Abstract
In order to identify the effects of soil properties on the transfer of Cd from soil to wheat under actual field conditions, 126 pairs of topsoil and wheat samples were collected from the Yangtze River delta region, China. Relevant parameters (Cd, Ca, Mg, Fe, Mn, Zn, N, P, K, S, pH, total organic carbon, and speciation of soil Cd) in soil and wheat tissues were analyzed, and the results were treated by statistical methods. Soil samples (19.8%) and 14.3% of the wheat grain samples exceeded the relevant maximum permissible Cd concentrations in China for agricultural soil and wheat grain, respectively. The major speciations of Cd in soil were exchangeable, bound to carbonates and fulvic and humic acid fraction, and they were readily affected by soil pH, total Ca, Mg, S and P, DTPA-Fe, Ex-Ca, and Ex-Mg. Cadmium showed a strong correlation with Fe, S, and P present in the grain and the soil, whereas there was no significant correlation in the straw or root. Generally, soil pH, Ca, Mg, Mn, P, and slowly available K restricted Cd transfer from soil to wheat, whereas soil S, N, Zn, DTPA-Fe, and total organic carbon enhance Cd uptake by wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of cadmium in an environment is of great concern as it is toxic to both crops and human health [1]. Cadmium present in the agricultural food chain is mainly imported from topsoil through its uptake by plants. With global economic development, many heavy metals have now entered soil–crop systems as a result of anthropogenic activities, such as industrial emissions, overuse of chemical fertilizers, and wastewater irrigation [2, 3].
Cadmium uptake by plants and its transfer to grain are influenced by soil properties, plant species, and crop management practices [4, 5]. Some publications report that soil pH, clay mineral content, salinity, organic matter content, and other soil properties, such as the presence of Zn, N, P, and Fe–Mn oxides, can affect Cd bioavailability and its transfer in soil–plants [6–9]. It is recognized that the effect of soil parameters on Cd transfer in the soil–wheat system is very complex. Many studies showed that soil pH was the most important factor [10–12]. However, some studies point out that organic matter is a more significant factor than soil pH [13, 14]. Besides their different effects on Cd transfer of soil properties, even the same soil parameter may affect Cd transfer differently under different conditions [15].
Earlier researchers studied the relationship between soil properties and Cd accumulation in cereals under field conditions and reported regression models predicting wheat grain Cd concentrations in relation to soil properties [11, 12, 16–19]. However, due to the complexity of soil–plant relationships and the heterogeneity of a natural agricultural system, the effect of soil properties on Cd transfer from soil to plant is not well understood, especially over a wide area under actual field conditions. Furthermore, the number of sampling sites or soil parameters in the studies mentioned above was relatively small and their study objects were mainly wheat grain and soil but few included speciation of Cd in coexisting wheat root and straw.
The Yangtze River delta region is one of the most famous alluvial deltas in the world. As a cradle of eastern civilization, agriculture is an important human activity, and rice (Oryza sativa L.) and wheat (Triticum aestivum L.) have been the most prominent crops. After the 1980s, economic development in this area (especially in the south) accelerated, and this had a great impact on the agricultural environment. The highly developed industrial area in the South Yangtze River delta and the traditional agricultural area in the north comprise a typical industry–agriculture transition area in the river delta region. The transfer and accumulation of heavy metals in soil and wheat here have practical significance for understanding global soil–plant relationships. Some reports have been published concerning Cd concentrations (contamination) in soil and crops in this region, but most previous work focused on special field areas, and the crops studied were mainly rice and vegetables [3, 20, 21]. There have been few investigations of Cd pollution and its transfer from soil to wheat under real field conditions. Therefore, a regional-scale survey was performed to study Cd contamination and the effects of soil properties on Cd transfer from soil to wheat under actual field conditions.
Materials and Methods
Study Area and Sampling
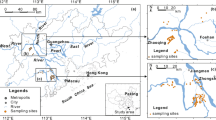
The study area is located between 30°00′ N–33°20′ N and 119°10′ E–121°40′ E in East China. According to Chinese soil taxonomy, anthrosol is the major soil order in this area.
A total of 126 pairs of topsoil and wheat (T. aestivum L.) samples (42 paired root and straw samples) at the same locations were collected from the study area shortly before harvesting at sites located with the aid of a Global Positioning System (Fig. 1). At each site, five topsoil (depth, 0–20 cm) subsamples were gathered in a 200-m2 area using a stainless steel trowel and mixed in a cloth bag. At the same time, five corresponding wheat plant subsamples (the sampling area of a subsample is 5 × 5 m) were collected and combined into a single composite sample, then separated into root, straw, and grain samples. The soil samples were air-dried and sieved through a 2-mm polyethylene sieve to remove larger debris, stones, and pebbles. Soil pH was measured on the unground sample first. After determining their pH, soil samples were ground to fine particles (<0.074 mm) for chemical analysis. The plant samples were carefully rinsed with deionized water, initially air-dried at room temperature, and ground into fine particles (<0.074 mm). The powdered samples of plant tissues were oven-dried at 60°C for 48 h before chemical analysis.
Chemical Analysis
Soil pH was determined in a paste with a ratio of 1:2.5 of unground soil sample to water using a pH meter (Model PHS-3C, Shanghai Precision and Scientific Instrument Co. Ltd., China) [22]. The total concentrations of Ca, Mg, K, Fe, Na, S, and P in soil samples were determined by powder X-ray fluorescence (ZSX primus II, Rigaku Japan, Osaka, Japan). The soil samples were first digested by HCl–HNO3–HClO4–HF, then Zn and Mn concentrations were determined by inductively coupled plasma–optical emission spectrometer (ICP-OES, Thermo Element iCAP6000 (Radial), Cambridge, BZ, UK). Cadmium concentrations were determined on the same digestions with an inductively coupled plasma–mass spectrometer (ICP-MS, Thermo Element X Series 2, Bremen, Germany). Total organic carbon (TOC) was determined by dichromate oxidation using a modified Tyurin method [23]. Total soil N was determined by the Kjeldahl method after digestion with H2SO4 [24].
Soil available Fe and available Zn were extracted using the diethylenetriamine penta-acetic acid (DTPA) method and analyzed by ICP-OES [25]. Soil available P was extracted using NH4F–HCl or NaHCO3 (based on pH) [26] and analyzed by ICP-OES. Soil available K was extracted with 1 M NH4OAc and concentrations measured by ICP-OES [27]. The concentration of slowly available K (the reservoir of available K, mainly comprises K in the clay fraction and mica [22]) was calculated from the amount of K extracted with 1 M hot HNO3 (i.e., HNO3–extractable K concentration minus available K concentration) [27], with HNO3–extractable K concentrations measured by ICP-OES. Soil ammonium nitrogen (NH4–N) was extracted with 1 M KCl and its concentration determined as per Bao [27]. Soil exchangeable Ca (Ex-Ca) and exchangeable Mg (Ex-Mg) were measured using the ammonium acetate method [28]. The six-step sequential extraction of soil Cd used for this study is described in Table 1. The extraction method was mainly modified from the methods of Tessier et al. (steps a, b, d, e, and f) and Donisa et al. (step c) [29, 30]. Soil phytoaccessible Cd (Phyto-Cd) generally comprises exchangeable, bound to carbonates and organic acid fractions Cd [31], so the present study estimates phyto-Cd by Σ (exchangeable Cd, bound to carbonates Cd, fulvic and humic acid fraction Cd).
To determine the concentrations of elements in wheat tissues, samples were digested using HNO3 and H2O2. Cadmium was determined by ICP-MS. The concentrations of S, P, K, Ca, Mg, Fe, Zn, and Mn were measured by ICP-OES. Measurement of N used the Kjeldahl method [24].
The accuracy of determinations was verified using the Chinese standardized reference materials GBW07402 and GBW07406 for soil samples and GBW10011 and GBW10014 for wheat samples; these standardized materials were purchased from National Institute of Metrology People’s Republic of China (18 E. North Third Ring Road, Beijing, 100013, China). The analytical quality control showed good precision throughout. The relative standard deviation (RSD) for soil Cd is 0.3–2.7%, for soil TOC, N, and S are <5.0%, and for other soil parameters is <3.0%, whereas RSDs for Cd present in wheat is 0.3–3.8%, and other elements in wheat is <5.0%.
Statistical Analysis
Data were statistically analyzed by SPSS version 16 program (SPSS Inc., USA). Spearman correlation coefficients were calculated to determine the relationship between soil parameters and wheat tissues.
Results
Soil Cd Concentration and Soil Properties
The soil properties in 126 soil samples and total soil Cd, TOC, and pH are shown in Fig. 2. The mean value of total soil Cd (0.215 μg g−1) apparently exceeds the local background value (mean = 0.116 μg g−1, range = 0.02–0.26 μg g−1, SD = 1.63, n = 157) [32], implying that the higher concentration of Cd in soil may derive from external sources such as industrial emissions, auto exhaust, and farming practices. Compared with soil environmental quality standards in China, 19.8% of the soil samples exceed the maximum allowable concentration of Cd in agricultural soil (0.3 μg g−1) [33]. Soil pH ranged from 4.80 to 8.28, i.e., strongly acid to mildly alkaline. Generally, the topsoils in the south Yangtze River delta region have lower acidity. This location-dependent soil acidity may be related to the soil parent materials and acidification, which was accelerated by the exterior sources such as acid rain and overuse of fertilizers [34, 35]. The wide range of soil pH and concentrations of Ca, Mg, N, P, etc. were suitable for studying the effect of soil properties on Cd uptake by wheat plants under actual field conditions. Compared with previous publications [11, 16, 18, 19, 36, 37], the total concentrations of soil Cd, TOC, and pH values in the present study generally lie in the worldwide range for corresponding soil parameter in normal agro-topsoil (Fig. 2, displays b–d).
Soil properties (n = 126) in the Yangtze River delta region and total concentrations of soil Cd, TOC, and pH values in different regions. A Concentrations of soil parameters in our study (SA-K slowly available K; the purple, red, and green bars on the columns indicate the maximum, mean, and minimum values, respectively). B–D Comparisons of total soil Cd, pH, and TOC between in the present study (blue columns and marked “a” at the bottom of columns) with those in previous publications (Norvell et al. study “b” [19], Eriksson and Söderström study “c” [11], Garrett study “d” [37], Nan et al. study “e” [18], Adams et al. study “f” [16], Chaudri et al. study “g” [36], respectively). As total Cd was not analyzed by Norvell et al. [19], the total Cd concentrations in the reports of it (B, b) is the concentrations of DTPA-Cd. Similarly, TOC was not analyzed by Adams et al., Eriksson and Söderström, and Nan et al. [11, 16, 18], so TOC concentrations in the reports of them (D, c–f) are the concentrations of total organic matter. The units of total Fe, Ca, Mg, Mn, K, P, S, and N, TOC, available K, and SA-K are g kg−1, of total Zn, total Cd, DTPA-Fe, DTPA-Zn, Available P and NH +4 –N are μg g−1, of Ex-Ca is cmol (1/2 Ca2+) kg−1, and of Ex-Mg is cmol (1/2 Mg2+) kg−1, respectively
Chemical Composition (Speciation) of Cd Under Sequential Extraction
Figure 3 shows the concentrations of Cd in each soil fraction presented in decreasing concentration of total Cd. The mean value of percentage recovery was 97% (ranging from 84 to 105%). The average concentrations of Cd in the different fractions were generally in the order of exchangeable (Ex-) > bound to carbonates (Car-) > fulvic and humic acid (HF-) > bound to Fe–Mn oxyhydroxides (Fe–Mn-) > bound to organic matter (OM-) and > residual (Res-). The major species of Cd were Ex-, Car-, and HF-Cd, and their sum accounted for about 60% of the total soil Cd.
Table 2 correlates the different fractions of Cd and soil properties. Each Cd fraction has a significant correlation with total soil Cd, S, and TOC. All the Cd fractions, except Ex-Cd and Res-Cd, show positive correlations with total soil N. Car and HF fractions of Cd have significant positive correlations with pH, total Ca, Mg, S, and P but have negative correlations with DTPA-Fe, Ex-Ca, and Ex-Mg. Each Cd fraction has a weak correlation with total soil Fe, available P, available K, slowly available K, and NH4–N. These results and correlations indicate that Ex-, Car-, and HF-Cd are the predominant fractions that affect the bioavailability of Cd and that they are susceptible to changes in soil properties, such as soil pH, total Ca, Mg, S, and P, DTPA-Fe, Ex-Ca, and Ex-Mg.
Cadmium and Some Relevant Elements in Wheat Grain, Straw, and Root
The concentrations of Cd and some relevant elements are presented in Table 3. The maximum permissible concentrations (MPC) for Cd in wheat grain are not consistent in different countries. The European Union set the MPC for Cd in wheat grain of 0.235 μg g−1 dry weight in 2001 [38], whereas Australia and New Zealand set it at 0.1 μg g−1 dry weight [39]. In China, the MPC is in agreement with Australia and New Zealand [40]. For safety for the consumer, the China-MPC value (0.1 μg g−1) is adopted in the present study. This means that 14.3% of the wheat grain samples exceeded this MPC, and Cd in wheat grain may be hazardous to consumers. Compared with studies on other regions, the concentrations of grain Cd in the present study are closest to those reported by Eriksson and Söderström and Gawalko et al. (the samples were also randomly gathered in the actual agricultural topsoil in both studies) [11, 41]. In our study, the mean concentration of Cd in root was higher than in both the straw and grain, whereas the CVs of Cd in different wheat tissues increased in the order of root < straw < grain. These results indicate that Cd concentrated preferentially in the root but had a wider variation in grain. Similarly, the mean concentration of Fe in different wheat tissues was in the order of grain < root < straw, whereas S, P, and Zn were in the order of root < straw < grain. Correlation analysis showed that grain Cd exhibited a significant correlation with grain Fe, S, and P; straw Cd had no significant correlation with straw Fe, S, and P; root Cd showed no significant correlation with root Fe, S, P, Ca, and Mg. In addition, soil Cd showed a positive correlation with soil S and P. These correlations imply that the major speciation of Cd in matured wheat grain and soil may closely correlate with Fe, S, and P. In addition, the close correlation between Cd and P may also indicate the presence of Cd in the P fertilizers applied. As a result of the overuse of fertilizers in the study area in the past, the total soil P contents range from 0.426 to 2.172 g kg−1 and so are generally above its the average concentration in Chinese topsoil (0.2–1.1 g kg−1) [42].
Correlations Between Cd and Other Parameters
Correlation analysis was performed to assess the effect of pH, TOC, N, P, S, K, Ca, Mg, Fe, and Mn on Cd concentrations in wheat (Table 4). Grain Cd correlated significantly with soil S, N, TOC, DTPA-Fe, DTPA-Zn, total Zn, soil total Cd, and phyto-Cd but correlated negatively with soil pH, Ca, Mg, Mn, P, and slowly available K. Meanwhile, correlations between root Cd and soil parameters are similar to that between grain Cd and soil parameters, although most are a little weaker. In general, the absolute values of the correlation coefficients between soil parameters and root Cd are generally 0.01–0.17 lower than those with grain Cd. This slight difference might be related to the intrinsic characteristic of wheat tissues and the smaller number of root samples. In addition, straw Cd shows a negative correlation with root Ca, Mg, Fe, and P, but a weak correlation with root S, indicating that root Ca, Mg, Fe, and P restrains Cd translocation from root to straw. This restraint by Ca, Mg, and Fe on Cd transfer may result from the synergism of the cations in the transfer process [9].
Discussion
Effects of Soil pH and TOC on Cd Transfer from Soil to Wheat
Both grain and root Cd show significant negative correlations with soil pH in our study. It is generally known that decreasing soil pH can enhance Cd uptake by plants, and some studies even report that soil pH was the most important factor in the uptake of heavy metals by plants [43, 44].
However, the effect of organic matter (OM) on Cd bioavailability and its transfer is more perplexing than pH. Soil OM affects the transfer of heavy metals in soils in two ways. Firstly, OM could reduce the bioavailability of heavy metals in soils by their adsorption or forming stable complexes with humic substances [17, 45]. Antoniadis et al. [46] reported enhanced heavy metal adsorption onto soil constituents with increasing OM contents in soils. Secondly, OM can affect soil pH [47], and it is also involved in supplying organic chemicals to the soil solution, which may serve as chelates and increase the bioavailability of metal to plants [48, 49].
Soil TOC correlates negatively with pH in our study area (r 2 = −0.205*), as this is mainly reflected in the reduction of OM. This also implies that the major reaction of OM in the topsoil may be the production of organic acid from OM decomposition and nitrification [47]. The dissolved organic acid could enhance the uptake of heavy metals by plants [48, 49]. In this case, soil OM should mainly contribute to Cd uptake by plants. In our study, as powerful proof, soil TOC correlated positively with both grain Cd and soil Cd. This indicates that the effect of soil OM in enhancing Cd bioavailability hid the effect of restraining its mobility in the soil–wheat system.
Effects of Ca, Mg, Mn, Fe, and Zn on Cd Transfer from Soil to Wheat
The effects of soil Ca and Mg on Cd uptake by plants may have two direct impacts and an indirect one. Firstly, the direct impact is to increase Cd2+ concentrations in soil solutions and enhance Cd bioavailability by desorption. Higher exchangeable Ca concentrations can increase the exchangeable Cd concentrations by reducing its sorption on soil, as there is competition between Ca2+ and Cd2+ in soil [47]. This desorption is intensified in acid soil, especially [50]. Secondly, the direct impact is to restrain Cd transfer from soil to plant. It is reported that there is a competition of Ca2+ with Cd2+ in uptake by plants and transfer in the cell plasma membrane [51, 52].
In our study, straw Cd shows a negative correlation with root Ca and Mg, reflecting that transfer of Cd was restrained by Ca and Mg and via a Ca pathway from wheat root to straw. However, soil Ex-Ca and Ex-Mg showed no significant correlations with either root Cd or grain Cd. The effect of Ca2+ and Mg2+ on Cd translocation from soil to root is more complex than that from root to straw. Besides, the suppression of the transfer of Cd, Ex-Ca, and Ex-Mg could induce desorption of Cd in soil and enhance its mobility and solubility.
However, the effect of soil Ex-Ca and Ex-Mg on Cd transfer can never be a surrogate for the effect of total soil Ca and total soil Mg. Either may indirectly influence Cd precipitation and its sorption by carbonates, which are hosts of Ca and Mg in soils. In our study, grain Cd and root Cd correlated negatively with total soil Ca and Mg, and soil Cd showed a positive correlation with soil Ca and Mg. These correlations indicate that Ca and Mg could retain Cd in soil and restrain its translocation from soil to wheat. Gil et al. [53] studied the relationship between Cd and carbonates and confirmed that Cd has the highest affinity for the fraction associated with carbonates in calcareous soils. Carbonate could coprecipitate with Cd present in a CdCO3 form [53], as it has the ability to bind Cd via sorption and occlusion, and thus increase soil pH [54]. Carbonates are the major mineral form of Ca in topsoils in our study area [55]. As one of the major Cd fractions in soil, Car-Cd in our study shows a significant positive correlation with total soil Ca and Mg. These phenomena suggest that the restraint by total soil Ca and Mg on Cd transfer is related to the deposition and sorption of Cd-containing carbonate.
Both grain Cd and root Cd show negative correlation with soil Mn, indicating that Mn restrained the uptake of Cd by wheat. Previous research showed that Fe and Mn oxyhydroxides could retain Cd in soil by sorption [12]. However, there is no significant correlation between total soil Fe and grain Cd or root Cd in our study; this may be because of speciation of Fe, but its effects are more complex than Mn in soils [56]. However, soil DTPA-Fe showed a significant positive correlation with grain Cd and root Cd but a negative correlation with soil Cd, indicating that soil DTPA-Fe enhanced Cd bioavailability and transfer from soil to wheat.
Grain Cd and root Cd show positive correlations with soil DTPA-Zn and total soil Zn in our study, indicating that soil Zn enhances Cd uptake by wheat. As Cd2+ can be taken up in plants by transporters of other divalent cations, such as Zn2+ and Fe2+ [9]; Zn present in soil, as well as DTPA-Fe, aids Cd transfer from soil to wheat. According to previous reports [18, 57], the effects of Zn on Cd transfer in soil–plants are not consistent. Besides our study, Nan et al. [18] and Smilde et al. [58] also reported the enhancement effect of Zn on Cd transfer and a synergy between Cd and Zn in soil–plant. On the other hand, some studies reported that Cd uptake and its accumulation in plants could be decreased as a result of the application of Zn [8, 57]. Hart et al. [59] attributed the competitive interaction between Cd and Zn for uptake to their common transport mechanism in the plasma membranes. These apparently different effects may be related to different soil Zn or Cd concentrations, species, and the complexity of the soil–wheat system. In our investigation, soil Zn and Cd concentrations ranged from 34.1 to 229.6 and 0.08 to 1.25 μg g−1, respectively. This may represent soil Zn and Cd concentrations in most agricultural land in the Yangtze River delta region, so the present assumption about the effect of Zn on Cd transfer in soil–plant may be more significant.
Effects of N, S, P, and K on Cd Transfer from Soil to Wheat
Depending on their different effects on wheat Cd concentration, N, S, P, and K can be divided into two categories. Soil S and N enhanced Cd concentrations in wheat, whereas soil P and slowly available K restrained its uptake.
Perilli et al. [57] reported that application of N fertilizer could increase Cd accumulation in wheat grains. Nitrogen can affect Cd bioavailability by altering soil properties and the osmotic strength of the soil solution [60]. Nitrogen can also affect Cd speciation and complexation, which are closely related to Cd translocation from soil to plant roots and its absorption onto roots [47, 61]. As nitrogen fertilizers have been overused in the study area [34, 35], the mean concentration of soil N apparently exceeds its mean concentration in Chinese tillage topsoil (1.05 g kg−1) [42]. Therefore, it is thought that increased soil N may contribute to the contamination of wheat grain by Cd.
In our study, soil S has a strongly positive correlation with soil Cd, indicating that sulfate- and sulfur-containing organic matter may be the major host of Cd in the Yangtze River delta soils. Organic S is the major form of S present in paddy soil, and one important speciation is organic sulfate (a major bioavailable speciation) [42, 62]. Cd-containing organic sulfate transforms into SO 2−4 easily in soil and releases Cd2+ synchronously, thus increasing the solubility and mobility of Cd via soluble CdSO4 [42]. This would then promote the uptake of Cd by wheat, accounting for the enhancement effect of soil S on wheat Cd concentration evident in our study.
Grain Cd has a negative correlation with soil P but shows no significant correlation with available P. In addition, soil Cd has a positive correlation with soil P. These correlations indicate that the main effect of soil P on the bioavailability and mobility of Cd in soil is the binding action by insoluble solid phase P. This agrees with the report by Brown et al. [63]. Additionally, negative correlation between root P and straw Cd showed that even P in the root restrains Cd transfer from root to straw. Little is known about this restraint (or correlation), and it may need further study.
Although there was no significant correlation between soil K and grain Cd or root Cd, soil slowly available K showed a significant negative correlation with grain Cd and root Cd. This slowly available K mainly comprises that present in the clay fraction and mica. Soil clays have a strong capacity to retain Cd in soil [53], and the sequestration of Cd on clay mineral surfaces can reduce Cd phyto-accessibility. Soil slowly available K may reflect this capacity of the clay fraction and mica in soil indirectly.
Conclusions
Compared with the maximum permissible Cd concentrations for agricultural soils and wheat grain in China, 19.8% of our soil samples from the Yangtze River delta and 14.3% of the wheat grain samples, respectively, are contaminated by Cd. The major speciations of Cd in soil were exchangeable, bound to carbonates or present in the fulvic and humic acid fractions, and were susceptible to soil parameters such as soil pH, total Ca, Mg, S, and P, DTPA-Fe, Ex-Ca, and Ex-Mg. Cadmium concentration shows a strong correlation with Fe, S, and P in the grain and the soil, whereas there is no significant correlation in the straw and the root. Correlation analysis indicates that root Ca, Mg, Fe, and P restrain the transfer of Cd from root to straw. In general, soil pH, Ca, Mg, Mn, P, and slowly available K restrict Cd transfer in the soil–wheat system, whereas soil S, N, Zn, DTPA-Fe, and TOC enhance Cd accumulation in wheat grain.
Abbreviations
- Car:
-
Bound to carbonates
- DTPA:
-
Diethylenetriamine penta-acetic acid
- Ex:
-
Exchangeable
- Fe–Mn:
-
Bound to Fe–Mn oxyhydroxides
- HF:
-
Fulvic and humic acid
- OM:
-
Organic matter
- Phyto-Cd:
-
Soil phytoaccessible Cd
- Res:
-
Residual
- TOC:
-
Total organic carbon
References
Grant CA, Buckley WT, Bailey LD, Selles F (1998) Cadmium accumulation in crops. Can J Plant Sci 78:1–17
Bakircioglu D, Kurtulus YB, Ibar H (2011) Investigation of trace elements in agricultural soils by BCR sequential extraction method and its transfer to wheat plants. Environ Monit Assess 175:303–314
Liu X, Wu J, Xu J (2006) Characterizing the risk assessment of heavy metals and sampling uncertainty analysis in paddy field by geostatistics and GIS. Environ Pollut 141:257–264
Cooper J, Sanderson R, Cakmak I et al (2011) Effect of organic and conventional crop rotation, fertilization, and crop protection practices on metal contents in wheat (Triticum aestivum). J Agric Food Chem 59:4715–4724
Rodda MS, Li G, Reid RJ (2011) The timing of grain Cd accumulation in rice plants: the relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant Soil 347:105–114
Bolan NS, Adriano DC, Mani PA, Duraisamy A (2003) Immobilization and phytoavailability of cadmiumin variable charge soils. I. Effect of phosphate addition. Plant Soil 250:83–94
Francois M, Grant C, Lambert R, Sauvé S (2009) Prediction of cadmium and zinc concentration in wheat grain from soils affected by the application of phosphate fertilizers varying in Cd concentration. Nutr Cycl Agroecosyst 83:125–133
Oliver DP, Wilhelm NS, McFarlane JD, Tiller KG, Cozens GD (1997) Effect of soil and foliar applications of zinc on cadmium concentration in wheat grain. Aust J Exp Agricult 37:677–681
Welch RM, Norvell WA (1999) Mechanisms of cadmium uptake, translocation and deposition in plants. In: McLaughlin MJ, Singh BR (eds) Cadmium in soils and plants. Kluwer, Dordrecht, pp 125–150
Amini M, Khademi H, Afyuni M, Abbaspour KC (2005) Variability of available cadmium in relation to soil properties and land use in an arid region in central Iran. Water Air Soil Pollut 162:205–218
Eriksson JE, Söderström M (1996) Cadmium in soil and winter wheat grain in southern Sweden. 1. Factors influencing Cd levels in soils and grain. Acta Agric Scand Sect B 46:240–248
Wenzel WW, Blum WEH, Brandstetter A et al (1996) Effects of soil properties and cultivar on cadmium accumulation in wheat grain. Z Pflanzenernaehr Bodenkd 159:609–614
Ge Y, Hendershot W (2005) Modeling sorption of Cd, Hg and Pb in soils by the NICA [non-ideal competitive adsorption]—Donnan model. Soil Sediment Contam 14:53–69
Sauvé S, Manna S, Turmel MC, Roy AG, Courchesne F (2003) Solid solution partitioning of Cd, Cu, Ni, Pb, and Zn in the organic horizons of a forest soil. Environ Sci Technol 37:5191–5196
Kirkham MB (2006) Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma 137:19–32
Adams ML, Zhao FJ, McGrath SP, Nicholson FA, Chambers BJ (2004) Predicting cadmium concentrations in wheat and barley grain using soil properties. J Environ Qual 33:532–541
Garrett RG, MacLaurin AI, Gawalko EJ, Tkachuk R, Hall GEM (1998) A prediction model for estimating the cadmium content of durum wheat from soil chemistry. J Geochem Explor 64:101–110
Nan ZR, Li JJ, Zhang JM, Cheng GD (2002) Cadmium and zinc interactions and their transfer in soil-crop system under actual field. Sci Total Environ 285:187–195
Norvell WA, Wu J, Hopkins DG, Welch RM (2000) Association of cadmium in durum wheat grain with soil chloride and chelate-extractable soil cadmium. Soil Sci Soc Am J 64:2162–2168
Huang XS, Wang HY, Zhou JM, Ma CL, Du CW, Chen XQ (2009) Risk assessment of potentially toxic element pollution in soils and rice (Oryza sativa) in a typical area of the Yangtze River Delta. Environ Pollut 157:2542–2549
Zhao KL, Liu XM, Xu JM, Selim HM (2010) Heavy metal contaminations in a soil–rice system: identification of spatial dependence in relation to soil properties of paddy fields. J Hazard Mater 181:778–787
Nanjing Institute of Soil Science of Chinese Academy of Science (1978) Soil chemical and physical analysis. Shanghai Scientific and Technological Press, Shanghai (in Chinese)
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL (ed) Methods of soil analysis. Part 3: chemical methods—SSSA book series no. 5. Madison, WI, USA, pp 961–1010
Bremner JM (1996) Nitrogen-total. In: Sparks DL (ed) Methods of soil analysis, Part 3, chemical methods – SSSA Book Series no. 5. Madison, Wisconsin, USA, pp1085-1121
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Shi JC, Xu JM, Huang PM (2008) Spatial variability and evaluation of status of micronutrients in selected soils around Taihu Lake, China. J Soils Sediments 8:415–423
Bao SD (2000) Analysis of soil and agricultural chemistry. Chinese Agriculture Press, Beijing (in Chinese)
Sumner ME, Miller WP (1996) Cation exchange capacity and exchange coefficients. In: Sparks DL (ed) Methods of soil analysis. Part 3: chemical methods—SSSA book series no. 5. Madison, WI, USA, pp 1085–1121
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Donisa C, Mocanu R, Steinnes E (2003) Distribution of some major and minor elements between fulvic and humic acid fractions in natural soils. Geoderma 111:75–84
Guan TX, He HB, Zhang XD, Bai Z (2011) Cu fraction, mobility and bioavailability in soil–wheat system after Cu-enriched livestock manure applications. Chemosphere 82:215–222
Xia ZL, Li SZ, Li TF, Ba Y (1987) The background value of soil element and study methods. Chinese Meteorological Press, Beijing (in Chinese)
State Environmental Protection Administration of China (1995) Environment quality standard for soils (GB15618-1995). Chinese National Standard Agency, Beijing
Guo JH, Liu XJ, Zhang Y et al (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010
Liu FC, Shi XZ, Yu DS (2006) Spatial and temporal variability of soil acidity in typical areas of Taihu Lake region in the last 20 years. Resour Environ Yangtze Basin 15:740–744 (in Chinese with English abstract)
Chaudri A, McGrath S, Gibbs P, Chambers B, Carlton-Smith C, Godley A, Bacon J, Campbell C, Aitken M (2007) Cadmium availability to wheat grain in soils treated with sewage sludge or metal salts. Chemosphere 66:1415–1423
Garrett RG (1994) The distribution of cadmium in a horizon soils in the prairies of Canada and adjoining United States. Current Research 1994-B, Geological Survey of Canada, pp 73-82
European Commission (2001) Commission Regulation (EC) No. 466/2001 of 8 March 2001 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Communities Legis 77:1–13
Food Standards Australia New Zealand (2000) Australia New Zealand food standards code. Standard 1.4.1. Contaminants and natural toxicants. Commonwealth of Australia Gazette No. P30
Ministry of Health of China (2005) Maximum level of contaminants in food (GB2762-2005)
Gawalko EJ, Garrett RG, Nowicki TW (2002) Cadmium, copper, iron, manganese, selenium, and zinc in canadian spring wheat. Commun Soil Sci Plant Anal 33:3121–3133
Chen HM (2005) Environmental soil science. Science Press of China, Beijing (in Chinese)
Basta NT, Ryan JA, Chaney RL (2005) Trace element chemistry in residual-treated soils: key concepts and metal bioavailability. J Environ Qual 34:49–63
Seuntjens P, Nowack B, Schulin R (2004) Root-zone modeling of heavy metal uptake and leaching in the presence of organic ligands. Plant Soil 265:61–73
Liu LN, Chen HS, Cai P, Liang W, Huang QY (2009) Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost. J Hazard Mater 163:563–567
Antoniadis V, Robinson JS, Alloway BJ (2008) Effects of short-term pH fluctuations on cadmium, nickel, lead, and zinc availability to ryegrass in a sewage sludge-amended field. Chemosphere 71:759–764
Chen HM (2002) Sulfur in soil and its relation with environmental quality. Science Press of China, Beijing (in Chinese)
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Heavy metal mobility and availability in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407:3972–3985
Impellitteri CA, Lu YF, Saxe JK, Allen HE, Peijnenburg WJGM (2002) Correlation of the partitioning of dissolved organic matter fractions with the desorption of Cd, Cu, Ni, Pb and Zn from 18 Dutch soils. Environ Int 28:401–410
Lee SZ, Allen HE, Huang CP, Sparks DL, Sanders PF, Peijnenburg WJGM (1996) Predicting soil–water partition coefficients for cadmium. Environ Sci Technol 30:3418–3424
He JY, Ren YF, Wang FJ, Pan XB, Zhu C, Jiang DA (2009) Characterization of cadmium uptake and translocation in a cadmium-sensitive mutant of rice (Oryza sativa L. ssp. japonica). Arch Environ Contam Toxicol 57:299–306
Tyler LD, McBride MB (1982) Influence of Ca, pH and humic acid on Cd uptake. Plant Soil 64:259–262
Gil C, Boluda R, Ramos J (2004) Determination and evaluation of cadmium, lead and nickel in greenhouse soils of Almería (Spain). Chemosphere 55:1027–1034
Plassard F, Winiarski T, Petit-Ramel M (2000) Retention and distribution of three heavy metals in a carbonated soil: comparison between batch and unsaturated column studies. J Contam Hydrol 42:99–111
Soil Survey Office of Jiangsu Province of China (1995) Soil of Jiangsu. Chinese Agriculture Press, Beijing (in Chinese)
Zhang H (2006) Soil environmental science. Chinese Chemical Industry Press, Beijing (in Chinese)
Perilli P, Mitchell LG, Grant CA, Pisante M (2010) Cadmium concentration in durum wheat grain (Triticum turgidum) as influenced by nitrogen rate, seeding date and soil type. J Sci Food Agric 90:813–822
Smilde KW, Luit BV, Driel WV (1992) The extraction by soil and absorption by plants of applied zinc and cadmium. Plant Soil 143:233–238
Hart JJ, Welch RM, Norvell WA, Kochian LV (2002) Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiol Plant 116:73–78
Mitchell LG, Grant CA, Racz GJ (2000) Effect of nitrogen application on concentration of cadmium and nutrient ions in soil solution and in durum wheat. Can J Sci 80:107–115
Hamon RE, McLaughlin MJ, Naidu R, Correl R (1998) Long-term changes in cadmium bioavailability in soil. Environ Sci Technol 32:3699–3703
Hu ZY, Zhao FJ, McGrath SP (2005) Sulphur fractionation in calcareous soils and bioavailability to plants. Plant Soil 268:103–109
Brown S, Chaney R, Hallfrisch J, Ryan JA, Berti WR (2004) In situ soil treatments to reduce the phyto- and bioavailability of lead, zinc, and cadmium. J Environ Qual 33:522–531
Acknowledgments
This research was sponsored by a project of China Geological Survey entitled “Evaluation of Regional Geochemical Ecology in the Lower Yangtze River Basin” ([2008] GZTR02-01) and the National Natural Science Foundation of China (40625012). Professor XY Yuan in Hohai University, China is gratefully acknowledged for reviewing this paper prior to submission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Ji, J., Yang, Z. et al. Effects of Soil Properties on the Transfer of Cadmium from Soil to Wheat in the Yangtze River Delta Region, China—a Typical Industry–Agriculture Transition Area. Biol Trace Elem Res 148, 264–274 (2012). https://doi.org/10.1007/s12011-012-9367-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9367-z