Abstract
The present study was performed to investigate the protective effects of selenium (Se), vitamin E (Vit E) and anthocyanins from purple carrots and their combination against the oxidative stress induced by d-galactose in rats. A total of 80 male rats were equally divided into 11 groups, one of which acted as control (I) just receiving intraperitoneal injections of physiological saline. The remaining ten groups (II–XI) were intraperitoneally injected with d-galactose at a dose of 400 mg kg−1 body weight (BW) per day for 42 consecutive days. Rats in groups III–XI were treated with antioxidants via gavage per day as follows: group III: Se-methylselenocysteine (SeMSC), IV: Se as sodium selenite (Na2SeO3), V: Se-enriched yeast (SeY), VI: Vit E as α-tocopherol acetate, VII: anthocyanin from purple carrots (APC), VIII: APC + Vit E, IX: SeMSC + APC+ Vit E, X: Na2SeO3 + APC + Vit E, XI: SeY + Ant + Vit E. The results showed that the rats treated with antioxidants (III–XI) showed significant decreases in the levels of malondialdehyde (MDA) and carbonyl protein (PCO) compared with the d-galactose-treated group (II) in the heart, liver, kidneys, and blood. Moreover, there were significant increases in the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), glutathione (GSH) concentration, and total antioxidant capacity (T-AOC) in the heart, liver, kidneys, and blood of antioxidant-treated animals (III–XI) than those in control group (I). In addition, the combined treatments of two or three antioxidants showed greater antioxidant activities than those of individual treatments, suggesting the synergistic antioxidant effects of Se, Vit E, and APC. In conclusion, all the antioxidants exhibited protective effects against d-galactose-induced oxidative damage in rats, and these antioxidants showed a synergistic effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress is a consequence of an imbalance between prooxidants and antioxidants, and the imbalance could overproduce the reactive oxygen species (ROS). Moreover, oxidative stress and ROS have been proposed to be major causes of aging [1]. The accumulated ROS may lead to the oxidative modification of biomolecules such as lipids, proteins, and nucleic acids [2]. It is widely accepted that consumption of diets high in plant-based foods is associated with a decreased risk of oxidative damage. Potential antioxidants in fruits and vegetables include vitamins, minerals, and numerous phytochemicals. It has been proven that antioxidants such as selenium (Se), vitamin E (Vit E), and phytochemicals could ameliorate the oxidative damage induced by ROS. Se, an essential micronutrient for human, has a strong ROS scavenging activity. It could protect the organs and tissues from oxidative damage and improve the immune system of body [3, 4]. Se could be decomposed to methylselenol in vivo, which is hypothesized to be a critical Se metabolite for the anticancer activity, and have different chemopreventive effects on some cancerous and non-cancerous cells [5]. Glutathione peroxidase (GSH-Px), a Se-containing enzyme, is responsible for the reduction of hydro and organic peroxides, and the synthesis of GSH-Px needs glutathione (GSH) [6]. On the other hand, Vit E, alpha-tocopherol, is the most important antioxidant in the lipid phase of cells [7]. Vit C and GSH are also strong free radical scavengers and can also transform Vit E to its active form [8]. In addition, anthocyanins have attracted lots of attention because of their potential biological and pharmacological benefits, such as antioxidant by donating hydrogen to ROS [9], anti-inflammatory [10] and antitumor properties [11], and decreasing lipid peroxidation and DNA damage [12]. It has been also demonstrated that anthocyanins from strawberry-ameliorated cardiovascular risk, oxidative stress, and platelet activation in human [13].
Moreover, there were synergistic effects among antioxidants to quench free radicals and peroxides. It has been shown that selenomethionine (200 μg/day) and Vit E (400 IU/day), alone or in combination (for 5.46 years), prevented prostate cancer in healthy men [14]. The synergistic effect of Se and Vit E was also found in lipid peroxidation preventive trials [15]. Both Vit E and anthocyanins could assist the treatment of many diseases, such as atherosclerosis, inflammation, and cancers [16]. Purple carrots were reported to be rich in anthocyanins and have high antioxidant activity in vitro [17]. Purple carrots can also help to improve memory and inhibit the proliferation of cancer cells, which were partly due to its anthocyanins [18]. However, little is known about the in vivo synergistic effects of Se, Vit E, and anthocyanins.
Therefore, the purpose of this study was to investigate and compare the synergistic antioxidant effects of individual and combined treatments with different forms of Se, Vit E, and purple carrot anthocyanins (APC) against the oxidative stress in rats.
Materials and Methods
Materials
Se-methylselenocysteine (SeMSC, 96 % purity) was provided by Chuan Qi Pharmaceutical Corporation. Se-enriched yeast (SeY, Se concentration 2000 mg/kg) was purchased from Angel Yeast Corporation. Vit E as α-tocopherol acetate powder (50 % purity) and Se as sodium selenite (Na2SeO3, 98 % purity) were purchased from Sigma-Aldrich (Steinheim, Germany). Anthocyanin extracts from purple carrot (APC) were obtained from Guo Yi Biological Technology Corporation (anthocyanins content 8.4 %). d-Galactose (d-gal, 98 % purity) was purchased from Aladdin Industrial Corporation. The total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), malondialdehyde (MDA), reduced glutathione (GSH), and protein carbonyl (PCO) kits were purchased from Nanjing Jiancheng Biotechnology Company (Nanjing, China).
Animals and Pretreatment
Male Sprague-Dawley rats [approximately 200–220 g body weight (BW)] were purchased from Shanghai Research Center of Experimental Animal of Academy in China (Batch No: SCXK 2013–2014), and the animals were acclimatized for 7 days prior to experiments. The experimental protocols were approved by Nanchang University Ethics Committee. All rats were raised in stainless cages in an ambient temperature controlled room (24 ± 2 °C) with a controlled photoperiod (12-h light/dark cycle) and relative humidity of 50 ± 5 %. The rats had free access to commercial pellet diet and water ad libitum. The basal diet consisted of 20 % vitamin-free casein, 40 % sucrose, 20 % corn starch, 5 % fiber, 10 % fat (soybean oil), 3.5 % mineral mix, and 1 % vitamin mix (without Vit E).
One week after acclimatization to laboratory conditions, the rats were randomly divided into 11 groups (n = 8). Treatments were carried out over a period of 42 days. The experimental feeds were formulated to have the same Se content (4.5 μg/kg).
The groups were as follows:
-
Group I: control (saline)
-
Group II: d-gal model (2 mL of d-gal solution, 400 mg/kg BW)
-
Group III: d-gal + SeMSC (4.5 μg/kg BW Se in the form of SeMSC per day)
-
Group IV: d-gal + Na2SeO3 (4.5 μg/kg BW Se in the form of Na2SeO3 per day)
-
Group V: d-gal + SeY (4.5 μg/kg BW Se in the form of SeY per day)
-
Group VI: d-gal + Vit E (8.4 mg/kg BW Vit E in the form of α-tocopherol acetate per day)
-
Group VII: d-gal + APC (100 mg/kg BW per day)
-
Group VIII: d-gal + Vit E + APC (100 mg/kg BW APC + 8.4 mg/kg BW Vit E in the form of α-tocopherol acetate per day)
-
Group IX: d-gal + SeMSC + Vit E + APC group (4.5 μg/kg BW Se in the form of SeMSC, 100 mg/kg BW APC and 8.4 mg/kg BW Vit E in the form of α-tocopherol acetate per day)
-
Group X: d-gal + Na2SeO3+ APC + Vit E (4.5 μg/kg BW Se in the form of Na2SeO3, 100 mg/kg BW APC and 8.4 mg/kg BW Vit E in the form of α-tocopherol acetate per day)
-
Group XI: d-gal + SeY + Vit E + APC (4.5 μg/kg BW Se in the form of SeY, 100 mg/kg BW APC and 8.4 mg/kg BW Vit E in the form of α-tocopherol acetate per day)
The doses were chosen based on the recommended intakes of individuals in China. They showed no toxic effect on rats in the pre-experiments (data not shown). Rats in group I were intraperitoneally injected with physiological saline as control group. The groups (II–XI) were intraperitoneally injected with d-gal at a dose of 400 mg kg−1 BW per day. Rats in group II were not treated with any antioxidant. Rats in groups III–XI were given 1.5 mL sample solutions by gavage per day.
After 42 days, all of the rats were culled by cervical decapitation to avoid stress. Blood was collected into ethylene diamine tetraacetic acid (EDTA) tubes and centrifuged at 3000 rpm for 10 min. Plasma samples were drawn and stored at −80 °C until analysis. The liver, heart, and kidneys were immediately dissected out, cleaned, and weighed. The organs were rinsed and homogenized (10 %, w/v) in an appropriate buffer (pH 7.4) and then centrifuged. The supernatants were used for the measurement of antioxidant activity. The SOD, GSH-Px acitivities, T-AOC and the contents of MDA, GSH, and PCO in blood and tissues were determined using the commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
HPLC-QTOF-MS/MS
An Agilent 1290 series high-performance liquid chromatography (Agilent Technologies, USA), coupled with an Agilent 7200 triple quadrupole time of flight mass spectrometer, were used to identify the anthocyanins from purple carrot extracts. The anthocyanin extract of purple carrot was dissolved in the water (1 mg/mL) and filtered through a 0.45-μm PTFE membrane filter for HPLC analysis immediately at an injection volume of 5 μL. The mobile phase was composed of A (0.2 % formic acid in water, v/v) and B (0.2 % formic acid in acetonitrile, v/v). The column was Agilent Zorbax SB-C18 column (4.6 mm × 250 mm, 5 μm) and the gradient elution program was as follows: 0–5 min, 85–80 % A; 5–10 min, 80–70 % A; 10–20 min, 70–60 % A; 20–30 min, 60–55 % A; 30–35 min, 55–40 % A; 35–40 min, 40–85 % A. The column temperature was 25 °C, the flow rate was 0.3 mL/min, and the DAD detector at 520 nm was used.
The ESI source was operated in the positive ion mode, and full scan mass spectral data were acquired over a range from m/z 100 to 22,000. The optimum values of the source parameter were as follows: capillary voltage, +3.5 kV; drying gas temperature at 350 °C at a flow rate of 11 L/min; nebulizer pressure, 30 psi. The HPLC-MS data were acquired and analyzed by the software Agilent MassHunter Acquisition B.09.01.
Biochemical Assays
The biochemical assays were performed using 96-well microplates (BD Falcon, USA) and a multi-well plate reader (Thermo Scientific varioskan flash, USA) with commercial kits (Nanjing, China). The SOD activity was measured with a SOD kit, which is based on its ability to inhibit the oxidation of hydroxylamine by the xanthine-xanthine oxidase system. The GSH-Px activity was determined using a GSH-Px assay kit, which is based on the oxidation of NADPH at 340 nm coupled with the reduction of glutathione oxidized by GSH-Px. The T-AOC was determined using a T-AOC assay kit, which is based on the ferric-reducing antioxidant power assay. The activities of the two antioxidant enzymes and T-AOC were expressed as per milligram of protein (U/mg prot) in tissues or units per milliliter (U/mL) in blood. The MDA content was measured using a commercial kit, which is based on thio-barbituric acid technique, and data were expressed as nanomoles per milligram of protein (nmol/mg prot) in tissues or nanomoles per milliliter (nmol/mL) in blood. The PCO content was measured using a commercial kit. The absorbance of samples was determined at 366 nm, and data were expressed as micromoles per milligram of protein (μmol/mg prot) in tissues or micromoles per milliliter (μmol/mL) in blood. The GSH content was measured using a commercial kit. The absorbance at 412 nm was measured at a 10-s interval. From the calibration curve made with commercial GSH, the concentration of GSH was determined and data were expressed as milligram per gram of protein (mg/g prot) in tissues or milligram per milliliter (mg/mL) in blood. The protein concentration in tissues was measured by using a protein kit, which is based on the method of Coomassie light blue, and the result was expressed as milligram per gram.
Statistical Analysis
Results were expressed as mean ± SD. Data were analyzed using one way analysis of variance (ANOVA), followed by Tukey’s post hoc comparisons. All statistical analyses were performed with the statistical program SPSS 17.0. The differences were considered to be statistically significant when p < 0.05.
Results
The Anthocyanins in Purple Carrots
A total of seven anthocyanins were found in purple carrot extracts. They were identified as cyanidin-3-(feruloyl)-sambubioside-5-glucoside (peak 1), pelargonidin-3-sambubioside-glucoside (peak 2), cyanidin-3-sambubioside (peak 3), cyanidin-3-(feruloyl)-diglucoside-5-glucoside (peak 4), cyanidin-3-(p-coumaroyl)-glucoside-5-glucoside (peak 5), cyanidin-3-galactoside-xyloside-glucoside-coumaric acid (peak 6), and cyanidin-3-glucoside (peak 7) by HPLC-QTOF-MS/MS (Fig. 1).
The HPLC profile of purple carrot anthocyanins. The peaks were as follows: (1) cyanidin-3-(feruloyl)-sambubioside-5-glucoside, (2) pelargonidin-3-sambubioside-glucoside, (3) cyanidin-3-sambubioside, (4) cyanidin-3-(feruloyl)-diglucoside-5-glucoside, (5) cyanidin-3-(p-coumaroyl)-glucoside-5-glucoside, (6) cyanidin-3-galactoside-xyloside-glucoside-coumaric acid, (7) cyanidin-3-glucoside
The MDA, PCO, and GSH Contents
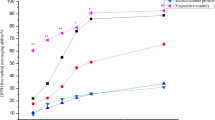
The MDA contents in the d-gal model group (blood, liver, heart, kidney: 9.52, 3.43, 1.98, and 5.16 nmol/mg, respectively) were significantly higher than those in the control group (4.78, 1.80, 0.97, and 3.08 nmol/mg, p < 0.001, Fig. 2), indicating that d-gal successfully induced oxidative damage in the rats. Meanwhile, the MDA contents in antioxidant-treated groups (III–XI) were much lower than those in the model group (p < 0.01). There was no significant difference among groups (III–VII) treated with individual antioxidants (SeMSC, Na2SeO3, SeY, Vit E, and APC, p > 0.05). Interestingly, the MDA contents in the blood, heart, and liver in group VIII were lower than those in individual antioxidant-treated groups (III–VII, p < 0.05), but much higher than those in combined antioxidant-treated groups (IX–XI). This phenomenon might be explained by the synergistic antioxidant effects of Se, Vit E, and APC. Moreover, it was found that the MDA contents in the heart in Se + Vit E + APC-treated groups (IX–XI) showed no significant differences with the control group. The combination of Se, Vit E, and APC could recover the damage by reducing the MDA content.
The changes of MDA contents in different groups. Data were presented as mean ± SD (n = 8); values not sharing a common superscript letter denote significant difference (p < 0.05). Rats were divided into 11 groups as follows: I: control, II: model, III: Se-methylselenocysteine (SeMSC), IV: Se as sodium selenite (Na2SeO3), V: selenium-enriched yeast (SeY), VI: Vit E as α-tocopherol acetate, VII: purple carrots anthocyanin extracts (APC), VIII: APC + Vit E, IX: SeMSC + APC+ Vit E, X: Na2SeO3 + APC + Vit E, XI: SeY + Ant + Vit E
Similarly, the PCO contents in the model group (blood, heart, liver, and kidneys: 2.99, 10.60, 7.30, and 8.08 μmol/mg prot, respectively) were higher than those in the control group (1.15, 6.30, 4.09, and 4.96 μmol/mg prot, p < 0.001, Fig. 3). The total PCO contents in the blood and heart in antioxidant-treated groups (III–XI) showed no significant differences (p > 0.05). However, the PCO contents of the liver and kidneys in the combined antioxidant-treated groups (IX–XI) were lower than those in the other treated groups (III–VIII, p < 0.05).
The highest GSH contents were found in the control group (blood, heart, liver, and kidneys: 123.99, 40.54, 38.78, and 39.05 U/mg prot, respectively, Fig. 4). The GSH contents in the model group (blood, heart, liver, and kidneys: 62.11, 24.47, 23.36, and 21.97 U/mg prot, respectively) were significantly lower than those in antioxidant-treated groups (p < 0.001). Meanwhile, the GSH contents in groups treated with combined antioxidants were significant higher than those in individual antioxidant-treated groups (p < 0.05), and there were no significant differences among groups treated with combined antioxidants (p > 0.05).
The Activities of T-AOC, SOD, and GSH-Px
T-AOC represents the non-enzymatic antioxidant defense system. Obviously, the T-AOC levels in the model group (blood, heart, liver, and kidneys: 7.20, 2.61, 3.10, and 3.37 U/mg, respectively) were significantly lower than those in the control group (13.36, 4.87, 5.77, and 6.08 U/mg, p < 0.001), which could prove again that this model was successfully established (Fig. 5). The combined antioxidant-treated groups (IX–XI) showed higher T-AOC (blood and tissues) than the individual treated groups. Moreover, the T-AOC in antioxidant-treated groups (III–XI) were increased significantly than those in the model group (p < 0.05). The T-AOC in tissues of three combined antioxidant-treated groups (IX–XI) were increased significantly than those in Vit E + APC group (p < 0.01). No significant differences were found among the groups treated with the individual antioxidants (III–VII, P > 0.05).
Similarly, the SOD activities in the model group (blood, heart, liver, and kidneys: 134.36, 59.82, 45.07, and 105.68 U/mg prot, respectively) were significantly lower than those in other groups (p < 0.001, Fig. 6). There were no significant differences among the groups treated with the individual antioxidants (p > 0.05). Meanwhile, no significant differences were found among the groups treated with three antioxidant combinations, which were significant higher than those in the other groups except the control group (p < 0.01).
Moreover, the highest GSH-Px activities were found in the control group (blood, heart, liver, and kidneys: 479.91, 99.18, 230.01, and 306.68 U/mg prot, respectively, Fig. 7). The levels of GSH-Px in antioxidant-treated groups were increased significantly than those in the d-gal group (p < 0.001). The GSH-Px activities of the blood and heart in SeMSC- or SeY-treated group were higher than those in Na2SeO3, Vit E, and APC groups. The GSH-Px activities of the heart and kidneys from the rats treated with APC + Vit E showed no significant differences with those from the Vit E or APC group (p > 0.05). Meanwhile, the GSH-Px activities in the combined antioxidant-treated groups were significantly higher than those in the other groups (p < 0.05).
Discussion
It has been reported that the damage of d-gal could be attributed to the overproduction of ROS, lipid peroxidation, and inhibition of antioxidant enzymes [19]. Oxidative stress and senescence phenomenon were found in rats and mice chronically treated with d-gal [20]. Thus, d-gal was widely used to induce the oxidative damage in animals [21]. In this study, the rats which received 400 mg/kg d-gal by intraperitoneal injection for 6 weeks showed oxidative stress, which was consistent with the previous reports [21, 22]. Moreover, higher antioxidant activities (T-AOC, SOD, GSH-Px, and GSH) and lower MDA and PCO contents were found in the rats treated with different antioxidants than in the model group. These data suggested that supplementation with APC, Vit E, or Se in diets may prevent or reduce oxidative stress.
Anthocyanins, a class of naturally occurring polyphenol compounds, showed multifunctional benefits to human beings. Dietary antioxidant phytochemicals, most notably phenolic acids and flavonoids from fruits and vegetables, played multiple protective roles at various stages of the oxidative process. For example, Devi et al. reported that anthocyanins could act as antioxidants by directly reducing free radicals with multiple hydroxyl and preventing oxidative damage to DNA from hydroxyl radical [12]. Cho et al. found that the antioxidant effects of anthocyanins included regenerating other antioxidants, enhancing antioxidant enzyme activity, and increasing mRNA expression of these enzymes [23]. In addition, other phenolic compounds in purple carrots were reported protecting oxidative stress in rats [24–26] which were consistent with our results.
Previous studies have shown that Vit E prevented or decreased the harmful effects of oxidative stress in different tissues [27]. Indeed, our results showed decreases in the levels of MDA and PCO in tissues and increases in SOD and GSH-Px activities and T-AOC, and GSH contents in DM + Vit E treated rats, when compared to DM group. These results were consistent with previous findings by Shireen et al. who found that Vit E supplementation protected soft tissues against impairment by toxic radicals [28].
The trace mineral Se plays important roles in the antioxidant activity, reproduction, endocrine, and immune systems [29]. Supplement of Se has been shown to improve the growth performance and antioxidant status of animals. For example, Shi et al. reported that dietary Se supplementation significantly improved the final weight and daily weight gain in male goats. Furthermore, blood GSH-Px, SOD, and CAT activities were higher and MDA was lower in Se-supplemented group than in the control [30]. Similar results were also found in chicken and pigs [31, 32], which were consistent with high GSH, GSH-Px, and SOD activity levels and low MDA content found in the study. GSH-Px, a Se-containing enzyme, could protect the organism from oxidative damage. SeMSC and SeY could be metabolized to methylselenol and then metabolized to H2Se, while Na2SeO3 could be directly metabolized to H2Se. The precursor H2Se could help the synthesis of selenocysteine and further accelerate the synthesis of GSH-Px [34]. Thus, either organic Se (SeMSC and SeY) or inorganic Se (Na2SeO3) could increase the GSH-Px activities due to the increased incorporation of selenocysteine. In addition, GSH-Px activities of the blood and heart in SeMSC and SeY groups were found to be significantly higher than those in Na2SeO3 group. Similarly, Liu et al. reported that Na2SeO3 had less effect on the hepatocarcinogenesis by inhibiting angiogenesis and relative cytokines to some extent compared to the Se-enriched malt [33]. Yao et al. also reported that the organic Se performed more effectively than inorganic Se in ethanol-induced rats [34].
It was reported that organic Se (SeMSC or SeY) was more effective in increasing the activities of blood antioxidant enzymes and Se retention in blood and some tissues than Na2SeO3 [30]. The better in vivo effects of organic Se might be due to the following reasons: Firstly, the absorption of inorganic Se (such as Na2SeO3) is lower than that of organic Se in organs [35]. Secondly, the methylseleno anion which disintegrated from SeMSC and SeY could react directly with O2, while selenite could not react directly with ROS [36]. Organic Se was also reported to be less accumulated in the body and have lower toxicity [37]. Meanwhile, free radicals could be eliminated by GSH-Px, and more oxidized glutathione could turn to GSH at low contents of free radicals [38]. Moreover, organic Se can avoid the depletion of GSH during the process of conversion from inorganic Se to H2Se, compared with inorganic Se (Na2SO3) [39].
It has been shown that two antioxidant combinations such as Vit E and Se exhibited a synergistic effect in ameliorating the oxidative damage in the body. Yao et al. found that Vit E worked synergistically with SeMSC to reduce the damages in tissues due to the effect of elevating GSH-Px activity [34]. Amara et al. reported that the nutritional supplementation of Se with Vit E may exert a synergistic effect of protecting the liver against dimethoate toxicity [40]. The synergistic effects of Se and Vit E were also proven in our study by increasing the activities of antioxidant enzymes, GSH contents, and T-AOC, as well as reducing the MDA and PCO contents in different tissues of rats. Shaheen et al. showed that rutin and Se had a synergistic protective effect against DENA-induced damage to the kidney by inhibiting the production of ROS via increasing the levels of antioxidant enzymes [41]. In our study, Vit E combined with APC also exhibited a better protection than the individuals by reducing MDA and PCO contents in the liver (p < 0.05).
In general, most of papers were focus on the synergistic effects of two different compounds, and the reasons were attributed to the changes of antioxidant enzymes (SOD, GSH-Px) and lipid peroxide (MDA) content. Here in our study, the synergistic effects of three compounds (Se, Vit E, and APC) were investigated, and the synergistic effects were clearly found in the antioxidant combinations (IX–XI: SeMSC + APC + Vit E, Na2SeO3 + APC + Vit E, and SeY + APC + Vit E). Se was reported to scavenge free radicals, chelate metals, and activate antioxidant enzymes in the body [36]. Anthocyanins have the ability to donate hydrogen to free radicals in addition to its activation of antioxidant enzymes and decrease oxidative stress through its antioxidant properties [9]. Gavage treatment of anthocyanins was reported to elevate the antioxidant capacity, including activating the expression of glutathione-related enzymes and increasing the GSH content [42]. Se-Vit E may protect against d-gal-induced toxicity by inhibition of the inactivation of GSH and antioxidant system, as well as upregulation of GSH-Px and Vit E in the tissues [7]. The GSH was also utilized in the regeneration of Vit E, which can be enhanced by the supplementation of Se [3]. Free radicals are produced by both enzymatic and non-enzymatic mechanisms present in the cells. The synergism of Se, Vit E, and APC was also affected not only by the enzyme systems, such as SOD and GSH-Px, but also by the non-enzyme systems through direct reaction of antioxidants and free radicals [43].
Conclusion
This study investigated the antioxidant effects of individual and combined treatments with Se (SeMSC, Na2SeO3, SeY), Vit E, and APC against the oxidative stress in rats. Treatment with SeMSC, Na2SeO3, SeY, Vit E, or APC decreased d-gal-induced oxidative damage in rats. Treatment with Se, Vit E, and APC reduced the contents of oxidation products (MDA and PCO) and increased the GSH content, T-AOC and the activities of antioxidant enzymes (SOD and GSH-PX). At the same dose, the in vivo antioxidant activities of SeMSC and SeY (organic Se) were higher than those of Na2SeO3 (inorganic Se). Vit E and APC worked synergistically with Se to ameliorate the oxidative damage in rats.
References
Valko M, Leibfritz D, Moncol J, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B 39:44–84
Dröge W (2003) Oxidative stress and aging. Adv Exp Med Biol 543:191–200
Brenneisen P, Steinbrenner H, Sies H (2005) Selenium, oxidative stress, and health aspects. Mol Asp Med 26:256–267
Wang Y, Wu YC, Luo K, et al. (2013) The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem Toxicol 58:61–67
Ebrahim AM, Eltayeb MAH, Shaat MK, et al. (2007) Study of selected trace elements in cancerous and non-cancerous human breast tissues from Sudanese subjects using instrumental neutron activation analysis. Sci Total Environ 383:52–58
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Nazıroǧlu M, Karaoğlu A, Aksoy AO (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195:221–230
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Algarra M, Fernandes A, Mateus N, et al. (2014) Anthocyanin profile and antioxidant capacity of black carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. J Food Compos Anal 33:71–76
Subarnas A, Wagner H (2000) Analgesic and anti-inflammatory activity of the proan-thoeyanidin shellegueain A from polypodium feei METT. Phytomedicine 7:401–405
Bagchi D, Sen CK, Bagchi M, et al. (2004) Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochem Mosc 69:75–80
Devi PS, Kumar MS, Das SM (2012) DNA damage protecting activity and free radical scavenging activity of anthocyanins from red Sorghum (Sorghum bicolor) bran. Bio Res Int 2012:1–9
Alvarez-Suarez JEM, Giampieri F, Tulipani S, et al. (2014) One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J Nutr Biochem 25:289–294
Lippman SM, Klein EA, Goodman PJ, et al. (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers. JAMA 301:39
Kara H, Cevik A, Konar V, et al. (2008) Effects of selenium with vitamin E and melatonin on cadmium-induced oxidative damage in rat liver and kidneys. Biol Trace Elem Res 125:236–244
Hagiwara A, Miyashita K, Nakanishi T, et al. (2001) Pronounced inhibition by a natural anthocyanin, purple corn color, of 2-amino-1-methyl-6-phenylimidazo. Cancer Lett 171:15–25
Cefola M, Pace B, Renna M, et al. (2012) Compositional analysis and antioxidant profile of yellow, orange and purple Polignano carrots. Ita J Food Sci 24:284–291
Netzel M, Netzel G, Kammerer DR, et al. (2007) Cancer cell antiproliferation activity and metabolism of black carrot anthocyanins. Innov Food Sci Emerg 8:365–372
Head E, Nukala VN, Fenoglio KA, et al. (2009) Effects of age, dietary, and behavioral enrichment on brain mitochondria in a canine model of human aging. Exp Neurol 220:171–176
Chiu C, Chiu Y, Wu L, et al. (2011) Diosgenin ameliorates cognition deficit and attenuates oxidative damage in senescent mice induced by D-galactose. Am J Chinese Med 39:551–563
Hao L, Huang H, Gao J, et al. (2014) The influence of gender, age and treatment time on brain oxidative stress and memory impairment induced by D-galactose in mice. Neurosci Lett 571:45–49
Yu Y, Bai F, Wang W, et al. (2015) Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol Biochem Be 133:122–131
Cho J, Kang JS, Long PH, et al. (2003) Antioxidant and memory enhancing effects of purple sweet potato anthocyanin and cordyceps mushroom extract. Arch Pharm Res 26:821–825
Coskun O, Kanter M, Korlmaz A, et al. (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res 51:117–123
Youdim KA, Shukitt-Hale B, MacKinnon S, et al. (2000) Polyphenolics enhance red blood cell resistance to oxidative stress: in vitro and in vivo. BBA-Gen Subjects 1523:117–122
Zhang Z, Fan S, Zheng Y, et al. (2009) Purple sweet potato color attenuates oxidative stress and inflammatory response induced by d-galactose in mouse liver. Food Chem Toxicol 47:496–501
Shireen KF, Pace RD, Mahboob M, et al. (2008) Effects of dietary vitamin E, C and soybean oil supplementation on antioxidant enzyme activities in liver and muscles of rats. Food Chem Toxicol 46:3290–3294
Mehmet K, Burhan A, Meryem A, et al. (2009) Vitamin E protects against oxidative damage caused by cadmium in the blood of rats. Eur J Gen Med 3:154–160
Lei C, Niu X, Ma X, et al. (2011) Is selenium deficiency really the cause of Keshan disease? Environ Geochem Hlth 33:183–188
Shi L, Xun W, Yue W, et al. (2011) Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Ruminant Res 96:49–52
Zhou X, Wang Y, Gu Q, et al. (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 291:78–81
Chaudhary M, Garg AK, Mittal GK, et al. (2010) Effect of organic selenium supplementation on growth, Se uptake, and nutrient utilization in guinea pigs. Biol Trace Elem Res 133:217–226
Liu J, Zhao H, Liu Y, et al. (2012) Effect of two selenium sources on hepatocarcinogenesis and several angiogenic cytokines in diethylnitrosamine-induced hepatocarcinoma rats. J Trace Elem Med Bio 26:255–261
Yao Z, Zhang Y, Li H, et al. (2015) Synergistic effect of Se-methylselenocysteine and vitamin E in ameliorating the acute ethanol-induced oxidative damage in rat. J Trace Elem Med Bio 29:182–187
Suzuki KT, Tsuji Y, Ohta Y (2008) Preferential organ distribution of methylselenol source Se-methylselenocysteine relative to methylseleninic acid. Toxicol Appl Pharm 227:76–83
Battin EE, Brumaghim JL (2009) Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem Biophys 55:1–23
Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: a review. Sci Total Environ 400:115–141
Rayman MP, Infante HG, Sargent M (2008) Food-chain selenium and human health: spotlight on speciation. Brit J Nutr 100:238–253
Hail N, Cortes M, Drake EN, et al. (2008) Cancer chemoprevention: a radical perspective. Free Radical Bio Med 45:97–110
Amara IB, Soudani N, Troudi A, et al. (2011) Antioxidant effect of vitamin E and selenium on hepatotoxicity induced by dimethoate in female adult rats. Ecotox Environ Safe 74:811–819
Shaheen NEM (2013) Oxidative stress of diethylnitrosamine on the functions of kidney in male rats and effective role of rutin and/or selenium. J Appl Sci Res 9:6684–6691
Shih P, Yeh C, Yen G (2007) Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agr Food Chem 55:9427–9435
Kasaikina MV, Turanov AA, Avanesov A, et al. (2013) Contrasting roles of dietary selenium and selenoproteins in chemically induced hepatocarcinogenesis. Carcinogenesis 34:1089–1095
Acknowledgments
This project was funded by the National Natural Science Funds of China (Grant No. 31301433) and the Postdoctoral Science Foundation of Jiangxi Province (Grant No. 2013KY04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures involving rats were conducted in accordance with the guidelines of China for the care and use of laboratory animals.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Li, X., Zhang, Y., Yuan, Y. et al. Protective Effects of Selenium, Vitamin E, and Purple Carrot Anthocyanins on d-Galactose-Induced Oxidative Damage in Blood, Liver, Heart and Kidney Rats. Biol Trace Elem Res 173, 433–442 (2016). https://doi.org/10.1007/s12011-016-0681-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0681-8