Abstract
Iodine-rich herbs such as seaweed, kelp, and sea tangle were widely used to treat various types of goiter with good effect and without any adverse side effects in China. When compared with potassium iodate (PI), iodine-rich herbs had a positive effect on the recovery of goiter resulting from iodine deficiency without any obvious harmful effects. In NOD.H-2h4 mice, an autoimmune thyroiditis-prone model, iodine excess can increase infiltration of lymphocytes and structural damage of the thyroid follicles, hence resulting in thyroiditis. Until now, there has been little research on the comparative effects of PI and iodine-rich herbs on thyroid in an autoimmune thyroiditis-prone model. This study was designed to compare the different effects of iodine-rich herbs and PI on the thyroid gland in iodine-deficient NOD.H-2h4 mice. Excessive intake of PI cause oxidative injury in the thyroid gland and increase the risk of autoimmune thyroiditis, while iodine-rich herbs cause less oxidative injury, significantly enhancing antioxidant capacity, and inhibit the high differentiation of Th17 cells in the thyroid glands of NOD.H-2h4 mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iodine-rich herbs such as seaweed, kelp, and sea tangle were widely used to treat various types of goiter with good effect and without any adverse side effects in ancient and present China. This is in contrast to potassium iodate (PI) which, when taken in excess, causes harmful effects on thyroid. Until now, there has been little research on the comparative effects of PI and iodine-rich herbs on thyroid in an autoimmune thyroiditis (AIT)-prone model.
Excess iodine is a recognized environmental factor for AIT [1]. Our earlier research found that excessive PI intake damaged thyroid follicular cells in the iodine-deficient Wistar rats. Increased dosage and duration of PI intake led to more obvious damage in the thyroid follicles [2]. But iodine-rich herbs had a positive effect on the recovery of iodine deficiency induced goiter without any obviously harmful effects [3, 4]. In a study with NOD.H-2h4 mice, an AIT-prone model, PI excess resulted in the infiltration of lymphocytes and structural damage of the thyroid follicles, thus leading to the occurrence of AIT [5]. Oxidative stress resulting from an imbalance between the oxidation and anti-oxidation systems due to PI excess is considered to be one of the main mechanisms causing structural damage of the thyroid follicular cells and lymphocyte infiltration. The thyroid oxidative stress due to PI excess may cause the over-production of aldehyde metabolites such as 4-hydroxynonenal (4-HNE), then damaging the cellular structures and causing protein cross-linking degeneration and DNA damage. Peroxide oxidation reduction enzyme 5 (PRDX5) may convert H2O2 to water and reduce intracellular lipid peroxide levels, which may give a more accurate representation of the level of oxidative stress in the thyroid [6].
Studies have shown that the IL-23/IL-17 axis may play an important role in the pathogenesis of AIT [7, 8]. Until now, there has been little research done concerning the effect of iodine-rich herbs on AIT in animal models with respect to both oxidative stress and IL-23/IL-17 axis.
Materials and Methods
Animals and Diet
All experimental protocols were approved by the ethics committee of the Liaoning University of TCM, and all procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Two hundred 8-week-old NOD.H-2h4 mice (no. 004447) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). They were raised in the Animal Research Center of the Liaoning University of TCM under a specific pathogen-free condition. Ordinary feed was provided by the Qianmin animal diet processing factory in Shenyang. Iodine-deficient feed, in which iodine level is 20 μg/kg, was made of maize, millet, and beans from severe iodine-deficient areas in Chengde City, Hebei province, China, as well as an appropriate amount of additives.
Main Reagents and Instruments
Main reagents were as follows: polyclonal antibodies of rabbit anti-mouse IL-17 and IL-23, polyclonal antibodies of goat anti-mouse 4-HNE (P-16:SC-130083), and polyclonal antibodies of rabbit anti-rat PRDX5 (FL-214:SC-33573), immunohistochemical SP kit, fluorescence quantitative PCR kit, cDNA synthesis kit, instant SABC peroxidase kit, malondialdehyde (MDA) kit, glutathione peroxidase (GSH-Px), superoxidase dismutase (SOD) kit and H2O2 kit (Wuhan Boster Biological Technology Co. Ltd.), total triiodothyronine (TT3), total thyroxine (TT4) RIA kit (S10930055-T3, S10930056-T4), thyroid-stimulating hormone (TSH) kit (S20083118-TSH) (Tianjin Jiuding Medical Biological Engineering Co. Ltd.), triozol, 4% paraformaldehyde, PBS buffer (Shenyang Dingguo Changsheng Biotechnology Co. Ltd.).
The main instruments were used as follows: electrophoresis apparatus (BIO-RAD, PowerPac200), vertical panel electrophoresis equipment (BIO-RAD, Mini-Protein III), DYY-40 B-transfer electrophoresis tanks (Beijing Liuyi Instrument Factory, China), high-speed refrigerated centrifuge (Sigma-31k 5C, USA), PCR amplification (Biometra, Germany), UV spectrophotometer (UV-visible Spectrometer, UV300), ABI amplification of 7500 m, DK-8B type electric heating constant temperature water tank (Shanghai Jinghong Laboratory Equipment Co. Ltd.), BIO-DAD 680 enzyme mark instrument, FJ-2008 PS gamma counter, slicing machine (Leica Company, Germany), microscope (Olympus, Japan), gastric lavage device (Shenyang Dingguo Changsheng Biotechnology Co. Ltd.).
Modeling, Grouping, and Treatment Factors
After being fed with standard diet for a week to adapt to their surroundings, 200 8-week-old NOD.H-2h4 female mice were fed with a low-iodine diet and double-distilled water for 90 days. The mice were then randomly divided into four groups with 50 mice per group. The normal control group (NC) was fed with a normal diet throughout the entire experiment. The model control group (MC) was fed with a low-iodine diet and a same daily gastric lavage volume of double-distilled water. The herbs with iodine excess group (HIE) was fed with a low-iodine diet and a daily herbal decoction including seaweed 15 g, kelp 15 g, and sea tangle 7.5 g (offered by Pharmacy of the Affiliated Hospital of Liaoning University of TCM) with iodine content of 1900.36 μg/L and a crude drug content of 4.7 g/kg [9]. The dosage of the herbs was calculated and converted according to equivalent human body surface area. The iodine excess group (IE) was fed with the low-iodine diet and double-distilled water with iodine content of 1900 μg/L, prepared with PI. After another 90 days of treatment, the mice were killed.
Specimen Collection
All the mice were killed after intraperitoneal anesthesia and being weighed. Blood samples were obtained from the orbital vein. The serum was separated by 3000 r/min and preserved in 1.5 -mL Eppendorf tubes for measuring indices of thyroid function and oxidative stress. The thyroid glands were separated and one lobe of each gland was fixed instantly by 4% paraformaldehyde. The thyroid specimens used for RT-PCR and Western blot detection were stored instantly at − 80 °C liquid nitrogen.
Thyroid Morphology and Identification of Thyroiditis
Thyroid follicular morphology was observed with hematoxylin and eosin (H&E) stain under light microscope. Thyroiditis was identified as follows: A ratio of the area of lymphocyte infiltration within the area of the whole lobe exceeding 2% was considered thyroiditis [ 10 ].
Measurement of Thyroid Function and Oxidative Stress
Serums TT3 and TT4 were measured by radioimmunoassay (RIA) method, and the serum TSH was measured by the immunoradiometric (IRMA) method. The levels of serum MDA were measured by thiobarbituric acid (TBA) method, GSH-Px by glutathione peroxidase method, SOD by xanthine oxidase method, and H2O2 by biochemical method.
The expressions of 4-HNE, PRDX5, CD68+, and IL-17 were measured by immunohistochemistry, using instant SABC peroxidase kit. Positive staining was localized in the cytoplasm and colored brown. The results were semi-quantitatively analyzed by MetaMorph Microscopy Automation & Image Analysis Software (UIC) to integrate optical density values to reflect the relative content of positive substances.
Statistical Analysis
T test was used for comparison of two samples; for comparison of three or more samples, analysis of variance was used. Statistical significance was defined as P < 0.05. All data were entered into an Excel spreadsheet and statistically managed with SPSS 17.0 software package.
Results
Comparison of Thyroid Function on the Effect of Herbs with Iodine Excess and PI Excess in Iodine-Deficient NOD.H-2h4 Mice
No significant difference was seen among groups as far as serum TSH, TT3, and TT4 levels were concerned (see Table 1).
The Incidence of Thyroiditis in Iodine-Deficient NOD.H-2h4 Mice
No significant lymphocyte infiltration was seen in NC and MC groups. In the mice of the IE group, lymphocyte infiltration was seen up to 30–50% view. Fewer lymphocyte infiltration and less damage to the thyroid follicules were seen in the mice of HIE group when compared with IE group. The incidence of thyroiditis in IE group was much higher than that of HIE group (see Table 2 and Fig. 1).
Comparison of the Effect of Herbs with Iodine Excess and PI Excess on Thyroid Oxidative Stress in Iodine-Deficient NOD.H-2h4 Mice
The activities of serum GSH-Px became much lower in IE and MC groups but not in HIE group when compared with NC group, both P < 0.05. No significant difference was seen in the activities of serum SOD among all the groups. The levels of serum MDA and H2O2 markedly decreased in MC group when compared with NC group, P < 0.05. The levels of serum MDA and H2O2 became much higher in IE group when compared with MC and NC groups, both P < 0.01. The levels of serum MDA and H2O2 in HIE group were much higher than model control but much lower than IE group, all P < 0.05 (see Table 3).
Comparison of the Expression of 4-HNE and PRDX5 Proteins in Thyroid of Each Group
The expression of 4-HNE protein in HIE and IE groups was much higher than NC group, P < 0.001. The expression of 4-HNE protein in HIE group decreased markedly when compared with IE group, P < 0.001. The expression of PRDX5 in IE group decreased significantly when compared with NC group, P < 0.01. However, the expression of PRDX5 in HIE group increased markedly when compared with IE group, P < 0.001 (see Table 4 as well as Figs. 2 and 3).
Comparison of the Expression of CD68+ and IL-17 Protein in the Thyroid of Each Group
The expression of CD68 and IL-17 in MC group was much lower than NC group, P < 0.01. The expression of CD68 and IL-17 in IE group was much higher than MC group, P < 0.01. The expression of CD68 and IL-17 in HIE group decreased markedly when compared with IE group, P < 0.01 (see Table 5 as well as Figs. 4 and 5).
The Expression of Thyroid IL-17 mRNA and IL-23 mRNA in Each Group
No significant difference was seen between the NC and MC groups in respect to the expression of IL-17 messenger RNA (mRNA) and IL-23 mRNA. The expression of IL-17 mRNA and IL-23 mRNA in IE group was much lower than the NC and MC groups, both P < 0.05. The expression of IL-17 mRNA and IL-23 mRNA in HIE group, however, markedly decreased when compared with IE group, P < 0.05 though significantly increasing when compared with the NC and MC groups, both P < 0.05 (see Tables 6 and 7).
The Expression of Thyroid IL-17 and IL-23 plus PRDX5 in Each Group of Mice (Western Blot)
No significant difference was seen between the NC and MC groups in respect to the protein expression of IL-17 and IL-23. The protein expression of IL-17 and IL-23 in IE group was much lower than the NC and MC groups, both P < 0.05. The protein expression of IL-17 and IL-23 in HIE group, however, markedly decreased when compared with IE group, P < 0.05 though significantly increasing when compared with the NC and MC groups, both P < 0.05.
At about 17K, each group uniformly showed a single band of especially distinguished anti-PRDX5 antibodies. Compared with NC (0.26) and IE group (0.13), the expression of PRDX5 protein in HIE group (0.47) increased significantly (see Table 8 as well as Figs. 6, 7, and 8).
Discussion
Iodine excess can induce and aggravate AIT in genetically predisposed autoimmune-susceptible animals. However, our study found that iodine-rich herbs and PI have different effects on the occurrence of AIT. Excessive PI given to AIT-susceptible mice showed an increased incidence of AIT as well as an increase in CD68+ thyroid interstitial cells. However, iodine-rich herbs did not increase the incidence of AIT.
In this study, the IE and HIE groups absorbed similar amounts of elemental iodine per day. In a strictly pathogen-free laboratory, adult mice in the IE group were given 4~7 mL of water per day, so their iodine intake was 7.6~13.3 μg/day. Mice in the HIE group were fed with the concentrated iodine-rich herbal decoction; their daily iodine intake was 6.65 μg.
Oxygen-free radicals may play an important role in thyroid autoimmunity. The thyroid is a very active organ of free radical generation. Oxidative stress produces excessive oxygen-free radicals, weakens the antioxidant defense mechanisms, and results in lipid peroxidation, thus damaging the structure and function of thyroid follicular cells during the development of AIT. We found that the iodine-rich herbs increased the expression of PRDX5 in the cytoplasm of thyroid cells but with no significant change in serum GSH-Px and SOD activity, or in serum H2O2 and MDA values. Excess PI, however, increased the H2O2 and MDA values significantly, but did not markedly increase PRDX5 levels and GSH-Px activity. These results were similar to those of Zhang et al. [11]. Furthermore, excess PI markedly increased the expression of 4-HNE in thyroid cells, when compared with HIE group, indicating that excessive PI could decrease antioxidant capacity and aggravate peroxidative injury in thyroid cells.
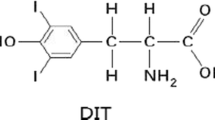
Compared with PI excess, iodine-rich herbs had a strong antioxidant capacity. Iodine-rich herbs not only significantly decreased the level of peroxide in the thyroid and serum, but also increased the antioxidant capacity in the thyroid. This mechanism may be associated with selenium, because iodine-rich herbs were also shown to be rich in selenium [6]. The different forms of iodine may have different effects on the thyroid. One study indicated that the effects of organic iodine (3,5-diiodotyrosine, DIT) and inorganic iodine (PI) on thyroid function and oxidative stress in iodine excess Wistar rats were different. The DIT did less damage to thyroid follicular cells than PI. Therefore, by balancing the antioxidant system, DIT had a more protective effect than potassium iodine [12].
The Okinawan food culture of Ryukyu Island in Japan is one of the world’s most interesting cultures because its consumers have long life expectancies and low disability rates, due in part to the everyday consumption of tofu and seaweed [13]. Another study found no association between seaweed intake and thyroid cancer in premenopausal or in postmenopausal women [14]. Recently, PI is being suggested for the treatment of Graves disease cases of exhibiting thionamide-associated side effects, Graves disease patients with moderate to severe hyperthyroidism, and Graves disease during the first trimester in Japan [15,16,17]. So iodine-rich herbs may be more beneficial to the abovementioned Graves disease patients though further research is really needed.
Oxidative damage is closely related to autoimmune inflammation. A new CD4+ T cell subset Th17 can secrete IL-17, which depends on IL-23 [18]. Th17 cells play an important role in mediating the inflammatory response, autoimmune diseases, transplant rejection, and tumor growth [19]. Increased differentiation of Th17 lymphocytes may be related to the pathogenesis and development of thyroid-specific autoimmunity in AIT [8, 20, 21]. Compared with PI, iodine-rich herbs decreased the thyroid expression of IL-23/IL-17 in iodine-deficient NOD.H-2h4 mice, which may be another important mechanism to reduce the incidence of AIT.
In summary, although the iodine content of the herbs used in this study is rich, there were no obvious harmful effects on the thyroid over the period of the study. This may be due to the fact that iodine-rich herbs cause light oxidative damage to the thyroid and inhibit the high expression of IL-23 and IL-17 in thyroid cells.
References
Luo Y, Kawashima A, Ishido Y et al (2014) Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int J Mol Sci 15(7):12895–12912
Gao T, Teng W (2004) Effect of mild and moderate iodine excess on thyroid function and morphology in iodine deficiency rats. Chin J Endocrinol Metab 20:353–356
Gao T, Cui P, Li H et al (2008) A study on the effect of Haizao Yuhu Decoction on thyroid function and morphology in iodine deficiency induced goiter rats. Chin J Basic Med Tradit Chin Med 14:113–116
Qi T, Gao T (2012) A comparative study on the effects of excess iodine and herbs with excess iodine on thyroid oxidative stress in iodine-deficient rats. Chin J Endocrinol Metab 28:858–861
Teng X, Shan Z, Teng W (2009) Experimental study on the effects of chronic iodine excess on thyroid function, structure and autoimmunity in autoimmune-prone NOD.H-2h4 mice. Clin Exp Med 9:51–59
Gao T, Shi R, Qi T et al (2014) A comparative study on the effects of excess iodine and herbs with excess iodine on thyroid oxidative stress in iodine-deficient rats. Biol Trace Elem Res 157:130–137
Ruggeri RM, Saitta S, Cristani M et al (2014) Serum interleukin-23 (IL-23) is increased in Hashimoto’s thyroiditis. Endocr J 61(4):359–363
Figueroa-Vega N, Alfonso-Perez M, Benedicto I et al (2010) Increased circulating proinflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroidits. J Clin Endocrinol Metab 95(2):953–962
Cui P, Gao T (2007) To measure the content of iodine in herbal medicine and compound prescription used in softening hard mass and disintegrating masses. Chin Arch Tradit Chin Med 25:1396–1398
Bagchi N, Brown T, Sundick R (1995) Thyroid cell injury is an initial event in the induction of autoimmune thyroiditis by iodine in obese strain chickens. Endocrinology 136:5054–5060
Zhang N, Tong Y, Shan Z et al (2006) Effect of chronic mild and moderate iodine excess on thyroid anti-oxidative ability of iodine-deficiency and non-iodine deficiency Wistar rats. Chin Med J 86:1274–1278
Liu D, Lin X, Yu F et al (2015) Effects of 3,5-diiodotyrosine and potassium iodide on thyroid function and oxidative stress in iodine-excess Wistar rats. Biol Trace Elem Res 168(2):447–452
Sho H (2001) History and characteristics of Okinawan longevity food. Asia Pac J Clin Nutr 10(2):159–164
Wang C, Yatsuya H, Li Y et al (2016) Prospective study of seaweed consumption and thyroid cancer incidence in women: the Japan collaborative cohort study. Eur J Cancer Prev 25(3):239–245
Okamura K, Sato K, Fujikawa M, Bandai S, Ikenoue H, Kitazono T (2014 Nov) Remission after potassium iodide therapy in patients with Graves hyperthyroidism exhibiting thionamide-associated side effects. J Clin Endocrinol Metab 99(11):3995–4002
Sato S, Noh JY, Sato S, Suzuki M, Yasuda S, Matsumoto M, Kunii Y, Mukasa K, Sugino K, Ito K, Nagataki S, Taniyama M (2015 Jan) Comparison of efficacy and adverse effects between methimazole 15 mg+inorganic iodine 38 mg/day and methimazole 30 mg/day as initial therapy for Graves’ disease patients with moderate to severe hyperthyroidism. Thyroid 25(1):43–50
Yoshihara A, Noh JY, Watanabe N, Mukasa K, Ohye H, Suzuki M, Matsumoto M, Kunii Y, Suzuki N, Kameda T, Iwaku K, Kobayashi S, Sugino K, Ito K (2015 Oct) Substituting potassium iodide for methimazole as the treatment for Graves disease during the first trimester may reduce the incidence of congenital anomalies: a retrospective study at a single medical institution in Japan. Thyroid 25(10):1155–1161
Harrington L, Mangan P, Weaver C et al (2006) Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol 18:349–356
Mucida D, Park Y, Kim G et al (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317:256–260
Kristensen B (2016). Regulatory B and T cell responses in patients with autoimmune thyroid disease and healthy controls. Dan Med J 63(2)
Xue H, Yang Y, Zhang Y et al (2015) Macrophage migration inhibitory factor interacting with Th17 cells may be involved in the pathogenesis of autoimmune damage in Hashimoto’s thyroiditis. Mediat Inflamm 2015:621072
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental protocols were approved by the ethics committee of the Liaoning University of TCM, and all procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of Interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Gao, Ch., Qu, Jq., Zhou, Xy. et al. Iodine-Rich Herbs and Potassium Iodate Have Different Effects on the Oxidative Stress and Differentiation of TH17 Cells in Iodine-Deficient NOD.H-2h4 Mice. Biol Trace Elem Res 183, 114–122 (2018). https://doi.org/10.1007/s12011-017-1115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1115-y