Abstract
This research was carried out to evaluate toxic effects of nanosilver (Ag-NPs) on liver function and some blood parameters of male and female mice Mus musculus. A group of 54 BALB/c mice was randomly divided into three groups (each with two replications): Ag-NP (2) and control (1), each with nine mice. The experiment lasted for 14 days. In the treatment groups, two different doses of 20 and 50 ppm of Ag-NP solution were administered orally, while in the untreated (control) group, no Ag-NP solution but distilled water was used. At the end of the experiment, the serum was obtained by centrifugation of the whole blood at 3000 rpm for 15 min. The biochemical levels including alanine aminotransferase (ALT), aspartate amino transferase (AST), and blood cells were assayed by an automatic biochemical analyzer. Also, liver biopsy was performed and samples were stained using hematoxylin and eosin (H&E) staining. The values of red blood cells (RBC), hemoglobin (Hb), and hematocrit (Hct) did not vary significantly in the control and Ag-NP-treated animals. There were significant changes in the treatment and control groups in the levels of liver enzymes so that at both doses, there were significantly elevated levels of ALT and AST in mice treated with Ag-NPs compared with the control (p < 0.05). Sexuality was not significantly involved in the results. Oral exposure to Ag-NPs produced changes in blood chemistry and hepatotoxicity as indicated by increased serum activity levels of both AST and ALT and histological damages to the liver with no significant changes between male and female mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the wide application of nanoparticles, there is a serious lack of information concerning their impact on human health and the environment [1]. Silver nanoparticles (Ag-NPs) are groups of silver atoms ranging in size, in at least one dimension (typically spherical diameter), from 1 to 100 nm. These nanoparticles have been considered as antibacterial made by human and could be used as an additive instead of antibiotics due to their antibacterial properties and their adaptability to biological systems [2–4].
In fact, nanomaterials are at the leading edge of the rapidly developing field of nanotechnology and their unique size-dependent properties make these materials superior and indispensable in many areas of human activity [5]. Recently, silver and silver nanoparticles (Ag-NPs) are widely being applied to consumer products and medical uses [6]. Ag-NPs are translocated into blood circulation and accumulated in some organs to cause hepatotoxicity when administered through oral, inhalation, or subcutaneously [7]. Liver appears to be a major accumulation site of circulatory silver nanoparticles [8]. In fact, silver nanoparticles have been shown to damage liver cells [9]. The toxic effect or heavy metal poisoning is defined as “any functional or morphologic change in the body produced by an ingested, injected, inhaled, or absorbed drug, chemical, or biological agent” [9].
Kim et al. [7] studied relation of histopathological responses and parameters of blood serum in poisonousness of nanoparticles and found significant changes in level of AST and ALT enzymes of blood serum. Pathological studies also prove damages to liver tissue, especially to hepatic lobules [7].
The first step for diagnosis of hepatic damage is simple blood test and then multiple biochemical tests [10]. Changes in enzyme activity of plasma are used as index of tissue damage, environmental stress, or disease position. An incresae in enzyme activity depends on the concentration of enzyme in cells, rate of leakage during damage, and pure natural path of enzyme from plasma. Changes in enzyme activity happened by increase or decrease of production of enzyme, natural path excretion, increase of the alter ability of cellular membrane, or disorder in blood flow [11].
The most important aspect to be considered for enzyme assays is determining activity of aminotransferase enzymes to indicate damage to hepatocytes or hepatic cells [12]. These enzymes usually are in hepatic cells but with damaging liver; these enzymes enter to blood flow [10–12]. Aminotransferase enzymes, i.e., ALT and AST, are used to evaluate damaging hepatic cells in rats, dogs, and nonhuman primates.
Our understanding of Ag-NPs in tissue deposition and related adverse effects is limited. In the current study, we therefore looked into Ag-NP tissue accumulation and toxicological impairments in mice exposed to Ag-NPs via oral administration. Many findings suggested that liver, among others, is the major organ for Ag-NP localization in mice. In addition, Kim et al. (2008) in Sprague-Dawley rats found that the kidneys showed a sex-dependent accumulation of silver, with a twofold higher accumulation in the female when compared with the male.
It must be noted that while the population exposed to silver nanoparticles continues to increase with ever new applications, silver nanoparticles remain a controversial research area as regards their toxicity to biological systems. In particular, the oral toxicity of silver nanoparticles is of particular concern to ensure public and consumer health. In addition, Kim et al. [7] studied oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma and/or Cheraghi et al. [9] just investigated in vivo effect of silver nanoparticles. This study could be new in that it specifically addressed the toxic effects of nanosilver on liver and some blood parameters. Overall, the study aimed to investigate the toxic effects of Ag-NPs with two different doses on the liver function and some blood and electrolyte parameters in male and female mice (Mus musculus) when administered orally.
Materials and Methods
Mice Holding
Animal test was performed with compliance of the local ethics committee. A group of BALB/c mice of about 9 weeks (weighting 27.2 ± 3.0 g) were purchased from Medical Faculty of Shahrekord University and then transferred to the laboratory. The animals were in a single group and maintained on commercial pellet diet, given deionized water ad libitum, and kept in plastic cages in a 20 ± 2 °C, 50–70 % relative humidity room with a 12-h light/dark cycle. The photoperiod was provided by fluorescent tubes (Thorn, 36 W, white light), and all lighting was excluded during the scotophase. A timer was used to turn the lights on and off. After 2-week acclimation, the mice were randomly divided into three groups (each with two replications): the Ag-NP (2) and control (1) groups, each with nine mice. The animals were kept fasting overnight before treatment. The mice were examined daily for infections. Equal numbers of male and female mice were used in this study.
Preparation of Ag-NPs
Silver nanoparticles (Ag-NPs) were purchased from Nano Pars Co., Iran with a purity of 95 %. The mean diameter of Ag-NPs averaged 40 nm (and ranged from 35 to 45 nm), according to the manufacturer.
Experiment
Anesthesia for experimentation was achieved with an intramuscular injection of 10 ml ketamine, 0.5 ml acepromazine, 2 ml diazepam, and about 0.5 ml xylazine solution at a dose of 50 mg/kg. Therefore, once a day at the same time, a volume of 50 μl from the nanosilver solution (20 and 50 ppm) was administered orally at a given time. The untreated (control) group received distilled water without Ag-NPs. Each group of mouse was housed separately. The experiment lasted for 14 days. Samplings (n = 9) were conducted on days 2, 7, and 14. The blood was obtained directly from heart using heparinized tubes. The serum was obtained by centrifugation of the whole blood at 3000 rpm for 15 min. Then, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using an ion autoanalyzer. In addition, a hematological autoanalyzer measured total red blood cell (RBC) count, hematocrit (Hct), hemoglobin (Hb) concentration, total white blood cell (WBC) count, and percent differential leukocytes.
Livers were taken from the mice and prefixed in 10 % (v/v) neutral buffered formalin for histopathological examination. The fixed tissues were trimmed, dehydrated, embedded in paraffin, sectioned and mounted on glass slides, stained with H&E, and examined by light microscopy.
Statistical analyses were performed using Student’s paired t test, one-way ANOVA, and Duncan post hoc test. A p value of 0.05 was considered significant. The results showed the average value ± standard deviation.
Results
The hematological and serum biochemical findings are shown in Table 1. The values of RBC, Hb, and Hct did not vary significantly in the control and Ag-NP-treated animals (Table 1). However, the total number of WBC in Ag-NP groups increased as compared to the control with a significant decrease in monocytes. Serum AST level in male and female mice with 20 and 50 ppm of nanosilver showed statistical increase compared to control (Figs. 1 and 2). Except for day 2 in which ALT level in male mice (50 ppm of nanosilver) elevated remarkably to control, trend of ALT level with a 50-ppm nanosilver was decreasing but it was increasing with a 20-ppm. (Figs. 3 and 4). Level of ALT in female mice again was increasing (with a 20-ppm), but after a sharp fall at day 7, it was increasing at day 14 when using 50 ppm of nanosilver.
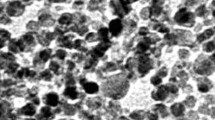
Histological studies of liver organ are seen in Fig. 5. There were vast damages to the liver tissue in both sexes which increased with time such as the necrosis, hepatocytic inflammation, and resultant aggregation of lymphocytes in liver tissue. In fact, all treated mice exposed to silver nanoparticle had minimal to moderate lymphocyte aggregation in hepatic area. More severe changes were observed in mice exposed to 50 ppm than to 20 ppm nanosilver. Histological findings (as pie diagrams) regarding the liver damage in both sexes and with two doses of Ag-NPs (20 and 50 ppm) are shown in Figs. 6, 7, 8, and 9. The histopathological examinations of the male and female mice livers revealed that while there was a dose-dependent deposition of silver nanoparticles, the effect of the silver nanoparticles on male was more prominent than female.
Histological changes in mice liver after oral administration of Ag-NPs. a Normal tissue. b–h Treatment groups. b Day 2, the hepatic cells with a generalized cytoplasmic granulation. c, Day 2, aquatic granular degeneration of hepatic cells. d Day 7, portal vein congestion with high blood cells. e Day 7, inflammation of hepatic sinusoids. f Day 14, cytoplasmic vacuolation of hepatocytes with necrosis. h Day 14, inflammation, necrosis, and degeneration of hepatic cells
Discussion
Nowadays, nanotechnology had rapid progress with the most effect on all parts of human, animal, and environmental and industrial life. The use of nanoparticles (NPs) in industrial and biomedical applications has increased significantly in recent years, yet their toxic effects have not been studied extensively [1, 3, 5, 17]. The results of the current study showed that silver nanoparticles was predominantly localized in liver in both sexes of mice, and this accumulation of nanoparticles in livers caused remarkable hepatic toxicity.
Many studies have demonstrated that exposure of silver nanoparticles may lead to clear accumulation in various organs including liver, as well as the kidneys, testes, lungs, and brain [4–8, 18]. For instance, accumulation of silver nanoparticles in the liver has been shown to induce hepatotoxicity in animal studies [18]. Studies have indicated that nanosilver has a strong toxicological effect in the range concentrations of 10–50 ppm (e.g., [19, 20]).Nanosilver enters the body through the skin, respiratory system and gastrointestinal tract. The most important way to contact it, especially in the gastrointestinal tract, is in colloidal form [13]. No consensus on the cytotoxicity of nanosilver has been reported; however, there is always reduced cell viability following exposure. As liver organ is able to actively remove compounds from the blood and transform them to chemical forms that can easily be excreted [9], so, silver nanoparticles might have impacted on the liver, as a major organ of detoxification. The hepatocytic inflammation in liver tissue of the current study is consistent with Lee et al. [18] study on rat liver following nanosilver administration, so that hepatocytes exhibited mild infiltration of inflammatory cells in portal vein area. Due to the accumulation of nanosilver in macrophages (Kupffer cells), they then concluded that Kupffer cells were involved in the process of inflammation following nanosilver exposure.
Hepatic function is evaluated by measuring AST and ALT. In the other words, liver damage induced by nanosilver particles of the present investigation physiologically affected AST (in particular) and also ALT in male and female. Increase serum AST and ALT levels indicated that liver tissues were damaged. These were confirmed by histological microscopy and by some other studies. For instance, in a histological analysis reported by Gatti et al. [14], inorganic particles, heterogeneous in nature but homogeneous in size, were identified in the liver. The results of our investigation are consistent with other studies (e.g., Cheraghi et al. [9] with nanosilver on these enzymes showing elevation of hepatic enzymes so that AST level in serum was elevated in male and female mice as compared to the control. On the other hand, the observed increase in ALT in the current study may be due to the free radicals released from the nanosilver particles when attacking hepatocytes and releasing ALT stored in them and entering into the blood serum [9–12]. Similarly, the increased level of WBC in the present study may follow phagocytosis of silver nanosilver [8, 9, 12]. The immune response of rats to an external factor has been the increase of the number of white blood cells for phagocytosis of nanosilver particles [9], whereas mice effects of nanosilver particles have been evaluated at different doses on serum. In this study, the level of ratio of WBC components changed which is in accordance with other studies. In fact, silver nanoparticles can lead changes in lymphocytes/granulocytes ratios so that the lymphocyte/granulocyte ratio may change sharply [15, 16]. Finally, while this study achieved a dose-dependent effect of the silver nanoparticles, the gender-related difference between the male and female mice livers has not been previously reported. Likewise, Kim et al. [7] found the gender-related distribution of silver nanoparticles in the rat kidneys (but not in the liver).
Conclusions
From the study, it was concluded that the oral exposure to silver nanoparticles produced changes in blood chemistry and hepatotoxicity as indicated by increased serum activity levels of both AST and ALT and histological damages to the liver with no significant changes between male and female mice.
References
The impact of silver nano particles on growth performance, lymphoid organs and oxidative stress indicators in broiler chicks. Global Veterinaria, 5: 366-370
Sawosza E, Bineka M, Grodzika M, Zieliñskaa M, Sysaa P, Szmidt M, Niemiec T, Chwalibog A (2007) Influence of hydrocolloidal silver nanoparticles on gastrointestinal microflora and morphology of enterocytes of quails. Arch Anim Nutr 61(6):444–451
Karimi M, Jeddi AND, Ahmadi F (2008) Evaluation of the effectiveness of different levels of silver nanoparticles on bursa of Fabricius development and on its histopathological lesions in broiler chicks. Acta Agraria Kaposvariensis 3(2):353–360
Mritunjai S, Singh S (2008) Nanotechnology in medicine and antibacterial effect of silver nano particles. J Nanomaterials Biostructures 3(3):115–122
Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK (2011) Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnology. 3;9:30
Park EJ, Bae E, Yi J, Kim Y, Choi K (2011) Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol 30:162–168
Kim S, Choi JE, Choi J, Chung K-H (2009) Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol in Vitro 23:1076–1084
Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, Schramel P, Heyder J (2001) Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect 4:547–551
Cheraghi J, Hosseini E, Hoshmandfar R (2013) In vivo effect of silver nanoparticles on serum ALT, AST and ALP activity in male and female mice. Adv Environ Biol 7(1):116–122
Woodrow Wilson International Center (2010) The Project on Emerging Nanotechnologies. Washington, USA. Available at: http://www.nanotechproject.org/inventories/consumer/analysis draft (accessed 23.06.10)
Farkas J, Christian P, Peter H, Roos N, Urrea JAG, Hassellِ VM, Tollefsen KE, Thomas KV, Hylland K (2011) Initial assessment of silver nanoparticles from washing machines. Environ Int 37(6):1057–1062
Griffitt RJ, Hyndman K, Denslow ND, Barber DS (2009) Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol Sci 107(2):404–415
Chang AL, Khosravi V, Egbert B (2006) A case of argyria after colloidal silver ingestion. J Cutan Pathol 33:809–811
Gatti AM, Montanari S, Monari E, Gambarelli A, Capitani F, Parisini B (2004) Detection of micro- and nano-sized biocompatible particles in the blood. J Mater Sci Mater Med 15:469–472
Tang J, Xiong L, Wang S (2009) Distribution, translocation and accumulation of silver nanoparticles in rats. J Nanosci Nanotechnol 9(8):4924–4932
Aniya Y, Koyama T, Miyagi C, Miyahira M, Inomata C, Kinoshita S, Ichiba T (2005) Free radical scavenging and hepatoprotective actions of the medicinal herb, Crassocephalum crepidioides from the Okinawa Islands. Biol Pharm Bull 28(1):19–23
Martínez-Gutierrez F, Thi EP, Silverman JM, de Oliveira CC, Svensson SL, Vanden HA et al (2012) Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomed 8(3):328–36
Lee TY, Liu MS, Huang LJ, Lue SI, Lin LC et al (2013) Bioenergetic failure correlates with autophagy and apoptosis in rat liver following silver nanoparticle intraperitoneal administration. Particle Fib Toxicol 10(40):1–13
Kvitek L, Vanickova M, Panacek A et al (2009) Initial Study on the Toxicity of Silver Nanoparticles (NPs) against Paramecium caudatum. J Phys Chem C 113(4296–4300):2009
Greulich C, Braun D, Peetsch A, Diendorf J et al (2012) The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv 2:6981–6987
Acknowledgments
While thanking Shahrekord University, the authors thank Mr Hatamei, Veterinary Section of Shahrekord University, for his technical assistance and Dr. Fattahian, a member of academic staff of Shahrekord University, for his sincere help in tissue identification and pathology.
Conflict of interests
The authors declare that they have no competing interests.
Authors’ contributions
MSH carried out the preliminary studies, established the methods, and drafted the manuscript. He performed experiments and drafted the manuscript. RJS and SA collected data, performed experiments, and provided substantial input in data interpretation and analysis. All authors gave final approval to the version to be published.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heydrnejad, M.S., Samani, R.J. & Aghaeivanda, S. Toxic Effects of Silver Nanoparticles on Liver and Some Hematological Parameters in Male and Female Mice (Mus musculus). Biol Trace Elem Res 165, 153–158 (2015). https://doi.org/10.1007/s12011-015-0247-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0247-1