Abstract
Aluminum (Al) is an accumulative toxic metal. Excessive Al accumulation inhibits osteoblasts mineralization and induces osteoporosis. However, the inhibition mechanism of Al on the mineralization is not fully understood. Thus, in this study, the rat osteoblasts were cultured and exposed to 0 mmol L−1 (control group, CG) and 0.52 mmol L−1 aluminum trichloride (AlCl3, treatment group, TG) for 7, 14, and 21 days, respectively. We found that mineralized matrix nodules, the activity of bone alkaline phosphatase, the concentration of extracellular calcium, the mRNA expression of type-I collagen, the mRNA and protein expressions of osteopontin, osteocalcin, and bone sialoprotein were all decreased, while the concentration of extracellular phosphorus was increased in TG compared with CG with time prolonged. Taken together, these results indicated that AlCl3 inhibited osteoblasts mineralization in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis threatens human with high incidence of disease [1, 2]. In Europe alone, 22 million women and 5.5 million men have osteoporosis [3]. Meanwhile, the residual of available aluminum (Al) in biologically ecosystem has been substantially increasing during recent years, so Al contamination becomes an increasing problem in human society [4]. Al compounds and derivatives are used in the preparation of various commercial products, including therapeutic agents, water purifiers, and food additives [5]. They are released into the environment both by natural processes and from anthropogenic sources, thus increasing the risk of consumer and occupational exposures [6]. Human exposure to Al may occur from the environment and through the drinking water and the consumption of certain medications. Al accumulation induces bone toxicity in human and is a risk factor for osteoporosis [7, 8]. Osteoporosis attributes to the abnormal bone mineralization. In particular, Al can inhibit bone formation through inhibiting bone mineralization [9, 10]. Therefore, the abnormal bone mineralization induced by Al may be the potential risk of osteoporosis. But the inhibition mechanism of aluminum trichloride (AlCl3) on the mineralization is not completely clarified.
Osteoblasts mineralization is an important osteoplastic link and specific biological process of the bone in which calcium (Ca) and phosphorus (Pi) deposit on type-I collagen (Col I) to form hydroxyapatite. Bone alkaline phosphatase (B-ALP), osteocalcin (OCN), osteopontin (OPN), and bone sialoprotein (BSP) are the important regulators of bone matrix mineralization and adjust the hydroxyapatite formation [11]. During the mineralization, Col I is the primary composition of extracellular matrix, which provides a mineralization scaffold for bone matrix. B-ALP secreted by osteoblasts can hydrolyze phosphate ester to provide the Pi for promoting the hydroxyapatite formation [12]. OCN is the most abundant non-collagen protein of the extracellular mineralized matrix. It can reside on the surfaces of hydroxyapatite crystals and maintain normal bone mineralization [13]. OPN regulates the hydroxyapatite crystals size and shape during its growth [14]. BSP, as a specific protein in mineralized tissue, can promote the formation of hydroxyapatite crystals and start-up the mineralization process [15]. However, it is unknown whether these regulatory factors were involved in the toxic effects of Al on osteoblasts mineralization.
Therefore, the components of bone matrix (Ca, Pi, and Col I) and main mineralization regulatory factors (B-ALP, OCN, OPN, and BSP) were measured to explore the effect of AlCl3 on osteoblasts mineralization, which will provide a theoretical foundation for revealing the mechanism of osteoporosis induced by AlCl3.
Materials and Methods
Rat Osteoblasts Isolation and Treatment
The design and procedure of experiment were approved by the Animal Ethics Committee of the Northeast Agricultural University (Harbin, China). The isolation and depuration of rat osteoblasts were done according to Matsuda et al. [16]. The healthy 3-day-old newborn SD rats were anesthetized by ether and then disinfected in 75 % ethanol for about 10 min. Calvarial osteoblasts were aseptically isolated from the skull of the rats. The endosteum and periosteum of calvarial were stripped off and washed with cold phosphate-buffered saline (PBS, pH 7.2). The rat cranium was cut into 1–2 mm2 pieces and digested with trypsin (2.5 g/L) for 15 min at room temperature and then digested with collagenase II (1.0 g/L) for 1 h in 37 °C water bath. The digested cranium was centrifuged at 1200×g for 10 min at room temperature. The precipitated rat calvarial osteoblasts were suspended in Dulbecco’s modified Eagle medium (DMEM; Gibco, Carlsbad, CA) containing 10 % fetal calf serum (FBS; Sigma, USA) and incubated overnight at 37 °C under an atmosphere containing 5 % CO2.
The rat osteoblasts (9 × 104 cells/mL) were cultured with DMEM until 90 % confluence in 24-well plates and then changed to mineralization medium containing 10 mmol L−1 β-glycerophosphate (Sigma, USA), 50 μg mL−1 ascorbic acid (Sigma, USA), and 0.1 μmol L−1 dexamethasone (Sigma, USA) and exposed to 0 mmol L−1 (control group, CG) and 0.52 mmol L−1 AlCl3 (1/10 IC50, treatment group, TG) for 7, 14, and 21 days, respectively.
Determination of Mineralized Matrix Nodules
The determination of mineralized matrix nodules was done according to Matsuda et al. [16]. The rat osteoblasts (9 × 104 cells/mL) were stained using alizarin red staining on days 7, 14, and 21 for assessing the mineralized matrix nodules. The supernatant was removed, and rat osteoblasts were fixed in 95 % ethanol for 10 min and then washed twice by PBS and stained with 0.1 % alizarin red S (ARS; Sigma, USA) for 30 min at room temperature. Finally, stained rat osteoblasts were observed under the inverted phase contrast microscope (Nikon, Japan). SigmaScan (Systat Software International, San Jose, CA) was used to count the number of red pixels per field [17].
Determination of the B-ALP Activity
The supernatant of rat osteoblasts (9 × 104 cells/mL) were collected on days 7, 14, and 21, and the activity of B-ALP was measured by ELISA kit (R&D Systems, Inc. Shanghai, China) according to the manufacturer’s instructions. The optical absorbance was measured at 450 nm on a microplate reader (Bio-Tek Epoch, USA).
Determination of Extracellular Ca and Pi Concentration
On days 7, 14, and 21, the concentrations of Ca and Pi in the supernatant of rat osteoblasts (9 × 104 cells/mL) were measured with the method of colorimetric and phosphomolybdate kit (Nanjingjiancheng Co., Ltd., Nanjing, China) according to the manufacturer’s instructions, respectively. The optical absorbance was measured at 610 nm (Ca) and 660 nm (Pi) on a microplate reader (Bio-TekEpoch Co., Ltd., VT, USA).
The mRNA Expressions of Col I, OCN, OPN, and BSP
The mRNA expressions of Col I, OCN, OPN, and BSP of rat osteoblasts (2 × 106 cells/mL) were determined according to Sun et al. [18]. On days 7, 14, and 21, the total RNA of rat osteoblasts were isolated using Trizol reagent according to the manufacturer’s instructions (Invitrogen Co., Ltd., Beijing, China). Total RNA was analyzed by spectrophotometry at 260 and 280 nm using a Gene Quant II RNA/DNA Calculator (Pharmacia, BiotechCo., Ltd., Cambridge, UK). Only samples with an optical density ratio at 260/280 nm >1.8 were used for cDNA synthesis with reverse transcription kit (TaKaRa Co., Ltd., Tokyo, Japan). Synthesized cDNA was diluted five times with sterile water and stored at −80 °C before use. Primer Premier Software (PREMIER Biosoft International Co., Ltd., CA, USA) was used to design specific primers for Col I, OCN, OPN, BSP, and β-actin based on rats sequences (Table 1). The gene expression was evaluated by real-time fluorescence relative quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis using the SYBR Green QuantiTect RT-PCR Kit (Qiagen Inc., Valencia, CA, USA). RT-FR-qPCR was performed on a 7000 Fast Real-Time PCR System (Applied Biosystems, ABI, USA). The relative mRNA expression was normalized to β-actin levels.
The Protein Expressions of OCN, OPN, and BSP
The protein expressions of OCN, OPN, and BSP were determined by western blotting method according to Sun et al. [18]. On days 7, 14, and 21, the total protein of rat osteoblasts (2 × 106 cells/mL) was extracted on the ice cake. The protein concentration was determined by BCA protein assay kit (Beyotime Institute of Biotechnology, USA). The extracted protein was separated by SDS-PAGE method, and the protein was transferred onto PVDF membranes. The PVDF membranes were then blocked with 5 % skimmed milk for 1 h, followed by incubation with primary antibodies (the OCN, sc-376835, the ratio of 1:500; OPN, sc-21742, the ratio of 1:400; BSP, sc-292394, the ratio of 1:200) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C, respectively. The membranes were subsequently incubated for 1 h at room temperature with secondary antibodies (Peroxidase-Conjugated AffiniPure Goat Anti-Mouse IgG (H + L) (ZB-2305), the ratio of 1:5000; Peroxidase-Conjugated AffiniPure Goat Anti-Mouse IgG (H + L) (ZB-2305), 1:2000; Peroxidase-Conjugated AffiniPure Goat Anti-Rabbit IgG (H + L) (ZB-2301), 1:2000) (ZSGB-BIO, Beijing, China), respectively. Mouse anti-beta actin monoclonal antibody (TA-09) was diluted at 1:200 and Peroxidase-Conjugated AffiniPure Goat Anti-Mouse IgG (H + L) (ZB-2305) was diluted at 1:4000 (ZSGB-BIO, Beijing, China). The protein was detected using the enhanced chemiluminescent (ECL) reagent (Beyotime Institute of Biotechnology). Quantitative analysis was carried out using ImageJ analysis system.
Statistical Analysis
Statistical analysis were done using SPSS 22.0 package programmer (SPSS Inc., Chicago, IL, USA). Shapiro-Wilk test was used to check the normal distribution of data. Levene’s test was used to assess the variance homogeneity. One-way ANOVA with LSD and Bonferroni’s method was used to conduct multiple comparisons. Data were shown as least square means and standard error (SE, bar on the top of each column). **P < 0.01 was considered markedly significant difference and *P < 0.05 was considered significant difference versus CG. ## P < 0.01 was considered markedly significant difference and # P < 0.05 was considered significant difference versus TG on day 7.
Results
Effects of AlCl3 on the Formation of Mineralized Matrix Nodules
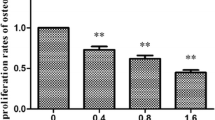
Osteoblasts were demonstrated by the red mineralized nodules (Fig. 1a). The number of mineralized matrix nodules were decreased gradually in TG with the time prolonged and were significantly lower than those in CG on days 7, 14, and 21 (P < 0.01) (Fig. 1b).
Effects of AlCl3 on mineralized matrix nodules (×100). a Rat osteoblasts were stained by the alizarin red staining. The arrows showed mineralized matrix nodules. b The number of red pixels per field was count by SigmaScan. Data are represented as the means ± SE. CG control group, TG treatment group. **P < 0.01 versus CG, ## P < 0.01 versus TG on day 7
The B-ALP Activity
The B-ALP activity was decreased gradually in TG with the time prolonged and was significantly lower than those in CG on days 7, 14, and 21 (P < 0.01) (Fig. 2).
The Concentration of Extracellular Ca and Pi
The concentration of extracellular Ca in all groups were increased gradually with the time prolonged, while the concentration of extracellular Ca in TG was significantly lower than those in CG on days 7, 14, and 21 (P < 0.01) (Fig. 3a). The concentration of extracellular Pi in all groups was decreased gradually with the time prolonged, while the concentration of extracellular Pi in TG was significantly higher than those in CG on days 7, 14, and 21 (P < 0.01) (Fig. 3b).
The mRNA Expressions of Col I, OCN, OPN, and BSP
The mRNA expressions of Col I was decreased gradually in TG with the time prolonged and were significantly lower than those in CG on days 7, 14, and 21(P < 0.05, P < 0.01) (Fig. 4a). The mRNA expression of OCN was decreased gradually in TG with the time prolonged and was significantly lower than those in CG on days 7, 14 (P < 0.01), and 21 (P > 0.05) (Fig. 4b). The mRNA expressions of OPN (Fig. 4c) and BSP (Fig. 4d) were decreased gradually in TG with the time prolonged and were significantly lower than those in CG on days 7, 14, and 21 (P < 0.01).
The Protein Expressions of OCN, OPN, and BSP
Figure 5a represents the protein bands and Fig. 5b–d represents the gray values of the OCN, OPN, and BSP, respectively. The protein expressions of OCN, OPN, and BSP were decreased gradually in TG with the time prolonged and were significantly lower than those in CG on days 7, 14, and 21 (P < 0.01).
Discussion
Osteoblasts mineralization cycle is still controversial from 7 to 21 days after adding mineralization medium [19, 20]. To investigate the effects of AlCl3 exposure on the mineralization process, we cultured rat osteoblasts with mineralization medium for 7, 14, and 21 days, respectively. Our results found that AlCl3 exposure suppressed the number of mineralized matrix nodule, disturbed metabolic balance of Ca and Pi, decreased the Col I deposition, and suppressed expressions of mineralization regulatory factors (B-ALP, OCN, OPN, and BSP), indicating that AlCl3 inhibits osteoblasts mineralization.
The aberrant bone mineralization inhibits bone formation and induces osteoporosis. Degeratu et al. confirmed that Al3+ inhibited growth of hydroxyapatite calcospherite and mineralization [21]. In our study, a clear decrease in mineralized nodule number was observed in osteoblasts with AlCl3 administration time prolonged. This result may be attributed to the imbalance of Ca and Pi caused by AlCl3. Ca and Pi are essential elements for bone mineralization, and Ca can combine with Pi to form hydroxyapatite [22]. Furthermore, imbalance of Ca and Pi could inhibit osteoblasts mineralization [23]. Sánchez et al. found that Al accumulation in bone disturbed the metabolism of Ca and Pi [24]. In this experiment, the Ca concentration was decreased and Pi concentration was increased with AlCl3 administration time prolonged, which might attribute to the direct effect of AlCl3 exposure or the antagonism effect of Ca and Pi. Moreover, Lossdörfer et al. found that the release of Ca and Pi to the cell medium was altered in a reciprocal manner of human osteoblasts. At low Pi concentration, Ca was released to a greater extent and at high Pi concentration, Ca was released to lesser extent [25]. Taken together, these results indicated that AlCl3 impaired bone mineralization by an imbalance of Ca and Pi.
B-ALP and Col I are secreted by osteoblasts and are reliable and sensitive markers for osteoblasts mineralization and bone formation. B-ALP can modulate ossification in mineralization [26, 27]. As a stent of bone mineralization, Col I plays a critical role in mediating mineralization of the extracellular matrix (ECM) [28] and presents high expression in the interim of mineralization [29].
In neonatal mouse osteoblasts, AlCl3 decreased ALP activity and Col I synthesis [30]. Moreover, Al induced a dose-dependent inhibition of nodule formation and calcification and may be related to its inhibition of collagen production in mouse osteoblasts [31]. Our previous research found that AlCl3 reduced B-ALP activity in rat serum and osteoblasts [32]. In this experiment, the B-ALP activity and Col I mRNA expression were significantly decreased with AlCl3 administration time prolonged, indicating that AlCl3 inhibited formation of hydroxyapatite crystals and synthesis, secretion, and mineralization of bone matrix.
OCN, OPN and BSP, synthesized and secreted by osteoblasts, are the main non-collagen proteins for regulating mineralization [33]. OCN is a hydroxyapatite-binding protein and is considered to be a specific functional marker of osteoblast mineralization [34]. Gomez-Alonso et al. found that Al exposure reduced the serum OCN levels in rats and led to the inhibition of bone formation [35]. Fanti et al. found AlCl3 decreased the OCN content of culture medium with a dose-dependent effect in osteoblasts [36]. In our study, the protein expression of OCN was significantly deceased with AlCl3 administration time prolonged; the mRNA expression of OCN was significantly decreased on days 7 and 14 in TG compared with CG, low expression levels on day 21 both in CG and TG, and there was no significant difference. This might be a reason for the high expression levels of OCN at the early mineralization and lower expression levels at the late stage of mineralization which marks the mineralization termination [13]. OPN is a highly acidic glycosylated phosphoprotein and a non-collagenous ECM protein in mineralized tissues [37, 38]. Moreover, OPN is involved in bone cell attachment to ECM and acts as a chemoattractant for bone cells during the early and late stage of bone development [39]. Attachment of osteoblasts to OPN was mediated by arginine-glycine-aspartate (RGD) peptide sequence [40]. Meanwhile, the RGD of OPN molecule combines with hydroxyapatite during the osteoblasts mineralization and can regulate size and shape of mineralized crystal [38, 39, 41]. BSP is an anionic phosphoprotein in the ECM of mineralized tissues and a promoter of biomineralization and osteoblast development [42, 43]. BSP, also contains RGD sequences [44, 45], is critical for hydroxyapatite formation activity [46]. The RGD of BSP molecule mediates cell attachment and promotes osteoblasts mineralization in vitro [47–50]. In this experiment, the mRNA and protein expressions of BSP were increased on day 21 in CG; this result may be attributed to the high expression levels of BSP during maturation from osteoblasts [51–53]. In another side, in this study, the mRNA and protein expressions of BSP and OPN were decreased with AlCl3 administration time prolonged, which indicated that AlCl3 inhibited mineralization of bone matrix. Moreover, our previous research found that AlCl3 exposure inhibited osteoblasts activity [32], which might be a reason for the inhibitory effect of AlCl3 on expressions of BSP and OPN. Collectively, these results indicated that AlCl3 inhibited the mineralization of osteoblasts on gene and protein levels.
Conclusion
AlCl3 exposure suppressed the formation of mineralized matrix nodule and reduced the expressions of main mineralization regulatory factors, indicating that AlCl3 inhibits osteoblast mineralization with time prolonged.
References
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R (2015) Erratum to: clinician is guide to prevention and treatment of osteoporosis. Osteoporos Int 26:2045–2047
Fine JP (2012) Bone-density testing interval and transition to osteoporosis in older women-NEJM. N Engl J Med 366:225–233
Gjoksi B, Ghayor C, Siegenthaler B, Ruangsawasdi N, Zenobi-Wong M, Weber FE (2015) The epigenetically active small chemical N-methyl pyrrolidone (NMP) prevents estrogen depletion induced osteoporosis. Bone 78:114–121
Sliva VS, Goncalves PP (2014) Effect of lysine acetylsalicylate on aluminium accumulation and (Na+/K+)ATPase activity in rat brain cortex synaptosomes after aluminium ingestion. Toxicol Lett 232:167–174
D’Souza SP, Vijayalaxmi KK, Naik P (2014) Assessment of genotoxicity of aluminium acetate in bone marrow, male germ cells and fetal liver cells of Swiss albino mice. Mutat Res Genet Toxicol Environ Mutagen 766:16–22
Sivakumar S, Khatiwada CP, Sivasubramanian J (2014) Studies the alterations of biochemical and mineral contents in bone tissue of Mus musculus due to aluminum toxicity and the protective action of desferrioxamine and deferiprone by FTIR, ICP-OES, SEM and XRD techniques. Spectrochim Acta A Mol Biomol Spectrosc 126:59–67
Aaseth J, Boivin G, Andersen O (2012) Osteoporosis and trace elements—an overview. J Trace Elem Med Biol 26:149–152
Crisponi G, Fanni D, Gerosa C, Nemolato S, Nurchi VM, Crespo-Alonso M, Lachowicz JI, Faa G (2013) The meaning of aluminium exposure on human health and aluminium-related diseases. Biomol Concepts 4:77–87
Rodriguez M, Felsenfeld AJ, Llach F (1990) Aluminum administration in the rat separately affects the osteoblast and bone mineralization. J Bone Miner Res 5:59–67
Cristinel ND, Guillaume M, Corneliu C, Daniel C (2013) Aluminum inhibits the growth of hydroxyapatite crystals developed on a biomimetic methacrylic polymer. J Trace Elem Med Biol 27:346–351
Pinto M, Nicolete LDF, Rodrigues ES, Palma PVB, Orellana MD, Kashima S, Covas DT (2013) Overexpression of hsa-miR-125b during osteoblastic differentiation does not influence levels of Runx2, osteopontin, and ALPL gene expression. Braz J Med Biol Res 46:676–680
Janckila AJ, Takahashi K, Sun SZ, Yam LT (2001) Tartrate-resistant acid phosphatase isoform 5b as serum marker for osteoclastic activity. Clin Chem 47:74–80
Lipton A (2010) Implications of bone metastases and the benefits of bone-targeted therapy. Semin Oncol 37:S15–S29
Chabas D, Baranzini SE, Mitchell D, Bermard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Oksenberg JR, Steinman L (2001) The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294:1731–1735
Holm E, Aubin JE, Hunter GK, Beier F, Goldberg HA (2015) Loss of bone sialoprotein leads to impaired endochondral bone development and mineralization. Bone 71:145–154
Matsuda S, Silva TL, Buzalaf MA, Rodrigues AC, De Oliveira RC (2014) Differential effects of fluoride during osteoblasts mineralization in C57BL/6J and C3H/HeJ inbred strains of mice. Biol Trace Elem Res 161:123–129
Johnson MG, Kristianto J, Yuan B, Konicke K, Blank R (2014) Big endothelin changes the cellular miRNA environment in TMOb osteoblasts and increases mineralization. Connect Tissue Res 55:113–136
Sun H, Hu C, Jia L, Zhu Y, Zhao H, Shao B, Wang N, Zhang Z, Li Y (2011) Effects of aluminum exposure on serum sex hormones and androgen receptor expression in male rats. Biol Trace Elem Res 144:1050–1058
Wang X, Harimoto K, Liu J, Guo J, Hinshaw S, Chang Z, Wang Z (2011) Spata4 promotes osteoblast differentiation through Erk-activated Runx2 pathway. J Bone Miner Res 26:1964–1973
Zhang X, Yang M, Lin L, Chen P, Ma KT, Zhou CY, Ao YF (2006) Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose-derived stem cells in vitro and in vivo. Calcif Tissue Int 79:169–178
Degeratu CN, Mabilleau G, Cincu C, Chappard D (2013) Aluminum inhibits the growth of hydroxyapatite crystals developed on a biomimetic methacrylic polymer. J Trace Elem Med Biol 27:346–351
Coe FL, Evan A, Worcester E (2005) Kidney stone disease. J Clin Investig 115:2598–2608
Bellows CG, Reimers SM, Heersche JNM (1999) Expression of mRNAs for type-I collagen, bone sialoprotein, osteocalcin, and osteopontin at different stages of osteoblastic differentiation and their regulation by 1, 25 dihydroxyvitamin D3. Cell Tissue Res 297:249–259
Ahlstrom M, Pekkinen M, Riehle U, Lamberg-Allardt C (2008) Extracellular calcium regulates parathyroid hormone-related peptide expression in osteoblasts and osteoblast progenitor cells. Bone 42:483–490
Lossdörfer S, Schwartz Z, Lohmann CH, Greenspan DC, Ranly DM, Boyan BD (2004) Osteoblast response to bioactive glasses in vitro correlates with inorganic phosphate content. Biomaterials 25:2547–2555
Collin P, Nefussi JR, Wetterwald A, Nicolas V, Boy-Lefevre M, Fleisch H, Forest N (1992) Expression of collagen, osteocalcin, and bone alkaline phosphatase in a mineralizing rat osteoblastic cell culture. Calcif Tissue Int 50:175–183
Gomez BJ, Ardakani S, Ju J, Jenkins D, Cerelli MJ, Daniloff GY, Kung VT (1995) Monoclonal antibody assay for measuring bone-specific alkaline phosphatase activity in serum. Clin Chem 41:1560–1566
Lynch MP, Stein JL, Stein GS, Lian JB (1995) The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp Cell Res 216:35–45
Hoyer B, Bernhardt A, Heinemann S, Stachel I, Meyer M, Gelinsky M (2012) Biomimetically mineralized salmon collagen scaffolds for application in bone tissue engineering. Biomacromolecules 13:1059–1066
Lieberherr M, Grosse B, Cournot-Witmer G, Hermann-Erlee MP, Balsan S (1987) Aluminum action on mouse bone cell metabolism and response to PTH and 1,25(OH)2D3. Kidney Int 317:36–743
Sprague SM, Krieger NS, Bushinsky DA (1993) Aluminum inhibits bone nodule formation and calcification in vitro. Am J Physiol Ren Physiol 264:F882–F890
Li XW, Han YF, Guan Y, Zhang L, Bai CS, Li YF (2012) Aluminum induces osteoblast apoptosis through the oxidative stress-mediated JNK signaling pathway. Biol Trace Elem Res 150:502–508
Gordon JAR, Tye C, Sampaio A, Underhill M, Hunter G, Goldberg H (2007) Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 41:462–473
Taichman RS, Hauschka PV (1992) Effects of interleukin-1 beta and tumor necrosis factor-alpha on osteoblastic expression of osteocalcin and mineralized extracellular matrix in vitro. Inflammation 16:587–601
Gomez-Alonso C, Menendez-Rodriguez P, Virgos-Soriano MJ, Femandez-Martin JL, Fernandez-Coto MT, Cannata-Andia JB (1999) Aluminum-induced osteogenesis in osteopenic rats with normal renal function. Calcif Tissue Int 64:534–541
Fanti P, Kindy MS, Mohapatra S, Klein J, Malluche HH (2006) Dose-dependent effects of aluminum on osteocalcin synthesis in osteoblast-like ROS 17/2 cells in culture. Am J Physiol Endocrinol Metab 263:E1113–E1118
Denhardt DT, Guo X (1993) Osteopontin: a protein with diverse functions. FASEB J 7:1475–1482
McKee MD, Nanci A (1996) Osteopontin at mineralized tissue interfaces in bone, teeth and osseointegrated implants: ultrastructral distribution and implication for mineralized tissue formation, turnover, and repair. Microsc Res Tech 33:141–164
Cho HJ, Cho HJ, Kim HS (2009) Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep 11:206–213
Somerman MJ, Prince CW, Butler WT, Foster RA, Moehring JM, Sauk JJ (1989) Cell attachment activity of the 44 kilodalton bone phosphoprotein is not restricted to bone cells. Matrix 9:49–54
Shin H, Zygourakis K, Farach-Carson MC, et al. (2004) Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. [J] Biomater 25(5):895–906
Mosig RA, Martignetti JA (2013) Loss of MMP-2 in murine osteoblasts upregulates osteopontin and bone sialoprotein expression in a circuit regulating bone homeostasis. Dis Model Mech 6:397–403
Chen J, Singh K, Mukherjee B, Sodek J (1993) Developmental expression of osteopontin (OPN) mRNA in rat tissues: evidence for a role for OPN in bone formation and resorption. Matrix 13:113–123
Espinoza J, Sanchez M, Sanchez A, Hanna P, Torrejon M, Buisine N (2010) Two families of Xenopus tropicalis skeletal genes display well-conserved expression patterns with mammals in spite of their highly divergent regulatory regions. Evol Dev 12:541–551
Shintani S, Kamakura N, Kobata M, Toyosawa S, Onishi T, Sato A (2008) Identification and characterization of integrin-binding sialoprotein (IBSP) genes in reptile and amphibian. Gene 424:11–17
Hunter GK, Goldberg HA (1993) Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A 90:8562–8565
Bernards MT, Qin C, Jiang S (2008) MC3T3-E1 cell adhesion to hydroxyapatite with adsorbed bone sialoprotein, bone osteopontin, and bovine serum albumin. Colloids Surf B: Biointerfaces 64:236–247
Bernards MT, Qin C, Ratner BD, Jiang S (2008) Adhesion of MC3T3-E1 cells to bone sialoprotein and bone osteopontin specifically bound to collagen I. J Biomed Mater Res A 86:779–787
Gordon JA, Hunter GK, Goldberg HA (2009) Activation of the mitogen-activated protein kinase pathway by bone sialoprotein regulates osteoblast differentiation. Cells Tissues Organs 189:138–143
Bianco P, Fisher LW, Young MF, Termine JD, Robey PG (1991) Expression of bone sialoprotein (BSP) in developing human tissues. Calcif Tissue Int 49:421–426
Aubin JE (2001) Regulation of osteoblast formation and function. Rev Endocr Metab Disord 2:81–94
Chen J, Shapiro HS, Sodek J (1992) Development expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner Res 7:987–997
Chen JK, Shapiro HS, Wrana JL, Reimers S, Heersche JN, Sodek J (1991) Localization of bone sialoprotein (BSP) expression to sites of mineralized tissue formation in fetal rat tissues by in situ hybridization. Matrix 11:133–143
Acknowledgments
The study was supported by a grant from the National Science Foundation Project (31372496) and the Science Foundation for Young Scientists of Jilin Province, China (20130522091JH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Miao Song and Hui Huo contributed equally to this study.
Rights and permissions
About this article
Cite this article
Song, M., Huo, H., Cao, Z. et al. Aluminum Trichloride Inhibits the Rat Osteoblasts Mineralization In Vitro. Biol Trace Elem Res 175, 186–193 (2017). https://doi.org/10.1007/s12011-016-0761-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0761-9