Abstract
Iodine is an integral component of thyroid hormones. Relationship between the level of iodine intake and the risk of occurrence of thyroid diseases is ‘U’ shaped, that indicates there is an increasing risk with both low and high iodine intake. There are studies to evaluate thyroid functions in iodine deficient regions on humans and animals. To prevent iodine deficiency iodized salt has been introduced through universal salt iodization programme and enormous benefits have been achieved. However, improper monitoring of iodine content in edible salt at consumer level have increased the risk of excessive iodine intake leading serious health consequences to thyroid as iodine induced hypothyroidism, hyperthyroidism, goitre, thyroid gland disruption, thyrocyte apoptosis, thyroiditis, etc. Further, excess iodine induces marked alteration in the morphology and histology of testis along with male accessory sex organs including functional characteristics of sperm which are associated with the antifertility potential; in female cyclic ovarian function changes leading to decreased fertility potential that prevents pregnancy. Hypothyroidism caused by excess iodine impairs glial cell structure causing functional as well as morphological impairment of the major areas of brain developing neurological disorders interrupting connectivity of neural networks that signals early cognitive impairments triggering cognitive weakening, memory impairment and depression. Iodine in excess has the immunomodulatory effect in thyroid developing autoimmunity. Prolonged exposure of excess iodine impairs carbohydrate and lipid metabolic pattern and the histoarchitecture of the pancreas, liver, kidney, as well as skeletal and cardiac muscles. All these show the emergence of several health consequences in post-salt iodization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary supplies of iodine in adequate amount is an essential constituent of thyroid hormones thyroxine (T4) and triiodothyronine (T3) synthesis and is necessary for regular growth, differentiation, maturity and metabolic processes (Pearce et al.2013). Thyroid gland functions are impaired both in iodine deficiency and in excess. The effects of iodine in thyroid are complex with a ‘U’ shaped relationship between iodine intake and risk of thyroid diseases (Laurberg et al. 2001). Iodine deficiency may cause thyroid dysfunction leading to severe thyroid diseases such as endemic goitre and congenital hypothyroidism (Medani et al. 2011; Kurtoğlu et al. 2014). To prevent and control iodine deficiency disorders (IDDs) addition of iodine to edible salt through universal salt iodization programme has been recommended globally (WHO/UNICEF/ICCIDD 2007). Potassium iodide (KI) and potassium iodate (KIO3) are in common use as salt iodization agents in most countries (Bürgi et al. 2001).
Iodine prophylaxis through iodized salt has been implemented almost globally to prevent and manage IDDs, resulting in enormous benefits in all aspects of life in the affected communities (Hetzel and Dunn 1989). However a single ‘one size fits all’ standard/uniform salt iodization ignoring the local specific conditions like bioavailability of iodine in food and water, goitrogenic//antithyroidal constituents present in consumed food has increased the risk of excess iodine intake (Leung and Braverman 2014) in many regions (Zimmermann 2008) Besides the improper monitoring of salt iodine content during post salt iodization period not only affect thyroid functions (Andersson et al. 2010) but may have adverse effect on thyroid hormone responsive organs like reproduction (Chakraborty et al. 2016; Mahapatra and Chandra 2017), brain (Mandal et al. 2017), metabolism (Sarkar et al. 2018) and immune system (Saha et al 2019). All these aspects based on available information have been reviewed in this article.
Median Urinary Iodine: An Indicator of Iodine Intake
Urinary excretion pattern of iodine is considered as a valuable indicator to understand the regular consumption of iodine because the major portion of iodine present in body is eliminated through urine, generally more than 90 per cent; thus, the iodine concentration in urine reflects the individual iodine intake (Dunn et al. 1993). In persons, elimination of body’s iodine alters time to time even in 24 h. Epidemiological criteria for assessing iodine nutrition is therefore based on median urinary iodine concentration of school-age children (6–12 years) in 30 urine samples from an area as defined by WHO/UNICEF/ICCIDD (2007).
After the key recommendations made in 2007 of WHO/UNICEF/IGN (Iodine Global Network), in the expert Technical Group Meeting in December, 2015, certain emerging recommendations came out in the revised ‘UNICEF Guidance on the Meeting of Salt Iodization Programmes and Determination of Population iodine Status’ (UNICEF and IGN 2018) that are highlighted below.
-
1.
Recommended intake of iodine in communities likely to be examined from all classes of population with special reference to most affected classes. This is because, median value of iodine that is eliminated through urine (mUIC) in the school children (6–12 years) does not reflect iodine nutritional status of lactating and pregnant mothers who need more.
-
2.
The ‘adequate’ range of iodine intake among school children can be widened from 100 to 199 µg/L to 100–299 µg/L. This is because the range 200–299 µg/L which has been defined earlier above requirement among school children (WHO/UNICEF/ICCIDD, 2007) however, mUIC range of 100–299 µg/L not associated with any dysfunction of thyroid.
-
3.
In currently available methods, the mUIC can only be used to define population iodine status and not to quantify the proportion of the population with iodine deficiency or iodine excess.
-
4.
Iodine content in processed foods must be checked through appropriate authority of the country.
Excess Iodine and Thyroid
Auto-regulatory Mechanism of Thyroid
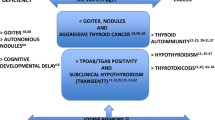
A temporary decline in production of hormones in thyroid gland that last for about one day found in rats after intake of iodine over and above requirement is defined as Wolff–Chaikoff effect (Wolff and Chaikoff 1948). When the plasma iodine level is elevated the binding of iodine in thyroid is decreased (Acute Wolff–Chaikoff effect); in presence of continued high plasma iodine concentration adaptation or escape from the acute effect takes place within 2 days. This is because increased intrathyroidal iodine decreases both sodium iodide symporter (NIS) mRNA and protein expression by transcriptional inhibition (Eng et al. 1999). In patients having specific risks factors, can’t adopt acute Wolff–Chaikoff effect probably for a damaged thyroid as a result of earlier pathological insults causing iodine induced hypothyroidism; on the contrary in some predisposed patients, an excess iodine is a rich substrate for increased production of thyroid hormones leading to iodine induced hyperthyroidism (the Jod-Basedow phenomenon) that may be transient or permanent (Leung and Braverman 2014). The generation of several inhibitory substances within the thyroid follicles viz. iodolipids, iodoacetones or iodoaldehydes are considered as the effectors for inhibition of thyroid peroxidase activity by reducing the formation of iodide to oxidized iodide (Pramyothin et al. 2011). Increased intrathyroidal iodine seemed to decrease deiodinase activity might also decreased thyroid hormone production (Hussein et al. 2012). In addition, propylthiouracil, ethyl mercaptoimidazole (MMI) and carbamizole are called thiourelene antithyroid drugs. The thioureylene drugs are potent inhibitors of TPO-catalysed iodination of protein and tyrosine (Taurog 1976). Thiocyanate is the detoxification product of cyanogenic plant foods which are naturally occurring goitrogen and a potent inhibitor of iodine transport through basal membrane of thyroid follicles (Erdoǧan 2003). Thiocyanate even in presence of adequate iodine developed goitre and led goitrous population towards hypo- and hyperthyroidism with hypoechoic thyroid and thyroiditis (Singh et al. 2021).
Excess Iodine and Thyroid Autoimmune Disorders
There are reports of normalization of iodine status in global scenario including India following universal salt iodization (Kapil and Singh 2004; Kapil et al. 2004). There are studies that showed an increase in autoimmune thyroiditis in school children who had been supplemented with excess iodine showing further a correlation between urinary iodine excretion (UIE) and thyroidal microsomal antibody (Palaniappan et al. 2017). More than adequate iodine intake could be a public health concern in terms of thyroid function and thyroid autoimmunity in the Chinese populations (Teng et al. 2011), school children in Delhi, (Gopalakrishnan et al. 2006), in North western Greece (Zois et al.2003) and iodine supplemented areas of Athens and suburbs (Kaloumenou et al. 2008). However, it has also been reported that introduction of iodized salt to severely iodine-deficient children does not provoke thyroid autoimmunity (Zimmermann et al. 2003). In spite of adequate iodine prophylaxis, goitre prevalence has not reduced as much as expected indicating the involvement of factors other than iodine deficiency in goitrogenesis in many areas of India; further enlargement of thyroid for autoimmunity may in part found responsible for existing goitre prevalence (Marwaha et al. 2003). In post salt iodization phase, the children in the state of Manipur of north east India found goitrous, in spite of their adequate iodine intake. However, their genetic susceptibility associated with excess thiocyanate exposure that comes for the consumption of bamboo shoots might have increased the risk for the development of autoimmune thyroid disorders in their latter phases of life (Chandra and Singh 2012). The coastal plains of the Gangetic West Bengal found environmentally iodine replete but iodine prophylaxis is also in vogue in the region. The people get iodine both from local food and water however endemic goitre found prevalent The large goitre that are found in the region were histomorphologically and biochemically examined after surgical removal of the gland and observed that the large goitrous subjects were affected by Graves’ disease and the possible reason for the development of this thyroid autoimmunity was for the consumption of dietary goitrogens (thiocyanate precursors) and iodine over and above the recommended level (Chandra et al.2018) as thiocyanate promotes thyroid gland size and thyroid autoimmunity (Singh et al. 2021) while iodine prophylaxis also induces the similar effect (Papanastasiou et al.2007).
Excess Iodine and Hypothyroidism
In individuals who could not adopt acute Wolf–Chaikoff effect because of their damaged thyroid for earlier pathological consequences or medication developed iodine-induced hypothyroidism as evident by their low thyroid hormone profile as well as enhanced TSH (Safran and Braverman 1982; Saberi and Utiger 1975; Eng et al. 1999).
Sodium–Iodide Symporter (NIS), the transmembrane glycoprotein found in the basal membrane of follicular cells in thyroid actively transport iodide from circulation into thyroid (Dai et al. 1996). High concentration of iodide (I−) decreased NIS expression and thyroid function through PI3K/AKt signalling pathway. The reactive oxygen species (ROS) that is generated through this pathway under the influence of excess I− inhibits NIS expression in thyrocytes indicating the role of thyroid oxidative status to the Wolff–Chaikoff effect (Serrano-Nascimento et al.2014, 2016).
Excess Iodine and Hyperthyroidism
Excess iodine load is a rich substrate for production of thyroid hormone in some susceptible subjects leading to iodine induced hyperthyroidism (Jod Basedow phenomena) (Leung and Braverman 2014). Wolff–Chaikoff effect is found in euthyroid persons only. The outcome of excess iodine on euthyroid subjects would not be compared with those who are susceptible to thyroid diseases. Iodine induced hyperthyroidism (Jod-Basadow phenomena) was described first in early 1800’s where endemic goitrous subjects were affected by thyrotoxocis after iodine supplementation than in individuals without goitre (Coindet 1821). Hyperthyroidism that developed after iodine supplementation might be transient or permanent that includes nontoxic or diffuse nodular goitre, latent Grave’s disease and long-standing iodine deficiency (Hussein et al. 2012). Iodine—induced hyperthyroidism in euthyroid individuals with nodular goitre in iodine—replete region has also been noticed after excessive iodine supplementation (Vagenakis et al.1972).
Excess Iodine and Thyroid Gland Disruption
The morphological and functional status of thyroid gland after the exposure of iodine (KI) over and above the recommended level, two different doses as physiological dose (100 times more than the recommended level) and pharmacological dose (500 times more than its recommended level) administered through gavage to adult female rats regularly for two different durations as short (30 days) and long (60 days) respectively (Lupachik et al.2007). Serum GOT and GPT levels of the KI exposed group of animals after both the doses for different durations were assayed and found that these levels were not elevated in any of the groups compared to normal rats suggesting that no toxicity developed after the exposure of iodine as mentioned (Chakraborty et al. 2016; Mahapatra and Chandra 2017). Iodine nutritional status, iodine content of thyroid, thyroid hormonal profiles following serum T4 and T3 and histology of thyroid were studied.
In all, the results revealed that excretion of iodine increased on dose and time dependent manner in comparison to normal group. Significant change in iodine content of thyroid noticed in all the groups except the group treated with physiological dose for short duration. In consistent with iodine content in thyroid, significant decrease in serum T3 level and increase in serum T4 level was found in all the groups except the group as mentioned. The relative thyroid gland weight was increased along with hypertrophy, hyperplasia of follicular cells and scalloping of colloid within thyroid follicles resulting in necrosis and atrophy of the follicles resembling goitre occurred in all the groups depending on dose and duration of iodine exposure. The study concludes that excess iodine even in physiological dose may develop biochemical hypothyroidism if the exposure is continued long while excessive iodine in pharmacological doses develop such a state of hypothyroidism both after short and prolonged exposure (Chakraborty et al. 2016; Mahapatra and Chandra 2017).
KI is reported also to diminish the hyperplasia as well as hypervascularity of thyroid which is characterized by the diffuse goiter of Graves’ disease. Therefore, treatment with KI is a common practice in surgical therapy for this disorder. It has been hypothesized that iodine binds to organic compounds that interfere with the metabolic processes required for development and maintenance of hyperplasia and the possible cause for thyroid involution after excess iodine (Pisarev and Itoiz 1972). The possible mechanism is that that intracellular KI is necessary to exert its effect on protein synthesis, and that this effect is mediated through an organic form of iodine, probably iodothyronines. This action of KI is specific for the thyroid gland only. Therefore, potassium iodide (KI) has shown to have an antigoitrogenic action and inhibits in vivo thyroid protein biosynthesis (Pisarev and Aiello 1976).
Excess Iodine and Thyrocyte Apoptosis
The toxicity of iodine excess has shown both in cell systems and in animals. Thyroid gland involution has been demonstrated in rats (Mahmoud et al.1986; Belshaw and Becker 1973). Iodide inhibits the growth of thyroid cell and induces morphological changes of porcine thyroid cells in in vitro studies (Takasu et al. 1985). Effects of iodide appears to be species specific because cytotoxic effect of iodide has been demonstrated in FRTL-5 cells of rats but not found in thyrocytes of dog in primary culture (Golstein and Dumont 1996) Iodide induced cytotoxic effect on thyrocytes of rat showed necrotic and apoptotic characteristics, showing the involvement of the controlled regulation of cell death leading to an active process of cell self-destruction that requires activation of the genetic programme, inducing changes in morphology, DNA fragmentation and protein cross-linking (Cohen 1993);Ellis et al. 1991). Excess iodide induced apoptosis showed that DNA damage is not the primary event in immortalized thyroid cell line (TAD-2) in primary cultures of human thyroid cells. This type of apoptosis is p53 independent, does not require protein synthesis, and is not induced by modulation of Bcl-2, Bcl-XL, or Bax protein expression. However, indicate that excess molecular iodide, produced by oxidation of ionic iodine through endogenous peroxidases, induces apoptosis in thyroid cells through a mechanism involving generation of free radicals (Vitale et al. 2000). (Fig. 1).
Excess Iodine and Thyroid Hormone Receptive Systems
Disruption in Reproduction
Thyroid hormones regulate development and growth of gonads and their functions throughout the different phases of life. Iodine prophylaxis through salt has increased iodine intake in population especially in iodine-rich regions and pose a serious risk in public health (Alsayed et al. 2008; Camargo et al. 2008) including infertility (Paulíková et al. 2002), still births, abortions and embryo toxicity (Han et al. 2012).
Disruption in Male Reproduction
Supplementations of excess iodine have increased iodine content in testis in proportion to the dose exposed. Excess iodine induces marked alteration in the morphology and histology of testis along with accessory sex organs viz. epididymis, ventral prostrate, seminal vesicles as well as coagulating gland in adult rats; in the seminiferous tubules, degeneration in spermatogonia and germ cell content was noticed. Electron microscopic observations also portrayed the absence of sperm in excess iodine exposed groups. Under the influence of iodine in excess the serum testosterone level was reduced which might be the probable cause for the changes as mentioned. Excess iodine also affects the functional characteristics of sperm i.e. acrosome integrity, motility, mitochondrial membrane potential, plasma membrane integrity, DNA fragmentation, cell cycle, apoptosis—all these are associated with the antifertility potential (Chakraborty et al. 2016; Chandra and Chakraborty 2017).
Disruption in Female Reproduction
The average duration of estrous cycle is 4.8 days in adult female rats and is almost consistent (Astwood 1939; Blandau et al.1941). Based on the characteristic of vaginal smear, the duration of individual steps of the estrous cycle of rats with a four- or five-day cycle are designated as proestrous (12–14 h), estrous (25–27 h), metestrous (6–8 h) and diestrous (55–57 h) respectively. Slight variations depending on species and age have also been reported (Everett 1980; Felicio et al. 1984). The rhythmical changes that take place in the ovary of a sexually mature female rat is dependent on the changes in the gonadal hormonal levels as well as pituitary gonadotropins (Lerner 1969; Neguin et al. 1975). Serum levels of estrogens, progesterone, LH and FSH are the major regulators in modulating the cyclical changes in females. Any exogenous or internal stimulus that brings about changes in the ovarian physiology consequently brings about respective changes in the estrous cycle periodicity.
After excess and excessive iodine (physiological and pharmacological doses respectively) ingestion in adult female rats, marked alteration in the periodicity at each stage resulting in overall increase in total duration of cycle was found. In ovary, iodine accumulation is also varied with the stages of estrous cycle in iodine ingested groups of animals; maximum accumulation of iodine was noticed in estrous stage followed by diestrous and least in proestrous stage in comparison to normal adult female rats. To understand the possible cause for the alteration in the duration of estrous cycle, serum gonadal hormones (estrogens and progesterone) and gonadotropins (LH and FSH) levels were assayed in excess and excessive iodine exposed groups of animals.
Exposure of iodine over and above the recommended level to experimental female animals serum gonadal hormonal levels were altered that regulate the changes in estrous cyclic pattern. Serum estradiol (E2) level was decreased in excess iodine administered group but remained elevated in excessive iodine exposed group throughout the stages of estrous cycle. In other words, there developed a hypoestrogenic state in excess iodine group but a hyperestrogenic state in excessive iodine exposed group compared to normal animals. These altered changes in gonadal hormonal profiles had induced alteration in the activities in steroidogenic enzymes in the different stages of estrous cycle. Activities of steroidogenic enzymes (viz Δ5 3β HSD, 17β HSD and aromatase) found elevated in excessive iodine exposed group and was responsible for the increased serum estrogen (E2) level developing a hyperestrogenic state; while the decreased activities of the steroidogenic enzymes in excess iodine exposed animals was responsible for the decreased serum estrogen and progesterone levels. The changes that occurred in gonadal hormonal levels has also reflected in the alteration with the activity of pituitary–gonadal axis that brings an alteration in serum LH and FSH levels (Mahapatra et al. 2017). Therefore, on exposure to excess and excessive iodine, the normal ovarian function was disrupted that has also been attributed in the fertility potential. It is evident from the number of neonates analysed after successful mating till delivery of the litter. The presence of sperm in vaginal smear is considered as day ‘0’ of pregnancy and after 21–23 days (gestation period of rats) the neonates were counted. Following this the number of neonates was analysed in normal and iodine exposed groups of experimental animals. In normal rats maximum number of pups indicates 100% fertility index while in both the excess and excessive iodine exposed groups of animals pregnancy does not occur indicating negative fertility index (Mahapatra et al. 2017).
Impairment of Iodine Nutritional Status of Breast Fed infants
Expression of NIS is high in the lactating mammary gland and thus capable to concentrate iodine even when the iodine intake is low and thus even in the phase of iodine deficiency iodine supply to the new born is maintained through breast milk (Tazebay et al. 2000). In coastal areas of West Bengal, India the environment is iodine replete; in addition iodine is supplemented through iodized salt. Median urinary iodine concentration (MIC) of both lactating mothers and their absolutely breast fed infants (0–3 months) were found higher than the recommended level as per WHO/UNICEF/ICCIDD (2007) guidelines. The overall UIC of the breast fed infants found significantly higher than their mothers. The median breast milk iodine concentration was significantly higher than the mothers’ median UIC. Therefore, the iodine nutritional status of the breast fed infants of the studied areas is at the limit of above requirement and excess to a certain portion or in other words they are at the risk of excess iodine (Pal et al. 2018).
The influence of excess iodine on intelligence in human is yet to establish. Epidemiological findings did not show the effects of excess iodine on intelligence (Gao et al. 2004). However, iodine excess can damage children's intelligence level (Liu et al. 2009) and it is also observed that iodine excess at a certain concentration can cause intelligence damage as observe through meta-analysis (Ren and Zhong 2014). The main reason of impairing intelligence is that iodine excess induces hypothyroidism (Burgi 2010), which in turn can cause mental retardation (Krassas et al. 2015); moreover there are in vitro and in vivo studies revealing that excess iodine damages nerve cells. Iodine in excess can cause impairment of learning and memory in 2-month-old male offspring (Zhao et al. 2019) and 4-month-old females (Cui et al. 2018).
Disruption in Functional and Morphological (Histological) Status of Brain
Functional Status
The brain is the largest and most complex organ in the body consists of billions of nerve cells that communicate in trillions of connections through synapses. The specialized areas of brain are cerebral cortex, cerebellum, hippocampus, and hypothalamus. Exposure of iodine over and above the recommended level in the functional status of the major areas of brain was studied following Na+-K+ ATPase, acetylcholine esterase (AchE) and monoamino oxidase (MAO) activities.
Sodium–potassium-ATPase (Na+-K+-ATPase) is involved in the maintenance of polarized condition in nerve cells because of its asymmetrical distribution of Na+ and K+ ions across the plasma membrane. This ion gradient determines the resting potential of cells and drive secondary processes (Blanco and Mercer 1998; Mobasheri et al. 2001). Cognitive deficit has been reported in situations where Na+-K+-ATPase activity was reduced such as Alzheimer disease and under oxidative stress (Hattori et al. 1998: Lehotský et al. 1999). In cerebral cortex and hippocampus, after exposure of iodine in excess (physiological dose) and excessive (pharmacological dose) doses for short and prolonged duration decreased Na+-K+-ATPase actively markedly however no such changes were noticed in hypothalamus and cerebellum in brain of adult male rats (Mandal et al. 2016).
In central nervous system acetyl choline esterase (AChE) alters neuronal activity, synaptic plasticity and co-ordinates firing the group of neurones by regulating the concentration of acetylcholine (Krakowiak et al. 2012). AChE activity was increased in cerebral cortex and hippocampus after excess iodine exposure in adult male albino rats but reverse effect was observed in hypothalamus and cerebellum (Mandal et al. 2016).
Monoamine oxidase (MAO) converts amines to their corresponding aldehydes. The monoamine hypothesis postulates that depression is due to deficiency of monoamine neurotransmitter nor-epinephrine (NE) and 5-hydroxy tryptophan (5-HT) in the important areas of brain ((Fišar et al. 2010). MAO activity was increased after excess iodine treatment in cerebral cortex, hippocampus and cerebellum but no such alteration was found in hypothalamus (unpublished results).
Certain biomarkers of stress in major areas of brain was studied and found that in cerebral cortex, lipid peroxidation level was augmented depending on dose and time of exposure of excess iodine, while catalase and SOD activity initially increased and then decreased. Similar changes were noticed in hippocampus but the severity was less than cerebral cortex. However such changes were not noticed in hypothalamus and cerebellum. Therefore all the concerned antioxidant enzyme activities failed to cope up the situation possibly for breakdown of antioxidant defence mechanism creating a state of cytotoxicity and brain damage in cortex and hippocampus (unpublished results).
Morphological (Histological) Status
To study the influence of iodine exposure over and above the recommended level on structural alteration in major areas of brain, the morphological and histological changes in cerebral cortex, hippocampus, hypothalamus and cerebellum were investigated.
A significant decrease in weight of the cerebral cortex, hippocampus, and cerebellum were noted depending on doses and duration of the exposure of iodine in excess however no such change was noticed in hypothalamus. Histological changes of the different areas of brain have been discussed in the preceding section.
-
(i)
Cerebral cortex
Histological alterations evident by changes in astrocytes, microglia, oligodendroglia and axon fibres in cerebral cortex have been studied using silver nitrate stain taking silver nitrate at different concentrations (Weil and Davenport 1933).
Typical astrogliosis (number and concentration of astrocytes) found after the exposure of excess iodine but the length of their processes decreased gradually. Astrocytes with their long radiating processes maintain the integrity of the cortex and they also convert T4 to T3 for the presence of deiodinase 2 (D2) (Guadaño-Ferraz et al. 1997. Astrogliosis occurs due to toxic and traumatic means through a complex process that includes morphological and functional changes (Colangelo et al. 2012). Exposure of excess and excessive iodine both for short and long durations respectively showed no toxic effect as serum SGOT and SGPT levels remained unaltered indicating the involvement of other mechanism. Presumably the developed hypothyroidism after excess iodine exposure induced astroglosis.
Microglia is the phagocytic cells of nervous system involved in cleaning the cell debris; during phagocytic reaction with foreign bodies microglia are degenerated. Presence of amoeboid microglia is a marker of inflammation (Arcuri et al. 2017). After exposure of excess and excessive iodine for long duration, hypothyroidism progresses that increased microglia concentration, further ramification of microglia took place that in course of time change the shape of amoeboid called amoeboid microglia (unpublished results).
Oligodendroglias are the glial cells of nervous system and are involved in the generation of myelin sheath (Nave and Werner 2014). Excess iodine exposure for long duration caused degeneration of nerve fibres for impaired myelin sheath (unpublished results).
Exposure of excess iodine for prolonged duration has detrimental effect on cerebral cortex manifested by astrogliosis, degeneration of axon fibres and other supporting cells that may lead to degenerative disease (unpublished results).
-
(ii)
Hippocampus
In hippocampus excess iodine exposure for longer duration had increased the number of astrocytes as well as disintegrated microglia (unpublished results) Disintegration of microglia is the sign of neuroinflamation in hippocampus (Bruccoleri and Harry 2000; Harry and Kraft 2008). Excessive iodine treatment had also increased oligodendrocytes concentration rather numbers but these cells undergo degenerative changes; such degenerative changes decrease the efficacy of these cells in the formation of myelin sheath (Bradl and Lassmann. 2010).
-
(iii)
Hypothalamus
This area is quite resistant to morphological rather structural changes induced by excess iodine in respect of glial cells (astrocytes, microglia and oligodendroglia) as well as nerve fibres. Excess iodine induced hypothyroidism results in less thyroid hormones (T4 and T3) in circulation that is responsible for histological changes in the different areas of brain however, the distribution of thyroid hormone receptors in this region found less in hypothalamus (unpublished results).
-
(iv)
Cerebellum
The density of astrocytes and oligodendrocytes in cerebellum was increased after prolonged excess iodine exposure. The concentration of oligodendrocytes was increased with the increase in perinuclear space for dissolution of the membrane with the dose and duration of iodine exposure. Further there occurred degeneration of axon fibres in the medulla of cerebellum containing white matter. The probable reason of such changes in cellular architecture is for increased lipid peroxidation after exposure of excess and excessive iodine for prolonged duration (unpublished results).
Excess iodine induced hypothyroidism therefore, impair glial cell functions causing functional as well as morphological impairment of the major areas of brain developing neurological disorders. Neurodegeneration begins for failure in brain homeostasis and alterations in connectivity of neural networks that signals for early cognitive impairments (Kimelberg and Nedergaard 2010; Heneka et al. 2010). Adult hypothyroid patients show cognitive weakening, memory impairment and depression (Boelaert and Franklyn 2005; Chen et al. 2012; Cortés et al. 2012) and the observations as found after excess iodine exposure are consistent with clinical symptoms.
Disruption in Immune System
Thyroid autoimmunity from iodine prophylaxis is another induction of excess iodine intake. Experiments conducted in animal models reveal that iodine could initiate or intensify thyroid autoimmunity however its effects in humans found not consistent because of inherited, racial and ecological variations ((Papanastasi et al.2007). The mechanisms that have been anticipated for iodine induced thyroid autoimmunity are.
-
(i)
Increased immunogenicity of a highly iodinated Tg (Ruwhof and Drexhage 2001)
-
(ii)
Toxic effect of iodine in thyroid follicular cells (Mahmoud et al. 1986) and
-
(iii)
Direct stimulation by iodine on immune and immune-related cells (Mooij et al. 1994; Sharma et al. 2005).
Iodine incorporation in thyroglobulin molecules increases its antigenicity by the formation of iodinated neo-epitopes in experimental animal models. In genetically pre-disposed animals, excess iodine leads to high incidence whereas low iodine intake leads to low incidence of auto immune thryroiditis (Ruwhof and Drexhage 2001). The other suggested mechanism by which iodine modulates thyroid auto immunity after intake of high dose of iodine to goitrous animals causes necrosis and inflammation of thyroid cells (Mahmoud et al. 1986). This is because relatively large amounts of iodine oxidized by TPO producing large amount of oxygen radicals that causes damage of cell membrane, which could be the first step of lymphocytic infiltration and autoimmunity. The third proposed mechanism is that iodine stimulates immune cells such as macrophages, T and B lymphocytes and dendritic cells. Thyroid hormones, iodinated Tg and the iodinated compounds enhance the transition of monocytes into dendritic cells (Mooij et al. 1994). In addition, iodine excess increases expression of intercellular adhesion molecules- I which promotes cell to cell interaction and enhances the inflammatory process in thyroid cell in genetically susceptible mice for the development of auto immune thyroiditis (Sharma et al. 2005).
It has also been reported from our laboratory that exposure of iodine in moderate and excessive doses in rat caused impairment of SOD, Catalase and GPx activities leading to elevated NO, LPO in lymphocytes with increased IL-6 and TNF-α level, lymphocytic proliferation and DNA damage depending on the doses of iodine that might be the cause of autoimmune thyroid disease in long run (Saha et al. 2019).
Iodine in excess therefore, has an immunomodulatory effect in thyroid developing autoimmunity. In one of our studies reported from Manipur of North East India, a classical goitre endemic belt in Sub Himalayan region mild goiter endemicity found prevalent of children and women in spite of effective salt iodization. A percentage of goitrous population has sub clinical and overt hypo- and hyperthyroidism with elevated thyroid auto antibodies and hypoechoic thyroid and they were at the risk of malignancy. In general there is no evidence of autoimmunity in those affected by endemic goitre (Weetman 2003). This study concludes that elevated thyroid auto antibodies along with exposure of iodine and thiocyanate in current level might be the possible reason for progression the goitrous population towards thyroid auto immune disorders (Singh et al. 2021).
Disruption in Carbohydrate and Lipid Metabolism
Prolonged exposure of excess iodine in carbohydrate and lipid metabolic pattern and the histoarchitecture of the pancreas, liver, kidney, as well as skeletal and cardiac muscles were studied in experimental animals. Under the influence of excess iodine, the body weight and the excretion of iodine in urine were increased while serum thyroid hormone levels decreased developing a state of primary thyroid dysfunction. In consistent with these, there was an increase in blood glucose, cholesterol, triglycerides, low density lipoprotein (LDL), and very low density lipoprotein (VLDL), while the levels of high density lipoprotein (HDL) was decreased. However, in the liver and skeletal muscle glycogen content was decreased and it was increased in the cardiac muscle and renal tissue. Histoarchitecture of the pancreas showed drastic disruption with any recognizable characteristics. The structure of liver revealed the enlarged central vein with degenerating hepatocytes, while the skeletal muscle showed dissolution of muscle fibre cells associated with glycogen loss from these organs. The heart shows the same features to that of a fatty heart with cardiac muscles mutilation, while the kidney shows an increase in glomerular tuft size with expansion of Bowman's space indicating structural disruption. Prolonged exposure of excess iodine causes a biochemical state of hypothyroidism; the hypothyroidism that developed induces the hyperglycaemic and hypercholestromic states, depletes glycogen at normal storage sites in the liver and skeletal muscle at the expense in other sites as cardiac muscle and kidney. Severe destruction of the pancreatic structure is also evident from histological sections. All such changes are prerogative for the pathogenesis of cardiovascular and renal diseases (Sarkar et al. 2018) (Fig. 2).
Conclusion
Iodine prophylaxis has increased iodine ingestion and gradually decreased disorders of iodine deficiency however a only ‘one size fits ‘ criterion not considering local setting like accessibility of iodine in food and water in iodine-replete milieu has augmented the danger of excess iodine intake in inhabitants of several regions. Excess intake of iodine caused iodine induced-thyroid disorders including autoimmune disorders in vulnerable individuals but its effects on thyroid hormone responsive systems have not been explored effectively. Experiments performed in animal models propose that iodine in excess even in physiological doses for long damages structural and functional status of thyroid, both male and female reproductive systems leading to infertility, affect major areas of brain causing neurodegenerative disorders, alter carbohydrate and lipid metabolic pattern, impair immune functions developing autoimmunity through diverse mechanisms depending on relative strength and length of excess iodine exposure.
Like its deficiency, iodine excess should be avoided not only for the protection of thyroid functions but also for other thyroid hormone receptive major systems as described. For the rectification of iodine deficiency iodine prophylaxis should be continued however proper follow-up over time and continuous monitoring on iodine intake have to sustain to avoid its unfavourable consequences.
References
Alsayed, A., A.M. Gad, H. Abdel-Baset, A. Abdel-Fattah, A. Ahmed, and A. Azab. 2008. Excess urinary iodine is associated with autoimmune subclinical hypothyroidism among Egyptian women. Endocrine Journal 55: 0805140141–0805140141.
Andersson, M., B. de Benoist, and L. Rogers. 2010. Epidemiology of iodine deficiency: Salt iodisation and iodine status. Best Practice & Research Clinical Endocrinology & Metabolism 24 (1): 1–11.
Arcuri, C., C. Mecca, R. Bianchi, I. Giambanco, and R. Donato. 2017. The pathophysiological role of microglia in dynamic surveillance, phagocytosis and structural remodelling of the developing CNS. Frontiers in Molecular Neuroscience 10: 191.
Astwood, E.B. 1939. Changes in the weight and water content of the uterus of the normal adult rat. American Journal of Physiology-Legacy Content 126 (1): 162–170.
Belshaw, B.E., and D.V. Becker. 1973. Necrosis of follicular cells and discharge of thyroidal iodine induced by administering iodide to iodine-deficient dogs. The Journal of Clinical Endocrinology & Metabolism 36 (3): 466–474.
Blanco, G., and R.W. Mercer. 1998. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. American Journal of Physiology-Renal Physiology 275 (5): F633–F650.
Blandau, R.J., J.L. Boling, and W.C. Young. 1941. The length of heat in the albino rat as determined by the copulatory response. The Anatomical Record 79 (4): 453–463.
Boelaert, K., and J.A. Franklyn. 2005. Thyroid hormone in health and disease. Journal of Endocrinology 187 (1): 1–15.
Bradl, M., and H. Lassmann. 2010. Oligodendrocytes: Biology and pathology. Acta Neuropathologica 119 (1): 37–53.
Bruccoleri, A., and G.J. Harry. 2000. Chemical-induced hippocampal neurodegeneration and elevations in TNFα, TNFβ, IL-1α, IP-10, and MCP-1 mRNA in osteopetrotic (op/op) mice. Journal of Neuroscience Research 62 (1): 146–155.
Burgi, H. 2010. Iodine excess. Best Practice & Research Clinical Endocrinology & Metabolism 24 (2010): 107–115.
Bürgi, H., T.H. Schaffner, and J.P. Seiler. 2001. The toxicology of iodate: A review of the literature. Thyroid 11 (5): 449–456.
Camargo, R.Y., E.K. Tomimori, S.C. Neves, I.G. Rubio, A.L. Galrao, M. Knobel, and G. Medeiros-Neto. 2008. Thyroid and the environment: Exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in Sao Paulo, Brazil. European Journal of Endocrinology 159 (3): 293.
Chakraborty, A., J. Mandal, C. Mondal, S. Sinha, and A.K. Chandra. 2016. Effect of excess iodine on oxidative stress markers, Steroidogenic—enzyme activities, testicular morphology, and functions in adult male rats. Biological Trace Element Research 172 (2): 380–394.
Chandra, A.K., and A. Chakraborty. 2017. Influence of iodine in excess on seminiferous tubular structure and epididymal sperm character in male rats. Environmental Toxicology 32 (6): 1823–1835.
Chandra, A.K., and L.H. Singh. 2012. Studies on the possible occurrence of autoimmune thyroid disorders in Goitrous children of Manipur in North-East India. Ind J Physiol Allied Sci 66: 51–57.
Chandra, A.K., M. Dutta, S. Kundu, A. Chakraborty, C. Mondal, and D. Sarkar. 2018. Histomorphological and biochemical analysis of large Goitres in iodine replete Gangetic plains of West Bengal, India: Is The Spectrum Shifting Towards Graves’ Disease? Indian Journal of Physiology and Pharmacology 62 (3): 286–297.
Chen, C., Z. Zhou, M. Zhong, Y. Zhang, M. Li, L. Zhang, M. Qu, J. Yang, Y. Wang, and Z. Yu. 2012. Thyroid hormone promotes neuronal differentiation of embryonic neural stem cells by inhibiting STAT3 signaling through TRα1. Stem Cells and Development 21 (14): 2667–2681.
Cohen JJ.1993. Apoptosis. Immunol Today 14:126–130
Coindet, J.F. 1821. Iodine: On its application as a medicine. Quarterly Journal of Science 11: 408.
Colangelo, A.M., G. Cirillo, M.L. Lavitrano, L. Alberghina, and M. Papa. 2012. Targeting reactive astrogliosis by novel biotechnological strategies. Biotechnology Advances 30 (1): 261–271.
Cortés, C., E. Eugenin, E. Aliaga, L.J. Carreño, S.M. Bueno, P.A. Gonzalez, S. Gayol, D. Naranjo, V. Noches, M.P. Marassi, and D. Rosenthal. 2012. Hypothyroidism in the adult rat causes incremental changes in brain-derived neurotrophic factor, neuronal and astrocyte apoptosis, gliosis, and deterioration of postsynaptic density. Thyroid 22 (9): 951–963.
Cui, Y., Z. Zhang, B. Zhang, L. Zhao, C. Hou, Q. Zeng, J. Nie, J. Yu, Y. Zhao, T. Gao, and A. Wang. 2018. Excessive apoptosis and disordered autophagy flux contribute to the neurotoxicity induced by high iodine in Sprague-Dawley rat. Toxicology Letters 297: 24–33.
Dai, G., O. Levy, and N. Carrasco. 1996. Cloning and characterization of the thyroid iodide transporter. Nature 379 (6564): 458–460.
Dunn, J.T., H.E. Crutchfield, R. Gutekunst, and A.D. Dunn. 1993. Two simple methods for measuring iodine in urine. Thyroid 3 (2): 119–123.
Ellis, R.E., J. Yuan, and H.R. Horvitz. 1991. Mechanisms and functions of cell death. Annual Review of Cell Biology 7 (1): 663–698.
Eng, P.H., G.R. Cardona, S.L. Fang, M. Previti, S. Alex, N. Carrasco, W.W. Chin, and L.E. Braverman. 1999. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology 140 (8): 3404–3410.
Erdoǧan, M.F. 2003. Thiocyanate overload and thyroid disease. BioFactors 19 (3–4): 107–111.
Everett, J.W. 1980. Reinstatement of estrous cycles in middle-aged spontaneously persistent estrous rats: Importance of circulating prolactin and the resulting facilitative action of progesterone. Endocrinology 106 (6): 1691–1696.
Felicio, L.S., J.F. Nelson, and C.E. Finch. 1984. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biology of Reproduction 31 (3): 446–453.
Fišar, Z., J. Hroudová, and J. Raboch. 2010. Inhibition of monoamine oxidase activity by antidepressants and mood stabilizers. Neuroendocrinology Letters 31 (5): 645.
Gao, T.S., W.P. Teng, Z.Y. Shan, Y. Jin, H.X. Guan, X.C. Teng, F. Yang, W.B. Wang, X.G. Shi, Y.J. Tong, and D. Li. 2004. Effect of different iodine intake on schoolchildren’s thyroid diseases and intelligence in rural areas. Chinese Medical Journal 117 (10): 1518–1522.
Golstein, J., and J.E. Dumont. 1996. Cytotoxic effects of iodide on thyroid cells: Difference between rat thyroid FRTL-5 cell and primary dog thyrocyte responsiveness. Journal of Endocrinological Investigation 19 (2): 119–126.
Gopalakrishnan, S., S.P. Singh, W.R. Prasad, S.K. Jain, V.K. Ambardar, and R. Sankar. 2006. Prevalence of goitre and autoimmune thyroiditis in schoolchildren in Delhi, India, after two decades of salt iodisation. Journal of Pediatric Endocrinology and Metabolism 19 (7): 889–894.
Guadaño-Ferraz, A., M.J. Obregón, D.L.S. Germain, and J. Bernal. 1997. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proceedings of the National Academy of Sciences 94 (19): 10391–10396.
Han, H., P. Xin, L. Zhao, J. Xu, Y. Xia, X. Yang, X. Sun, and L. Hao. 2012. Excess iodine and high-fat diet combination modulates lipid profile, thyroid hormone, and hepatic LDLr expression values in mice. Biological Trace Element Research 147 (1): 233–239.
Harry, G.J., and A.D. Kraft. 2008. Neuroinflammation and microglia: Considerations and approaches for neurotoxicity assessment. Expert Opinion on Drug Metabolism & Toxicology 4 (10): 1265–1277.
Hattori, N., K. Kitagawa, T. Higashida, K. Yagyu, S. Shimohama, T. Wataya, G. Perry, M.A. Smith, and C. Inagaki. 1998. Cl−-ATPase and Na+/K+-ATPase activities in Alzheimer’s disease brains. Neuroscience Letters 254 (3): 141–144.
Heneka, M.T., M.K. O’Banion, D. Terwel, and M.P. Kummer. 2010. Neuroinflammatory processes in Alzheimer’s disease. Journal of Neural Transmission 117 (8): 919–947.
Hetzel, B.S., and J.T. Dunn. 1989. The iodine deficiency disorders: Their nature and prevention. Annual Review of Nutrition 9 (1): 21–38.
Hussein, A.E.A.M., A.M. Abbas, G.A. El Wakil, A.Z. Elsamanoudy, and A.A. El Aziz. 2012. Effect of chronic excess iodine intake on thyroid function and oxidative stress in hypothyroid rats. Canadian Journal of Physiology and Pharmacology 90 (5): 617–625.
Kaloumenou, I., G. Mastorakos, M. Alevizaki, L.H. Duntas, E. Mantzou, C. Ladopoulos, A. Antoniou, D. Chiotis, I. Papassotiriou, G.P. Chrousos, and C. Dacou-Voutetakis. 2008. Thyroid autoimmunity in schoolchildren in an area with long-standing iodine sufficiency: Correlation with gender, pubertal stage, and maternal thyroid autoimmunity. Thyroid 18 (7): 747–754.
Kapil, U., and P. Singh. 2004. Current status of urinary iodine excretion levels in 116 districts of India. Journal of Tropical Pediatrics 50 (4): 245–247.
Kapil, U., P. Singh, and P. Pathak. 2004. Current status of iodine nutriture and iodine content of salt in Andhra Pradesh. Indian Pediatrics 41 (2): 165–169.
Kimelberg, H.K., and M. Nedergaard. 2010. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7 (4): 338–353.
Krakowiak, P., C.K. Walker, A.A. Bremer, A.S. Baker, S. Ozonoff, R.L. Hansen, and I. Hertz-Picciotto. 2012. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129 (5): e1121–e1128.
Krassas, G., S.N. Karras, and N. Pontikides. 2015. Thyroid diseases during pregnancy: A number of important issues. Hormones 14 (1): 59–69.
Kurtoğlu, S., Ş Köroğlu, O. Baştuğ, G. Daar, A. Yıkılmaz, and F. Elmalı. 2014. The comparison of thyroxine versus thyroxine plus oral iodine in the treatment of congenital hypothyroidism due to iodine deficiency. Hormone Research in Paediatrics 81 (6): 409–415.
Laurberg, P., I.B. Pedersen, N. Knudsen, L. Ovesen, and S. Andersen. 2001. Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid 11 (5): 457–469.
Lehotský, J., P. Kaplan, P. Račay, M. Matejovičová, A. Drgova, and V. Mézešová. 1999. Membrane ion transport systems during oxidative stress in rodent brain: Protective effect of stobadine and other antioxidants. Life Sciences 65 (18–19): 1951–1958.
Lerner, G. 1969. The lady and the mill girl: Changes in the status of women in the age of Jackson. American Studies 10 (1): 5–15.
Leung, A.M., and L.E. Braverman. 2014. Consequences of excess iodine. Nature Reviews Endocrinology 10 (3): 136–142.
Liu, H.L., L.T. Lam, Q. Zeng, S.Q. Han, G. Fu, and C.C. Hou. 2009. Effects of drinking water with high iodine concentration on the intelligence of children in Tianjin, China. Journal of Public Health (oxford, England) 31 (1): 32–38. https://doi.org/10.1093/pubmed/fdn097.
Lupachik, S.V., L.I. Nado, Z.V. Netsetskaya, and V.V. Vinogradov. 2007. Effect of long-term injection of high doses of potassium iodide on iodine metabolism in rat thyroid gland. Biochemistry (moscow) Supplement Series b: Biomedical Chemistry 1 (1): 53–57.
Mahapatra, D., and A.K. Chandra. 2017. Biphasic action of iodine in excess at different doses on ovary in adult rats. Journal of Trace Elements in Medicine and Biology 39: 210–220.
Mahapatra, D., S. Chattopadhyay, and A.K. Chandra. 2017. Iodine in excess: Impact on ovarian and uterine histology in adult rats. Indian Journal of Physiology and Pharmacology 61 (2): 166–174.
Mahmoud, I., I. Colin, M.C. Many, and J.F. Denef. 1986. Direct toxic effect of Iodide in excess on Lodine-deficient thyroid glands: Epithelial necrosis and inflammation associated with lipofuscin accumulation. Experimental and Molecular Pathology 44 (3): 259–271.
Mandal, J., A. Chakraborty, and A.K. Chandra. 2016. Altered Acetyl cholinesterase and Na+-K+ ATPase activities in different areas of brain in relation to thyroid gland function and morphology under the influence of excess iodine. Int J Pharm Clin Res 8: 1564–1573.
Marwaha, R.K., N. Tandon, N. Gupta, A.K. Karak, K. Verma, and N. Kochupillai. 2003. Residual goitre in the postiodization phase: Iodine status, thiocyanate exposure and autoimmunity. Clinical Endocrinology 59 (6): 672–681.
Medani, A.M.M., A.A. Elnour, and A.M. Saeed. 2011. Endemic goitre in the Sudan despite long-standing programmes for the control of iodine deficiency disorders. Bulletin of the World Health Organization 89: 121–126.
Mobasheri, A., D. Oukri, S.P. Dawodu, M. Sinha, P. Greenwell, D. Stewart, M.B.A. Djamgoz, C.S. Foster, P.M. Vasallo, and R. Mobasheri. 2001. Isoforms of Na+, K+-ATPase in human prostate; specificity of expression and apical membrane polarization. Histology and Histopathology 16 (1): 141–154.
Mooij, P., H.J. De Wit, and H.A. Drexhage. 1994. A high iodine intake in Wistar rats results in the development of a thyroid-associated ectopic thymic tissue and is accompanied by a low thyroid autoimmune reactivity. Immunology 81 (2): 309.
Nave, K.A., and H.B. Werner. 2014. Myelination of the nervous system: Mechanisms and functions. Annual Review of Cell and Developmental Biology 30: 503–533.
Neguin, L.G., J. Alvarez, and N.B. Schwartz. 1975. Steriod control of gonadotrophin release. Journal of Steroid Biochemistry 6: 1007.
Pal, N., S.K. Samanta, A. Chakraborty, N.K. Chandra, and A.K. Chandra. 2018. Interrelationship between iodine nutritional status of lactating mothers and their absolutely breast-fed infants in coastal districts of Gangetic West Bengal in India. European Journal of Pediatrics 177 (1): 39–45.
Palaniappan, S., L. Shanmughavelu, H.K. Prasad, S. Subramaniam, N. Krishnamoorthy, and L. Lakkappa. 2017. Improving iodine nutritional status and increasing prevalence of autoimmune thyroiditis in children. Indian Journal of Endocrinology and Metabolism 21 (1): 85.
Papanastasiou, L., I.A. Vatalas, D.A. Koutras, and G. Mastorakos. 2007. Thyroid autoimmunity in the current iodine environment. Thyroid 17 (8): 729–739.
Paulíková, I., G. Kovac, J. Bires, S. Paulik, H. Seidel, and O. Nagy. 2002. Iodine toxicity in ruminants. Veterinarni Medicina-Praha 47 (12): 343–350.
Pearce, E.N., M. Andersson, and M.B. Zimmermann. 2013. Global iodine nutrition: Where do we stand in 2013? Thyroid 23 (5): 523–528.
Pisarev, M.A., and L.O. Aiello. 1976. Studies on the mechanism of action of potassium iodide on thyroid protein biosynthesis. European Journal of Endocrinology 82 (2): 298–305.
Pisarev, M.A., and M.E. Itoiz. 1972. Action of KI on stimulated thyroid protein biosynthesis. Endocrinology 90 (5): 1409–1412.
Pramyothin, P., A.M. Leung, E.N. Pearce, A.O. Malabanan, and L.E. Braverman. 2011. A hidden solution. New England Journal of Medicine 365 (22): 2123–2127.
Ren, S., and Z. Zhong. 2014. Meta-analysis on the relationship between Chinese children’s intelligence and excessive iodine. Wei Sheng Yan Jiu= Journal of Hygiene Research, 133–138.
Ruwhof, C., and H.A. Drexhage. 2001. Iodine and thyroid autoimmune disease in animal models. Thyroid 11 (5): 427–436.
Saberi, M., and R.D. Utiger. 1975. Augmentation of thyrotropin responses to thyrotropin-releasing hormone following small decreases in serum thyroid hormone concentration. Journal of Clinical Endocrinology and Metabolism 40: 435–441.
Safran, M., and L.E. Braverman. 1982. Effect of chronic douching with polyvinylpyrrolidone-iodine on iodine absorption and thyroid function. Obstetrics and Gynecology 60 (1): 35–40.
Saha, A., S. Mukherjee, A. Bhattacharjee, D. Sarkar, A. Chakraborty, A. Banerjee, and A.K. Chandra. 2019. Excess iodine-induced lymphocytic impairment in adult rats. Toxicology Mechanisms and Methods 29 (2): 110–118.
Sarkar, D., A. Chakraborty, A. Saha, and A.K. Chandra. 2018. Iodine in excess in the alterations of carbohydrate and lipid metabolic pattern as well as histomorphometric changes in associated organs. Journal of Basic and Clinical Physiology and Pharmacology 29 (6): 631–643.
Serrano-Nascimento, C., S. da Silva Teixeira, J.P. Nicola, R.T. Nachbar, A.M. Masini-Repiso, and M.T. Nunes. 2014. The acute inhibitory effect of iodide excess on sodium/iodide symporter expression and activity involves the PI3K/Akt signalling pathway. Endocrinology 155 (3): 1145–1156.
Serrano-Nascimento, C., J.P. Nicola, S. da Silva Teixeira, L.L. Poyares, C. Lellis-Santos, S. Bordin, A.M. Masini-Repiso, and M.T. Nunes. 2016. Excess iodide downregulates Na+/I− symporter gene transcription through activation of PI3K/Akt pathway. Molecular and Cellular Endocrinology 426: 73–90.
Sharma, R.B., J.D. Alegria, M.V. Talor, N.R. Rose, P. Caturegli, and C.L. Burek. 2005. Iodine and IFN-γ synergistically enhance intercellular adhesion molecule 1 expression on NOD H2h4 mouse thyrocytes. The Journal of Immunology 174 (12): 7740–7745.
Singh, L.H., A.K. Chandra, S.D. Yumnam, D. Sarkar, R.K. Manglem, T. Dhabali, S. Mookerjee, and I. Ray. 2021. Thiocyanate in excess develops goiter followed by auto immune thyroid diseases even after effective salt iodization in a rural community of north east India. Ecotoxicology and Environmental Safety 208: 111711.
Takasu, N., Y. Handa, A. Kawaoi, Y. Shimizu, and T. Yamada. 1985. Effects of iodide on thyroid follicle structure and electrophysiological potentials of cultured thyroid cells. Endocrinology 117 (1): 71–76.
Taurog, A. 1976. The mechanism of action of the thioureylene antithyroid drugs. Endocrinology 98 (4): 1031–1046.
Tazebay, U.H., I.L. Wapnir, O. Levy, O. Dohan, L.S. Zuckier, Q.H. Zhao, H.F. Deng, P.S. Amenta, S. Fineberg, R.G. Pestell, and N. Carrasco. 2000. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nature Medicine 6 (8): 871–878.
Teng, X., Z. Shan, Y. Chen, Y. Lai, J. Yu, L. Shan, X. Bai, Y. Li, N. Li, Z. Li, and S. Wang. 2011. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: A cross-sectional study based on two Chinese communities with different iodine intake levels. European Journal of Endocrinology 164 (6): 943.
UNICEF and IGN. 2018. Updating guidance on the monitoring of salt iodization programs and population iodine status. IDD Newsletter 46 (2): 5–7.
Vagenakis, A.G., C.A. Wang, A. Burger, F. Maloof, L.E. Braverman, and S.H. Ingbar. 1972. Iodide-induced thyrotoxicosis in Boston. New England Journal of Medicine 287 (11): 523–527.
Vitale, M., T. Di Matola, F. D’Ascoli, S. Salzano, F. Bogazzi, G. Fenzi, E. Martino, and G. Rossi. 2000. Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinology 141 (2): 598–605.
Weetman, A.P. 2003. Autoimmune thyroid disease: Propagation and progression. European Journal of Endocrinology 148 (1): 1–9.
Weil, A., and H.A. Davenport. 1933. Staining of oligodendroglia and microglia in celloidin sections. Archives of Neurology & Psychiatry 30 (1): 175–178.
WHO/UNICEF/ICCIDD. 2007. Assessment of iodine deficiency disorders and monitoring their elimination: A guide for programme managers. WHO Publications, pp. 1–97.
Wolff, J., and I.L. Chaikoff. 1948. Plasma inorganic iodide as a homeostatic regulator of thyroid function. Journal of Biological Chemistry 174 (2): 555–564.
Zhao, L., B. Zhang, Y. Cui, C. Hou, Q. Zeng, T. Gao, Z. Zhang, J. Yu, Y. Wang, A. Wang, and H. Liu. 2019. 3-Methyladenine alleviates excessive iodine-induced cognitive impairment via suppression of autophagy in rat hippocampus. Environmental Toxicology 34 (8): 912–920.
Zimmermann, M.B. 2008. Iodine requirements and the risks and benefits of correcting iodine deficiency in populations. Journal of Trace Elements in Medicine and Biology 22 (2): 81–92.
Zimmermann, M.B., D. Moretti, N. Chaouki, and T. Torresani. 2003. Introduction of iodized salt to severely iodine-deficient children does not provoke thyroid autoimmunity: A one-year prospective trial in northern Morocco. Thyroid 13 (2): 199–203.
Zois, C., I. Stavrou, C. Kalogera, E. Svarna, I. Dimoliatis, K. Seferiadis, and A. Tsatsoulis. 2003. High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern Greece. Thyroid 13 (5): 485–489.
Acknowledgements
Dr. Arijit Chakraborty (UGC Research Fellow), Dr. Dakshayani Mahapatra (CSIR-NET, Research Fellow), Dr. Jagadish Mandal (Honorary Research Fellow), Miss. Adipa Saha (UGC Rajib Gandhi National Fellow) and Dr. Deotima Sarkar (DST-INSPIRED Fellow) are acknowledge for their work on male reproduction, female reproduction, nervous system, immune system and carbohydrate including lipid metabolism respectively under the direct supervision of the author in the Endocrinology & Reproductive Physiology Laboratory, Department of Physiology, University of Calcutta, Kolkata.
Funding
UGC Major Research Project Grant/ Rajib Gandhi National Project Grant for providing fund to work on excess iodine. The author has supervised the entire work as UGC Emeritus Fellow during 2017–2020 in the Department of Physiology, University of Calcutta, Kolkata.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chandra, A.K. Iodine in Disruption of Thyroid and Thyroid Hormone Receptive Systems. Proc Zool Soc 74, 494–506 (2021). https://doi.org/10.1007/s12595-021-00413-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-021-00413-2