capacity methods, followed by the FRAP method. Among the species of coffee silverskin, especially Ethiopia-A, Brazil-Rio Minas-A, Guatemala-A, and Indonesia-R, India-R can be used to enrich more nutritious and healthy food products with their high antioxidative properties.
When the results of the study are evaluated in general, the differences between the results of the FRAP, CUPRAC and ABTS antioxidant capacity methods are related to many reasons. The extraction of phenolic compounds in foods is affected by many factors such as the structure and composition of the food to be analyzed, particle size, and the extraction method to be applied. In the literature, there are many different methods used for the determination of the antioxidant capacity of foods. Considering the mechanisms, selectivity, sensitivity and applicability of the methods used, it is recommended to compare the antioxidant capacities of foods using more than one method. For that reason, ABTS, CUPRAC and FRAP methods were used in order to determine the antioxidant capacity of the coffee samples [17, 19]. One of the most important factors regarding the different results of the antioxidant capacity analyzes in this study is the physiological pH. FRAP operates in acidic conditions, CUPRAC in neutral conditions, and the Folin-Ciocalteu method in alkaline conditions. Since FRAP operates in more acidic conditions, its reducing capacity is suppressed due to the protonation of phenolic antioxidants. It is also known that the ABTS and CUPRAC methods can measure both hydrophilic and lipophilic antioxidants, while FRAP and Folin-Ciocalteu can only measure hydrophilic antioxidants [19, 44, 45]. In addition, it has been reported that the number and position of hydroxyl groups and the degree of conjugation of the whole molecule are important for efficient electron transfer, which affects antioxidant capacity measurements [46, 47]. It is thought that this result is related to the different kinetic behavior of the reagents used in the analysis methods and to the relative sensitivity of the reagents to various parameters such as air, sunlight, pH and humidity.
For the determination of the phenolic antioxidants in the coffee samples used in our study, the CUPRAC method showed the highest antioxidant capacity (AC) results for the sample food matrix compared to other methods. It has been reported that the compounds that make up the antioxidant capacity are quite diverse, and that these compounds include phenolic acids, caffeine, tocopherols, melanoidins, phenylalanines, caffeoyl-tryptophan and some other bioactive compounds formed during the roasting process [48, 49]. In another study, it has been stated that phenolic compounds in coffee (caffeic acid, caffeine, and hydroxycinnamic acid), melanoidins, which are the products of the Maillard reaction, and chlorogenic acid are important factors in terms of the antioxidant capacity of coffee [50]. Aguiar et al. [51] in their study, reported that the antioxidant effect of coffee was caused by caffeic acid, which is one of the phenolic acids, and chlorogenic acids (3-O-caffeolquinic acid (3-CQA), 4-O-caffeolquinic acid and 5-O-caffeolquinic acid). Among the compounds showing the highest antioxidant capacity in the CUPRAC method, caffeic acid, which is one of the phenolic acids that is predominantly found in coffee, has also been reported. [45, 52, 53]. The advantageous aspects of this method – the reason why the CUPRAC method is more suitable than other AC analysis methods – have been reported in the studies as follows [52,53,54,55]; (I) it can be applied to both hydrophilic and lipophilic antioxidants, (II) it has a selective effect on antioxidant compounds without affecting sugars and citric acid commonly found in foodstuffs, as well as having the capacity to analyze antioxidants containing -SH (III) it is a simple and widely applicable method of antioxidant capacity for flavonoids, phenolic acids, hydroxycinnamic acids, thiols, synthetic antioxidants, vitamin C, and vitamin E. (IV) Since the CUPRAC reagent does not contain any radical reagents, it is not affected by physical parameters such as temperature, sunlight, pH, and humidity. (V) it is used in environments close to physiological pH (VI) the CUPRAC method is an effective method in order to determine the antioxidant capacity of both synthetic mixtures and real mixtures because the principle of additiveness of absorbances is valid and antioxidant components in complex matrices do not interact with each other in a way that shows chemical deviations from Beer’s Law.
Conclusion
The antioxidant properties of coffee beans are affected by the species of coffee as well as growing conditions, harvest, processing methods, and storage conditions. Although the total phenolic content and antioxidant capacity of the coffee beans were determined to be higher in the Robusta coffee beans, it was determined that the Arabica coffee beans were higher in terms of bioaccessibility. It was determined that the total phenol content of the green coffee beans and the antioxidant capacity of the roasted coffee beans were higher. The results of measuring the antioxidant capacity of these methods (FRAP, CUPRAC, ABTS) do not correspond to the results of phenolic content. This situation is thought to be related to the fact that the dominant antioxidant components of the different coffee bean and coffee silverskin samples analyzed were affected by different chemical compounds other than phenolic structures. In addition, the reagent used in the CUPRAC method is much more stable than chromogenic radical reagents such as ABTS. Citric acid and simple sugars that can be oxidized with the Folin reagent are not oxidized with the CUPRAC reagent. Hydroxycinnamic acids, which are concentrated in coffee, are the biosynthetic precursors of flavonoids. Such phenolic acids are usually found in plants combined with sugar, organic acids or oils or as esters, and an effective conversion is observed between them. It has been stated that the antioxidant activities of phenolic acids and their esters depend on the number of hydroxyl groups in the molecule which are strengthened by steric hindrance. The Folin method, which is an electron-transfer (ET)-based method operates at unrealistic pH, away from physiological pH; therefore, there may be cases of underestimating or overestimating the total capacity. It has been reported that since most antioxidants are protonated at acidic pHs, their oxidation is difficult, and that since antioxidants donate their protons, oxidation becomes extremely easy at basic pH. Therefore, the mechanisms, sensitive compounds, selectivities and operating conditions of the Folin-Ciocalteu method used to determine the total phenolics content and the methods used to determine the antioxidant capacity (FRAP, CUPRAC, ABTS) are completely different from each other. For that reason, although the total phenolic content is a guide for antioxidant capacity, they cannot be considered to support each other completely. It was found out that the most appropriate method among the antioxidant capacity methods used was the CUPRAC method, followed by the ABTS method. As a result, coffee species belonging to different geographical regions are thought to have the potential to provide many benefits to human health due to their high total phenol contents, antioxidant capacity, and high bioaccessibility. With their high antioxidative properties, green and roasted coffee beans and their coffee silverskin can be recommended for daily coffee consumption and the development of functional foods.
References
E.M.S. Machado, R.M. Rodriguez-Jasso, J.A. Teixeira, S.I. Mussatto, Growth of fungal strains on coffee industry residues with removal of polyphenolic compound. Biochem. Eng. J. 60, 87–90 (2012). https://doi.org/10.1016/j.bej.2011.10.007
ICO, International Coffee Organization (ICO) Retrieved from, Trade statistics tables (2019) http://www.ico.org/prices/newconsumption-table.pdf
M. Moeenfard, A. Alves, New trends in coffee diterpenes research from technological to health aspects. Food Res. Int. 134, 109207 (2020). https://doi.org/10.1016/j.foodres.2020.109207
H.D. Belitz, W.P. Grosch, Schieberle Food Chemistry, 4th edn. (Springer Science & Business Media, Germany, 2009)
Y. Narita, K. Inouye (2015) Chapter 21 – Chlorogenic Acids from Coffee. V. R. Preedy (Ed.) Coffee in Health and Disease Prevention. San Diego Academic Press
B. Cheng, A. Furtado, H.E. Smyth, R.J. Henry, Influence of genotype and environment on coffee quality. Trends in Food Sci. Technol. 57, 20–30 (2016). https://doi.org/10.1016/j.tifs.2016.09.003
L. Bresciani, L. Calani, R. Bruni, F. Brighenti, D.D. Rio, Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food Res. Int. 61, 196–201 (2020)
A. Jiménez-Zamora, S. Pastoriza, J.A. Rufián-Henares, Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT - Food Sci. Technol. 61, 12–18 (2015). https://doi.org/10.1016/j.lwt.2014.11.031
P.F. Esquivel, V.H. Jimenez, Functional properties of coffee and coffee by-products. Food Res. Int. 46, 488–495 (2012). https://doi.org/10.1016/j.foodres.2011.05.028
G. Faustmann, C. Cavin, A. Nersesyan, S. Knasmüller (2009) Chemopreventive Properties of Coffee and Its Constituents. Chemoprevention of Cancerand DNA Damage by Dietary Factors Wiley-VCH Verlag Gmb H&Co KGaA 579–594
W. Kim, S. Kim, D. Kim, B. Kim, M. Baik, Puffing, a novel coffee bean processing technique for the enhancement of extract yield and antioxidant capacity. Food Chem. 240, 594–600 (2018). https://doi.org/10.1016/j.foodchem.2017.07.161
L.Z. Wang, Q.W. He (2005) Chinese Radish Scientific and Technical Documents Publishing House Beijing 292–370 (in Chinese)
A. Iriondo-DeHond, M.B. Ríos, T. Herrera, A. Rodríguez-Bertos, F. Nuñez, M.I. Andrés, S. Sánchez-Fortún, M.D. Castillo, Coffee silverskin extract: nutritional value, safety and effect on key biological functions. Nutrients 11, 2693 (2019). https://doi.org/10.3390/nu11112693
B. Ciaramelli, A. Palmioli, C. Airoldi, Coffee variety, origin and extraction procedure: Implications for coffee beneficial effects on human health. Food Chem. 278, 47–55 (2019). https://doi.org/10.1016/j.foodchem.2018.11.063
S. Perez-Burillo, T. Mehta, A. Esteban-Muñoz, S. Pastoriza, O. Paliy, J.A. Rufián-Henares, Effect of in vitro digestion-fermentation on green and roasted coffee bioactivity: The role of the gut microbiota. Food Chem. 279, 252–259 (2019)
M. Naczk, F. Shahidi, Extraction and analysis of phenolics in food. J. Chromatogr. A. 1054, 95–111 (2004). https://doi.org/10.1016/j.chroma.2004.08.059
D. Vitali, I. Vedrina Dragojevic, B. Šebecic, Effects of incorporation of integral raw materials and dietary fiber on the selected nutritional and functional properties of biscuits. Food Chem. 114, 1462–1469 (2009). https://doi.org/10.1016/j.foodchem.2008.11.032
M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger et al., A standardised static in vitro digestion method suitable for food — An international consensus. Food Funct. 5, 1113–1124 (2014)
R. Apak, K. Güçlü, M. Özyürek, S.E. Karademir, Novel total antioxidant capacity index for dietary polyphenols, vitamin C and E using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 52, 7970–7981 (2004). https://doi.org/10.1021/jf048741x
R. Apak, K. Güçlü, B. Demirata et al., Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Mol. 12, 1496–1547 (2007). https://doi.org/10.3390/12071496
A. Priftis, D. Stagos, K. Konstantinopoulos, C. Tsitsimpikou, D.A. Spandidos, A.M. Tsatsakis, M.N. Tzatzarakis, D. Kouretas, Comparison of antioxidant activity between green and roasted coffee beans using molecular methods. Mol. Med. Rep. 12, 7293–7302 (2015). https://doi.org/10.3892/mmr.2015.4377
M. Jeszka-Skowron, R. Frankowski, A. Zgoła-Grześkowiak, Comparison of methylxantines, trigonelline, nicotinic acid and nicotinamide contents in brews of green and processed Arabica and Robusta coffee beans? Influence of steaming, decaffeination and roasting processes on coffee beans. LWT - Food Sci. Technol. 125, 109344 (2020). https://doi.org/10.1016/j.lwt.2020.109344
F.G. Gemechu, Embracing nutritional qualities, biological activities and technological properties of coffee byproducts in functional food formulation. Trends in Food Sci. Technol. 104, 235–261 (2020). https://doi.org/10.1016/j.tifs.2020.08.005
A.S. Costa, R.C. Alves, A.F. Vinha, E. Costa, C.S. Costa et al., Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 267, 28–35 (2018). https://doi.org/10.1016/j.foodchem.2017.03.106
A. Panusa, R. Petrucci, R. Lavecchia, A. Zuorro, UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of arabica and robusta coffee silverskin: Antioxidants vs phytotoxins. Food Res. İnt. 99, 155–165 (2017). https://doi.org/10.1016/j.foodres.2017.05.017
B. Somporn, A. Kamtuo, P. Theerakulpisut, S. Siriamornpun, Effects of roasting degree on radical scavenging activity, phenolics and volatile compounds of Arabica coffee beans (Coffea arabica L. cv. Catimor). Int. J. Food Sci. Technol. 46, 2287–2296 (2011). https://doi.org/10.1111/j.1365-2621.2011.02748.x
P. Tripetch, C. Borompichaichartkul, Effect of packaging materials and storage time on changes of colour, phenolic content, chlorogenic acid and antioxidant activity in arabica green coffee beans (Coffea arabica L cv Catimor). J. Stored Products Res. 84, 101510 (2019). https://doi.org/10.1016/j.jspr.2019.101510
B. Cämmerer, L.W. Kroh, Antioxidant activity of coffee brews. Eur Food Res Technol. 223, 469–474 (2006). https://doi.org/10.1007/s00217-005-0226-4
D. Rio, A. Stalmach, L. Calani, A. Crozier, Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutr. 2, 820–833 (2019). https://doi.org/10.3390/nu2080820
J. Pokorná, P.R. Venskutonis, V. Kraujalytė, P. Kraujalis, P. Dvořák, B. Tremlová, V. Kopriva, M. Ošťádalová, Comparison of different methods of antioxidant activity evaluation of green and roast C. Arabica and C. Robusta coffee beans. Acta Aliment. 44, 454–460 (2015). https://doi.org/10.1556/066.2015.44.0017
Y. Narita, K. Inouye, High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem. 135, 943–949 (2012). https://doi.org/10.1016/j.foodchem.2012.05.078
O. Babova, A. Occhipinti, M. Maffei, Chemical partitioning and antioxidant capacity of green coffee (Coffea arabica and Coffea canephora) of different geographical origin. Phytochem. 123, 33–39 (2016). https://doi.org/10.1016/j.phytochem.2016.01.016
R.M. Alonso-Salces, F. Serra, F. Reniero, K. Héberger, Botanical and geographical characterization of green coffee (Coffea arabica and Coffea canephora): chemometric evaluation of phenolic and methylxanthine contents. J Agric. Food Chem. 57, 4224–4235 (2009). https://doi.org/10.1021/jf8037117
J.L. Song, T.S. Asare, M.Y. Kang, S.C. Lee, Changes in bioactive compounds and antioxidant capacity of coffee under different roasting conditions. Korean J. Plant Res. 31, 704–713 (2018). https://doi.org/10.7732/KJPR.2018.31.6.704
A. Hečimović, D. Belščak-Cvitanović, D. Horžić, Komes, Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 129, 991–1000 (2011). https://doi.org/10.1016/j.foodchem.2011.05.059
Y. Liu, D. Kitts, Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 44, 2418–2424 (2011). https://doi.org/10.1016/j.foodres.2010.12.037
N. Martinez-Saez, M. Ullate, M.A. Martin-Cabrejas, P. Martorell, S. Genovés, D. Ramon, M.D. del Castillo, A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 150, 227–234 (2014). https://doi.org/10.1016/j.foodchem.2013.10.100
V.S. Ribeiro, A.E. Leitão, J.C. Ramalho, F.C. Lidon, Chemical characterization and antioxidant properties of a new coffee blend with cocoa, coffee silverskin and green coffee minimally processed. Food Res. Int. 61, 39–47 (2014). https://doi.org/10.1016/j.foodres.2014.05.003
D. Gocmen, Y. Şahan, E. Yıldız, M. Coşkun, İA. Aroufai, Use of coffee silverskin to improve the functional properties of cookies. J Food Sci. Technol. 56, 2979–2988 (2019)
E. Fernández-García, I. Carvajal-Lérida, A. Pérez-Gálvez, In-vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 29, 751–760 (2009). https://doi.org/10.1016/j.nutres.2009.09.016
J. Carbonell-Capella, M. Buniowska, F. Barba, M.J. Esteve, A. Frígola, Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: a review. Compr. Rev. Food Sci. Food Safety 13, 155–171 (2014). https://doi.org/10.1111/1541-4337.12049
F. Barba, L.R. Mariutti, N. Bragagnolo, A. Mercadante, G. Barbosa-Cánovas, V. Orlien, Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends in Food Sci. Technol. 67, 195–206 (2017). https://doi.org/10.1016/j.tifs.2017.07.006
F. Barba, N.S. Terefe, R. Buckow, D. Knorr, V. Orlien, New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res. Int. 77, 725–742 (2015). https://doi.org/10.1016/j.foodres.2015.05.015
D.B. Huang, R.L. Prior, 2005, The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53, 1841–1856 (2005)
R. Apak, Current issues in antioxidant measurement. J. Agric. Food Chem. 67, 9187–9202 (2019). https://doi.org/10.1021/acs.jafc.9b03657
C. Rice-evans, N.J. Miller, G. Paganga, Antioxidant properties of phenolic compounds. Trends Plant Sci. 2, 152–159 (1997). https://doi.org/10.1016/S1360-1385(97)01018-2
R. Apak, K. Güçlü, B. Demirata, M. Özyürek, E.S. Çelik, B. Bektaşoğlu, K.I. Berker, D. Özyurt, Comparative evaluation of total antioxidant capacity assays applied to phenolic compounds and the CUPRAC Assay. Mol. 12, 1496–1547 (2007). https://doi.org/10.3390/12071496
M.C. Nicoli, M. Anese, L. Manzocco, C.R. Lerici, Antioxidant properties of coffee brews in relation to the roasting degree. LWT-Food Sci. Technol. 30, 292–297 (1997). https://doi.org/10.1006/fstl.1996.0181
R.C. Alves, I.M. Almeida, S. Casal, M.B. Oliveira, Isoflavones in coffee: influence of species, roastdegree, and brewing method. J. Agric Food Chem. 58, 3002–3007 (2010). https://doi.org/10.1021/jf9039205
M.D. Del Castillo, J.M. Ames, M.H. Gordon, Effect of roasting on the antioxidant activity of coffee brews. J. Agric. Food. Chem. 50, 3698–3703 (2002). https://doi.org/10.1021/jf011702q
J. Aguiar, B.N. Estevinho, L. Santos, Microencapsulation of natural antioxidants for food application – The specific case of coffee antioxidants – A review. Trends Food Sci. Technol. 58, 21–39 (2016). https://doi.org/10.1016/j.tifs.2016.10.012
R. Apak, K. Güçlü, M. Özyürek, Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim Acta 160, 413–419 (2008). https://doi.org/10.1007/s00604-007-0777-0
M. Bener, M. Ozyürek, K. Güçlü, R. Apak, Development of a low-cost optical sensor for cupric reducing antioxidant capacity measurement of food extracts. Anal Chem. 82, 4252–4258 (2010). https://doi.org/10.1021/ac100646k
R. Apak, M. Özyürek, K. Güçlü, E. Çapanoğlu, Antioxidant activity/Capacity measurement 1 classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J Agric Food Chem. 64, 997–1027 (2016). https://doi.org/10.1021/acs.jafc.5b04739
K. Güçlü, M. Altun, M. Özyürek, S.E. Karademir, R. Apak, Antioxidant capacity of fresh, sun- and sulphited-dried Malatya apricot (Prunus armeniaca) assayed by CUPRAC, ABTS/TEAC and folin methods. Int. J. International Journal of Food Sci. Technol. 41, 76–85 (2006). https://doi.org/10.1111/j.1365-2621.2006.01347.x
Acknowledgements
The authors would like to thank Bayramefendi Osmanlı Kahvecisi and Mrs. Sümeyra Caglak for the support of coffee and CSS supply. This study is a part of the Master’s thesis of the first author.
Author information
Authors and Affiliations
Contributions
İAA Performed the experiments. MS Performed the experiments, analyzed the data. DDA Designed the experiment, analyzed the data, wrote the manuscript, YS Designed the experiment, wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involved in human and animal rights
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aroufai, İ.A., Sabuncu, M., Dülger Altiner, D. et al. Antioxidant properties and bioaccessibility of coffee beans and their coffee silverskin grown in different countries. Food Measure 16, 1873–1888 (2022). https://doi.org/10.1007/s11694-021-01271-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01271-4