Abstract
Zinc oxide (ZnO) and copper oxide (CuO) nanoparticles (NPs) are widely used in medicine and industrial fields. They have negative effects such as hematoxic, cytotoxic, and genotoxic on animals. This research aimed to investigate the blood physiological and biochemical responses induced by ZnO-NP and CuO-NP individually or in combination in male Swiss albino mice. For purpose, NPs were given to mice with 100 μl of water by oral gavage for 14 days. Three sublethal NP dose groups (1, 5, 25 mg/kg/day) and one control group (only received 100 μl of water) were used in the experiments and serum metabolite (glucose, total protein, total cholesterol, triglyceride, cortisol, blood urea nitrogen, immunoglobulin G, and M), ions (Na, K, Cl, Mg, and Ca), and enzyme (ALT, AST, ALP, and LDH) levels were measured. ZnO-, CuO-, and ZnO+CuO-NPs especially higher doses (5 and 25 mg/kg/day) decreased all serum metabolite (except blood urea nitrogen), ions, and ALP while these nanoparticles increased ALT, AST, LDH, and blood urea nitrogen. These increases/decreases in all serum parameters were generally higher in mice treated with the ZnO+CuO-NP mixture compared to the ZnO-NP and CuO-NP groups alone. The study shows that serum biochemistry profiles can be used as indicators to assess nanoparticle toxicity on lipid, protein, and energy metabolisms, immune and enzyme systems, ion regulation, and tissue functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, toxic effects of metal oxide nanoparticles on animal health and ecosystems are of increasing concern. The adverse effects of these nanoparticles on organisms have been the main focus of nanotoxicology researches [1,2,3]. The animals including mice exposed to nanoparticles have shown important toxic alterations including oxidative stress, apoptosis, and genotoxicity and hemotoxicity [4, 5]. Therefore, much more toxicological studies are required to estimate the potential hazards associated with exposure to nanoparticles [4]. Blood hemato-biochemical responses are major indicators of toxicity caused by metal oxide nanoparticles.
Most nanotechnology applications use metal oxide nanoparticles, which can be toxic to organisms. Zinc oxide and copper oxide nanoparticles are important metal oxide nanoparticles. Zinc oxide nanoparticles (ZnO-NPs), semiconductor metal oxide nanoparticles, are widely used in biomedical applications as an anticancer drug, and in cosmetic products. Depending on the antimicrobial and fungicidal properties of ZnO-NPs, they are also used in the food industry and agriculture [6]. Copper oxide nanoparticles (CuO-NPs) are increasingly used in industrial applications, or as consumer products, in medicine, and as pesticides [7]. ZnO-, CuO-, or Ag-NPs are frequently used as bactericides, and after releasing into environment, these nanoparticles can cause toxic effects on non-target organisms [8]. ZnO- and CuO-NPs have been shown to have negative effects on the survival and growth of organisms. Therefore, there is an urgent need to understand their toxicity to organisms and the environment through the processes of absorption, biodistribution, metabolism, and excretion of nanomaterials in vivo [9]. As a result of the wide use of ZnO- and CuO-NPs in different fields, it is necessary to investigate their toxicity, especially in mice.
Certain biological indicators are used to identify a regular connection between the interaction of toxic chemicals and their toxicological effects on animals [10]. Of these, blood serum biochemical parameters can be measured easily and can be used as useful markers to assess the health status of animals [2]. Therefore, studying animal blood may be a convenient way to reveal the toxic effects of nanoparticles. It is no surprise that many researchers have studied the effects of some metal oxide nanoparticles on blood biochemical and physiological parameters in various animals including mice [2, 11, 12]. Enzymatic or nonenzymatic indices in blood serum/plasma are also regularly used to evaluate the toxic effects of nanoparticles. Glucose, cortisol, total protein, ions, enzymes, etc. are important components of serum and are widely used as biochemical indicators for evaluating the adverse effects of toxic substances.
Mice have been used extensively as animal models for biomedical research in genetics, oncology, toxicology, and immunology as well as cell and developmental biology. These mammalians are an important model organism for humans and are used to evaluate the effects of toxicants or stressors. The physiological and biochemical parameters of the blood are important indicators of the general health status. This research aimed to investigate the blood physiological and biochemical responses induced by CuO-NP and ZnO-NP individually or in combination in male Swiss albino mice by measuring serum metabolites (glucose, total protein, total cholesterol, triglyceride, cortisol, blood urea nitrogen, immunoglobulin G and M), ions (Na, K, Cl, Mg, and Ca), and enzymes (ALT, AST, ALP, and LDH).
Materials and Methods
Experimental Animals and Treatment
Experimental animals (Male Swiss Albino Mice) were purchased from Cukurova University’s Experimental Animal Research Center and the animals were placed in stainless steel cages in a ventilated animal room. Control and experimental mice were kept under standard conditions at a temperature of 22 ± 2 °C and 50% moisture for 12 h in a light/dark cycle and provided standard mice food with water. Their adaptation to this environment was ensured for 7 days before dosing. The weight of the mice was found to be approximately 25–30 g. Sixty male mice were divided into 10 groups (N = 6 for each group), consisting of one control group and nine experimental groups. During the experiments, they were fed with standard mice food (Rat Food, DSA Agrifood Products Inc., Kırıkkale, Turkey). NPs were purchased from Sigma-Aldrich (Germany) companies. The properties of NPs are as follows; CuO (∼ 40 nm, > 99% purity, > 29 m2/g surface area, 6.48 g/cm3 density) and ZnO (∼ 40 nm, > 99% purity, > 10–25 m2/g surface area, 5.61 g/cm3 density). Scanning electron microscope images of NPs were taken to see the size of the metals and also to compare them with the size data obtained from the manufacturer. NP suspensions were vigorously mixed, and sonicated for 20 min prior to processing (Bandelin HD2200, Germany).
After strong sonication (Bandelin HD2200, Germany) for 20 min before the experiments, CuO-NP and ZnO-NP stock solutions were prepared in distilled water. NPs were given to mice with 100 μl of water by oral gavage. Three sublethal NP dose groups (1, 5, 25 mg/kg/day) and one control group (only received 100 μl of water) were used in the experiments. These nanoparticle doses were chosen as sublethal doses as they were not lethal [13, 14]. After 14 days, the mice were killed with high doses of anesthesia (ketasol 10%, Harson Lab., India) and carefully dissected using sterile equipment. Blood samples were taken from the heart using syringes and placed in tubes. Serum was obtained by centrifuging blood at 3000 rpm for 10 min.
Serum Biochemical Analysis
Analysis of serum biochemical parameters is carried out at the Central Laboratory of Cukurova University’s Medical Faculty Hospital (Adana, Turkey). Hormone levels and other serum parameters were analyzed on Beckman UniCel DXC 800 and DXI 800 Synchron (Beckman Coulter Inc., CA, USA) automatic analyzers. Beckman Coulter reagents were used to perform serum parameters on automated analyzers. It was used UV test technique for ALT, AST, and LDH activities [15] and colorimetric assay for ALP activity [16], electrochemiluminometric assay for cortisol levels [17], colorimetric test/UV test for levels of other serum parameters [15, 18], and ion-selective electrodes for ion levels [19]. Immunoglobulin (IgG, IgM) levels in serum were analyzed by nephelometric methods [20] using a Siemens BN II nephelometer.
Statistical Analysis
Data are presented as mean ± standard error. An SPSS statistical software (SPSS 17, Chicago, IL) was used to analyze the data. One-way ANOVA test was applied to compare the doses of metal oxide nanoparticles. The differences were considered significant at p < 0.05.
Results and Discussion
There was no mice mortality and these animals did not indicate any apparent health problem and abnormal behaviors after oral exposures of all tested metal oxide nanoparticles for 14 days. Changes in serum metabolites and ions in mice exposed with ZnO-NP and CuO-NP either individually or in combination are given in Tables 1 and 2.
Glucose and cortisol are stress metabolites and play important roles in stressful organisms, especially in energy metabolism. These parameter levels in the serum are affected by toxic substances. In this study after the 14-day administration period of ZnO-NPs and CuO-NPs, separately or in a mixture, serum glucose and cortisol levels indicated significant decreases relative to the control (Table 1). These declines at the highest dose of ZnO-, CuO-, and ZnO+CuO-NPs were found to be 38%, 33%, and 43% for glucose and 44%, 47%, and 63% for cortisol, respectively. Decreases in the glucose level show that metal oxide nanoparticles cause hypoglycemia. These findings are similar to the study conducted by Lotfi et al. [21] who concluded that the administration of Ag-NPs for 14 days caused a decrease in the serum glucose levels of rats and hypo-glycemic effects. Serum cortisol levels may be reduced by suppressing the synthesis or release of this hormone by these nanoparticles. Similarly, Zha et al. [22] found that chromium (III) nanoparticles significantly reduced serum cortisol levels in rats. It was reported that Al2O3-NPs decreased the glucose levels in the serum of rats while they did not cause any significant change in the cortisol level [2].
The total serum protein (TP) is synthesized in the liver and used as an indicator of getting information about the liver functions [12]. In the current work, the TP was significantly lowered by all tested nanoparticles (Table 1). Significant reductions in TP levels were observed with the treatments of ZnO-NPs (59%) and CuO-NPs (30%), while a marginally significant reduction (71%) in this parameter was noted in mice exposed to ZnO+CuO-NPs. The decline in the TP level might be due to reduced protein synthesis and/or increased protein degradation as a result of nanoparticle toxicity. The Wistar rats dosed with 50 mg/kg of TiO2-NP have shown similar decreasing trends in the serum TP levels [23]. Also, previous results have indicated that stress caused by nanoparticles might decrease the TP levels in serum of rats [24].
The levels of serum total cholesterol and triglyceride were determined for evaluating effects of nanoparticles on lipid metabolism. Cholesterol is an important component of the cell membrane. Triglycerides are the main constituents of body fat in humans and other vertebrates. In the present study, the total cholesterol levels were significantly lowered by ZnO- and ZnO+CuO-NPs while the triglyceride levels were depleted in exposures of all tested nanoparticle groups relative to the control group (Table 1). Decreasing the cholesterol levels indicates that nanoparticles cause hypocholesterolemia. Reduced serum triglyceride level may be a result of direct or indirect toxic effects of nanoparticles on this metabolite. Similarly, Canli et al. [2] reported that serum triglyceride levels of rats were significantly lowered by the highest dose (50 mg/kg) of Al2O3- and CuO-NPs. In another study, Lee et al. [25] found decreased cholesterol levels in the serum of rats following exposure to a 28-day oral dose of 400 mg/kg/CuO-NPs.
Blood urea nitrogen (BUN) levels were measured to determine whether the nanoparticles caused kidney damage in mice. The BUN level is an important serum parameter that provides information about renal functions. In the present research, the highest dose (25 mg/kg) of all tested nanoparticles, especially ZnO-NP, induced significant elevations in serum BUN levels (Table 1). These increases were found to be 38%, 18%, and 22% for ZnO-, CuO-, and ZnO+CuO-NPs, respectively. The elevated serum BUN levels in response to metal oxide nanoparticles may show that these nanoparticles have nephrotoxicity. The results found by Wang et al. [26] revealed that titanium dioxide particles in mice after oral administration for 2 weeks caused a significant increase of serum BUN levels and that elevated BUN levels may indicate the important pathological change of kidneys associated with nanoparticles stress. In agreement with the present study, Vasantharaja et al. [23] found a similar increase in the serum BUN levels, showing the renal damage in the TiO2-NP-treated adult male Wistar rats.
The serum immunoglobulin G (IgG) and M (IgM) levels were measured to determine the effects of metal oxide nanoparticles on immune systems. It was observed that the mice dosed with ZnO-, CuO-, and ZnO+CuO-NPs indicated significant declines in levels of IgG and IgM and marginally significant decreases in these parameters were at the highest dose of all tested nanoparticles (Table 1). Decreases in the immunoglobulin levels in 25 mg/kg dose of ZnO-, CuO-, and ZnO+CuO-NPs were found as 39%, 43%, and 59% for IgG and 48%, 69%, and 64% for IgM, respectively. Our study shows that metal oxide nanoparticles suppress the immune system by reducing the level of immunoglobulins under their effect alone or together. These results indicate that metal oxide nanoparticles under their effect alone or mixtures suppress the immune system and have an immunotoxic effect by reducing the levels of immunoglobulins. Similar to the presented study results, Vandebriel et al. [27] found that Ag-NP administration in male Wistar rats for 28 days induced suppression of the functional immune system. The significant decreases were also determined in serum IgM levels of Wistar albino Sprague-Dawley rats following oral administration of CuO-NPs [2].
In the current study, treatment of CuO-NPs did not cause any significant alterations in serum Na and Ca levels while they declined K, Cl, and Mg levels (Table 2). However ZnO- and ZnO+CuO-NPs caused significant decreases in all analyzed serum ions levels. The decreases in the serum ions levels at the highest dose of ZnO-, CuO-, and ZnO+CuO-NPs were found to be 16%, 36%, and 47% for Cl and 41%, 40%, and 46% for Mg, respectively. Serum ion levels in animals play important roles in maintenance of homeostasis. In the present study, it was determined that metal oxide nanoparticles caused ionic disturbances by decreasing serum ion levels in mice. Alterations in the serum ion balance may be a consequence of the action of nanoparticles on organs involved in ion uptake and excretion, on the endocrine system or on active transport processes. Mangalampalli et al. [4] found significant decreases in serum Ca levels in rat in response to MgO-NP exposure. In another similar study, oral administration of rats to different doses (100, 1000, and 5000 mg/kg) of AgNPs for 7, 14, and 21 days caused a decline in levels of serum ions, especially Na [28]. Andjelkovic et al. [29] also reported that acute exposure to the Cd and Pb, more traditional and common pollutants, administered alone or in mixture form resulted in the decreased serum Ca and Mg levels of Wistar rats.
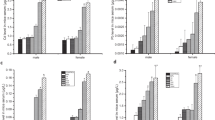
In oral exposures of ZnO-, CuO-, and ZnO+CuO-NPs, significant changes were statistically determined in serum enzyme activities of mice due to nanoparticle doses (Figs. 1, 2, 3, and 4). All tested nanoparticles increased activities of ALT, AST, and LDH while they declined ALP activity. Elevations in the enzyme activities in serum of mice treated with 25 mg/kg dose of ZnO-, CuO-, and ZnO+CuO-NPs were found to be 64%, 52%, and 39% for ALT, 91%, 142%, and 35% for AST, and 14%, 14%, and 17% for LDH, respectively. Decreases in the ALP activity in 25 mg/kg dose of ZnO-, CuO-, and ZnO+CuO-NPs were determined as 58%, 27%, and 77%, respectively.
Serum enzyme (ALT, AST, ALP, and LDH) activities are frequently used as a standard measure of hepatotoxicity in evaluating the effects of chemicals including nanoparticles on the liver tissues of animals. The presence of these enzymes in the serum in significant levels indicates tissue damage. In the present research, the changes in ALT, AST, ALP, and LDH activities were investigated to determine functional status of the liver upon oral administration of metal oxide nanoparticles. The elevations in the activities of serum ALT, AST, and LDH may be a result of liver tissue damage caused by ZnO-, CuO- and ZnO+CuO-NPs. These enzymes originate from the liver and are intracellular enzymes. Therefore, there is no significant change in serum levels unless the cells are damaged. In our study, it is predicted that enzyme activities increase due to the leakage of these enzymes into the blood tissue as a result of cellular damage caused by metal oxide nanoparticles in the liver tissue. Similar increasing trends in serum enzyme activities were also observed by Mangalampalli et al. [4] for female albino Wistar rats after magnesium oxide nanoparticle treatments. The researches reported that the acute doses of magnesium oxide nanoparticles induced significant elevations in the rat serum AST and ALT activity as a conclusion of nanoparticle toxicity on liver tissue damage. Similar to our study findings, Garcia et al. [12] observed that oral administration of different doses of silver nanoparticles in rats caused a decline in serum ALP activity. The researchers found that the administration of 200 mg/kg silver nanoparticles resulted in an approximately 25% reduction in ALP enzyme activity compared to the control group.
Conclusion
The current research has shown that serum physiological and biochemical parameters were significantly affected by metal oxide nanoparticles investigated. The 14-day administration period of ZnO-NP and CuO, separately or in a mixture, lowered serum glucose, total protein, total cholesterol, triglyceride, cortisol, IgG, IgM, Na, K, Cl, Mg, Ca, and ALP and raised BUN, ALT, AST, and ALP. These increases/decreases in all serum parameters were generally higher in mice treated with the CuO+ZnO-NP mixture compared to the CuO-NP and ZnO-NP groups alone. The study shows that serum biochemistry profiles can be used as indicators to assess nanoparticle toxicity on lipid, protein, and energy metabolisms, immune and enzyme systems, ion regulation, and tissue functions.
Data Availability Statement
Raw data are available upon request.
References
Patlolla AK, Hackett D, Tchounwou PB (2015) Silver nanoparticle-induced oxidative stress-dependent toxicity in Sprague-Dawley rats. Mol Cell Biochem 399:257–268. https://doi.org/10.1007/s11010-014-2252-7
Canli EG, Atli G, Canli M (2017) Response of the antioxidant enzymes of the erythrocyte and alterations in the serum biomarkers in rats following oral administration of nanoparticles. Environ Toxicol Pharmacol 50:145–150. https://doi.org/10.1016/j.etap.2017.02.007
Fırat O, Bozat RC (2019) Assessment of biochemical and toxic responses induced by titanium dioxide nanoparticles in Nile tilapia Oreochromis niloticus. Hum Ecol Risk Assess 25(6):1438–1447. https://doi.org/10.1080/10807039.2018.1465338
Mangalampalli B, Dumala N, Grover P (2017) Acute oral toxicity study of magnesium oxide nanoparticles and microparticles in female albino Wistar rats. Regul Toxicol Pharmacol 90:170–184. https://doi.org/10.1016/j.yrtph.2017.09.005
Canli EG, Ila HB, Canli M (2019) Response of the antioxidant enzymes of rats following oral administration of metal-oxide nanoparticles (Al2O3, CuO, TiO2). Environ Sci Pollut Res 26:938–945. https://doi.org/10.1007/s11356-018-3592-8
Abbasalipourkabir R, Moradi H, Zarei S, Asadi S, Salehzadeh A, Ghafourikhosroshahi A, Mortazavi M, Ziamajidi N (2015) Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food Chem Toxicol 84:154–160. https://doi.org/10.1016/j.fct.2015.08.019
Kiaune L, Singhasemanon N (2011) Pesticidal copper (I) oxide: environmental fate and aquatic toxicity. Rev Environ Contam Toxicol 213:1–26. https://doi.org/10.1007/978-1-4419-9860-6-1
Exbrayat J-M, Moudilou EN, Lapied E (2015) Harmful effects of nanoparticles on animals. J Nanotech 8:1–10. https://doi.org/10.1155/2015/861092
Chang Y-N, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5:2850–2871. https://doi.org/10.3390/ma5122850
Gomes T, Pinheiro JP, Cancio I, Pereira CG, Cardoso C, Bebianno MJ (2011) Effects of copper nanoparticles exposure in the mussel Mytilus galloprovincialis. Environ Sci Technol 45(21):9356–9362. https://doi.org/10.1021/es200955s
Heydrnejad MS, Samani RJ, Aghaeivanda S (2015) Toxic effects of silver nanoparticles on liver and some hematological parameters in male and female mice (Mus musculus). Biol Trace Elem Res 165:153–158. https://doi.org/10.1007/s12011-015-0247-1
Garcia T, Lafuente D, Blanco J, Sanchez DJ, Sirvent JJ, Domingo JL, Gomez M (2016) Oral subchronic exposure to silver nanoparticles in rats. Food Chem Toxicol 92:177–187. https://doi.org/10.1016/j.fct.2016.04.010
Xu M, Fujita D, Kajiwara S, Minowa T, Li X, Takemura T, Iwai H, Hanagata N (2010) Contribution of physicochemical characteristics of nano-oxides to cytotoxicity. Biomaterials 31:8022–8031. https://doi.org/10.1016/j.biomaterials.2010.06.022
Jarrar Y, Al-Doaiss A, Alfaifi M, Shati A, Al-Kahtani M, Jarrar B (2020) The influence of five metallic nanoparticles on the expression of major drugmetabolizing enzyme genes with correlation of inflammation in mouse livers. Environ Toxicol Pharmacol 80:103449. https://doi.org/10.1016/j.etap.2020.103449
Bergmeyer HU, Horder M, Rej R (1985) International federation of clinical chemistry (IFCC) scientific committee. J Clin Chem Clin Biochem 24:481–495
Empfehlungen D (1972) Deutschen Gesselschaft für Klinische Chemie. Z Klin Chem Klin Biochem 10:182–192
Chiu SK, Collier CP, Clark AF, Wynn-Edwards KE (2003) Salivary cortisol on ROCHE Elecsys immunoassay system: pilot biological variation studies. Clin Biochem 36:211–214. https://doi.org/10.1016/S0009-9120(02)00471-X
Schmidt FH (1961) Enzymatic determination of glucose and fructose simultaneously. Klin Wochenschr 39:1244–1250
Tietz NW, Logan NM (1987) Fundamentals of clinical chemistry. WB Saunders, Philadelphia, PA, USA
Winter WE, Hardt NS, Fuhrman S (2000) Immunoglobulin E: importance inparasitic infections and hypersensitivity responses. Clin Chem Lab Med 124:1382–1385
Lotfi A, Aghdam EG and Narimani-Rad M (2017) Effect of chemically-synthesized silver nanoparticles (Ag-NP) on glycemic and lipidemic status in rat model. In: Badnjevic A. (eds) CMBEBIH 2017. IFMBE Proceedings, vol 62. Springer, Singapore https://doi.org/10.1007/978-981-10-4166-2-25
Zha L, Zeng J, Sun S, Deng H, Luo H, Li W (2009) Chromium (III) nanoparticles affect hormone and İmmune responses in heat-stressed rats. Biol Trace Elem Res 129:157–169. https://doi.org/10.1007/s12011-008-8282-9
Vasantharaja D, Ramalingam V, Aadinaath Reddy G (2015) Oral toxic exposure of titanium dioxide nanoparticles on serum biochemical changes in adult male Wistar rats. Nanomed J 2:46–53. https://doi.org/10.7508/NMJ.2015.01.005
Adeyemi OS, Adewumi I (2014) Biochemical evaluation of silver nanoparticles in Wistar rats. Int Scholarly Res Not 8:1–8. https://doi.org/10.1155/2014/196091
Lee I-C, Ko J-W, Park S-H, Shin N-R Shin I-S, Moon C, Kim J-H, Kim H-C, Kim J-C (2016) Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Part Fibre Toxicol 13:56–71. https://doi.org/10.1186/s12989-016-0169-x
Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168:176–185. https://doi.org/10.1016/j.toxlet.2006.12.001
Vandebriel RJ, Tonk ECM, Fonteyne-Blankestijn LJ, Gremmer ER, Verharen HW, van der Ven LT, van Loveren H, Jong WH (2014) Immunotoxicity of silver nanoparticles in an intravenous 28-day repeated-dose toxicity study in rats. Part Fibre Toxicol 11:21–28
Adeyemi OS, Adewumi I, Faniyan TO (2015) Silver nanoparticles influenced rat serum metabolites and tissue morphology. J Basic Clin Physiol Pharmacol 26(4):355–361. https://doi.org/10.1515/jbcpp-2013-0092
Andjelkovic M, Djordjevic AB, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019) Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16:1–21. https://doi.org/10.3390/ijerph16020274
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kargin, D. Changes in Serum Physiological and Biochemical Parameters of Male Swiss Albino Mice After Oral Administration of Metal Oxide Nanoparticles (ZnO, CuO, and ZnO+CuO). Biol Trace Elem Res 199, 4218–4224 (2021). https://doi.org/10.1007/s12011-020-02560-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02560-7