Abstract
A hydroponic experiment was conducted to determine the possible effect of exogenous glutathione (GSH) in alleviating chromium (Cr) stress through examining plant growth, chlorophyll contents, antioxidant enzyme activity, and lipid peroxidation in rice seedlings exposed to Cr toxicity. The results showed that plant growth and chlorophyll content were dramatically reduced when rice plants were exposed to 100 μM Cr. Addition of GSH in the culture solution obviously alleviated the reduction of plant growth and chlorophyll content. The activities of some antioxidant enzymes, including superoxide dismutase, catalase (CAT) and glutathione reductase in leaves, and CAT and glutathione peroxidase in roots showed obvious increase under Cr stress. Addition of GSH reduced malondialdehyde accumulation and increased the activities of these antioxidant enzymes in both leaves and roots, suggesting that GSH may enhance antioxidant capacity in Cr-stressed plants. Furthermore, exogenous GSH caused significant decrease of Cr uptake and root-to-shoot transport in the Cr-stressed rice plants. It can be assumed that GSH is involved in Cr compartmentalization in root cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is the 21st most abundant element in Earth's crust and is toxic to plants without any function in plant metabolism [1]. In recent years, Cr contamination has become a serious issue worldwide for agricultural production and environment due to poor Cr management in industrial and agricultural practices [2]. Chromium occurs naturally in several oxidation states, with the trivalent (Cr (III)) and hexavalent (Cr(VI)) being the most stable and common forms [3]. Although both Cr (III) and Cr (VI) are toxic to plants, hexavalent Cr is considered to be much more toxic than its trivalent form. Chromium inhibits seed germination and plant growth, disrupts nutrient and water relations, induces inactivation of mitochondrial electron transport, decreases the activity of antioxidant enzymes, and causes ultrastructural damage of the chloroplast and cell membrane [4–7].
Oxidative stress is one of the most important damages for plants exposed to various abiotic stresses [8]. It was reported that Cr stress enhanced lipid peroxidation and produced reactive oxygen species (ROS), including H2O2, O −2 , and OH−, resulting in oxidative damage to plants [5, 9, 10]. In order to control the level of ROS and protect the cells from oxidative injury, plants have developed an antioxidant defense system, which is mainly composed of antioxidant enzymes, such as superoxide dismutase (SOD), guaiacol peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR), and non-enzymatic antioxidants, such as ascorbate, glutathione (GSH), flavonoids, carotenoids, and tocopherols [8, 11].
A low molecular weight tripeptide (γ-glutamylcys-teinyl-Gly), i.e., reduced GSH, is the most abundant source of non-protein thiols in plant cells, which is related to regulation of cell differentiation, death and senescence, pathogen resistance, and enzymatic activity [12]. GSH also participates in the maintenance of redox homeostasis in the cells and plays a crucial role as an antioxidant in cell defense and protection against various stresses, such as chilling, drought, salinity, and heavy metal [13–15]. Acting as a free radical scavenger, GSH is a part of the ascorbate–glutathione cycle, essential for scavenging of hydrogen peroxide (H2O2) [15]. Furthermore, GSH also works as a precursor in the synthesis of phytochelatins, which have a high affinity in binding heavy metals and are transported as complexes into the vacuole, leading to inactivation of heavy metals in the plant cells [16]. It is well documented that GSH plays an important role for organisms in their tolerance to heavy metals. Zhu et al. [17, 18] found that overexpression of glutathione synthetase and γ-glutamyl-cysteine synthetase increased Cd accumulation and enhanced Cd tolerance in Brassica juncea. Xiang et al. [19] also reported that genetically modified Arabidopsis plants with low GSH levels were hypersensitive to Cd stress.
The importance and physiological mechanism of endogenous GSH in fighting against abiotic stresses have been intensively studied [15, 20]. However, little research has been focused on the possible effect of exogenous GSH in alleviating Cr toxicity. In this study, a hydroponic experiment was conducted to investigate the effect of GSH in alleviating Cr toxicity.
Materials and Methods
Plant Growth and Treatments
Two rice genotypes (Oryza sativa L. cvs. Xiushui 113 and Dan K5) were used in this experiment. In our previous study, Xiushui 113 and Dan K5 were found to have low- and high-grain Cr accumulation, respectively [21]. Rice seeds of the two genotypes were surface sterilized in a 2% H2O2 solution for 10 min and then rinsed with tap water for 20 min, followed by washing with deionized water five times. The seeds were soaked in deionized water 25°C for 2 days and germinated at 35°C in the dark for 1 day. The seedlings were transferred to a plastic net floating on deionized water in a controlled growth chamber with photoperiod of 16 h light/8 h dark and light intensity of 225 ± 25 μmol m−2 s−1. The light/dark temperatures were set at 30/22°C, and relative humidity was kept at 85%. After 2 weeks, 14 uniform rice seedlings were transplanted into a 4-L plastic container containing nutrient solution (seedlings per pot) in a greenhouse with ambient light intensity, temperature (34°C day/25°C night), and humidity (approximately 75%). The rice plants were grown in a hydroponic solution prepared according to the protocols of Yoshida et al. [22] with the following salts (in millimolars): NH4NO3, 1.45; NaH2PO4, 0.32; K2SO4, 0.5; CaCl2, 1.0; MgSO4·7H2O, 1.7; MnCl2·4H2O, 9.1 × 10−3; (NH4)6MoO24·4H2O, 5.2 × 10−4; H3BO3, 1.8 × 10−2; ZnSO4·7H2O, 1.5 × 10−4; CuSO4·5H2O, 1.6 × 10−4, and Fe-citrate, 3.6 × 10−2. The pH value of the culture solution was adjusted to 5.1 ± 0.01 using 1 M HCl or NaOH solution as required. Culture solutions were renewed every 4 days. Half strength nutrient solution was applied for the first 4 days and then changed to complete nutrient solution for 1 week. Thereafter, potassium dichromate (K2Cr2O7) and GSH were added to the nutrient solutions to form six treatments as follows: (1) control (without GSH and Cr), (2) GSH 50 (50 μM GSH), (3) GSH 100 (100 μM GSH), (4) Cr (100 μM Cr), (5) Cr + GSH 50 (100 μM Cr + 50 μM GSH), and (6) Cr + GSH 100 (100 μM Cr + 100 μM GSH).
Measurement of Plant Growth and Metal Concentration
After a 20-day treatment, rice seedlings were sampled, immersed into 20 mM EDTA–Na2 for 3 h, and rinsed in running distilled water. Fifteen plants with similar size were selected from three replications, and the lengths of shoot and root were simultaneously measured with a centimeter scale. Then, the plants were separated into shoots and roots, dried at 80°C for 48 h, and weighed. Plant sample (1 g) was digested with 6 ml concentrated HNO3 at 150°C for 1 h and then with 2 ml concentrated HClO4 at 215°C for 2 h [23]. The final digestion was diluted to 25 ml with deionized water and filtered. Chromium concentration was determined via flame atomic absorption spectrometry (AA6300, Shimadzu, Kyoto, Japan). The translocation factor (TF) of Cr was calculated as TF (percent) = [Cr]shoot/[Cr]root × 100%.
Estimation of Photosynthetic Pigments
After the 20-day treatment, about 0.2 g of fresh leaves was extracted with a 10-ml mixture of acetone and ethanol (v/v = 1:1) in the dark at room temperature until leaf color disappeared completely. Light absorbance at 663, 645, and 470 nm was determined using a spectrophotometer (AA6300, Shimadzu, Tokyo, Japan). The concentrations of chlorophyll (a, b) and carotenoids were calculated using adjusted extinction coefficients [24].
Estimation of Malondialdehyde
The level of lipid peroxidation was expressed as malondialdehyde (MDA) content and was determined as 2-thiobarbituric acid (TBA) reactive metabolites according to the method of Hodges et al. [25]. After the 20-day treatment, fresh samples (both leaf and root, approximately 0.5 g) were homogenized in 4.0 ml of 1% trichloroacetic acid (TCA) solution and centrifuged at 10,000×g for 10 min. The supernatant was added to 1 ml 0.5% (w/v) TBA made in 20% TCA. The mixture was incubated in boiling water for 30 min, and the reaction was stopped by placing the tubes in an ice bath. The samples were centrifuged at 10,000×g for 5 min, and the absorbance of the supernatant was recorded at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The amount of the MDA–TBA complex (red pigment) was calculated from the extinction coefficient of 155 mM−1 cm−1.
Assay of Antioxidant Enzyme
After the 20-day treatment, fresh samples (both leaf and root, approximately 0.5 g) were homogenized with 8 ml 50 mM phosphate buffer solution (pH 7.8) in an ice bath and then centrifuged at 10,000×g for 15 min at 4°C The supernatant was designated as crude enzyme extract and stored at 4°C for the assays of various antioxidant enzyme activities and soluble protein content.
Superoxide Dismutase (EC 1.15.1.1)
SOD activity was assayed by the nitroblue tetrazolium (NBT) method [26] by measuring the photoreduction of NBT at 560 nm. The reaction mixture (3 ml) contained 50 mM sodium phosphate buffer (pH 7.8), 13 mM methionine, 75 μM NBT, 10 μM EDTA, 2 mM riboflavin, and enzyme extract (100 μl). The reaction was started by placing the tubes below two 15-W fluorescent lamps for 10 min and then stopped by switching off the light. The absorbance was measured at 560 nm. One unit of SOD was defined as the quantity of enzyme that produced 50% inhibition of NBT reduction under the experimental conditions.
Guaiacol Peroxidase (EC 1.11.1.7)
POD activity was assayed according to the method of Putter [27] with some modification. The reaction mixture (3 ml) consisted of 100 μl enzyme extract, 100 μl guaiacol (1.5%, v/v), 100 μl H2O2 (300 mM), and 2.7 ml 25 mM potassium phosphate buffer with 2 mM EDTA (pH 7.0). Increase in the absorbance was measured spectrophotometrically at 470 nm (ε = 26.6 mM−1 cm−1).
Catalase (EC 1.11.1.6)
CAT activity was assayed using the method described by Aebi [28]. The assay mixture (3.0 ml) contained 100 μl enzyme extract, 100 μl H2O2 (300 mM), and 2.8 ml 50 mM phosphate buffer with 2 mM EDTA (pH 7.0). The CAT activity was assayed by monitoring the decrease in the absorbance at 240 nm as a consequence of H2O2 consumption (ε = 39.4 mM−1 cm−1).
Ascorbate Peroxidase (EC 1.11.1.11)
APX activity was determined according to Nakano and Asada [29]. The reaction mixture consisted of 100 μl enzyme extract, 100 μl ascorbate (7.5 mM), 100 μl H2O2 (300 mM), and 2.7 ml 25 mM potassium phosphate buffer with 2 mM EDTA (pH 7.0). The oxidation of ascorbate was determined by the decrease in absorbance at 290 nm (ε = 2.8 mM−1 cm−1).
Glutathione Reductase (EC 1.6.4.2)
GR activity was assayed according to Garcia-Limones et al. [30]. The reaction mixture consisted of 100 μl enzyme extract, 100 μl NADPH (2.4 mM), 100 μl oxidized glutathione (GSSG, 10 mM), and 2.7 ml 25 mM potassium phosphate buffer with 2 mM EDTA (pH 7.0). The oxidation of NADPH was determined by its absorbance decrease at 340 nm (ε = 6.2 mM−1 cm−1).
Glutathione Peroxidase (EC 1.11.1.7)
GPX activity was assayed according to Nagalakshmi and Prasad [31]. The reaction mixture consisted of 200 μl enzyme extract, 100 μl NaCl (1.14 M), 100 μl GSH (5 mM), 100 μl NADPH (2 mM), 100 μl H2O2 (2.5 mM), 10 μl GR (25 U ml−1), and 380 μl 25 mM potassium phosphate buffer with 2 mM EDTA (pH 7.0). The oxidation of NADPH was determined by its absorbance decrease at 340 nm (ε = 6.2 mM−1 cm−1).
Statistical Analysis
The whole experiment was set up in a randomized block design. Statistical analysis was performed by a statistical package, SPSS version 13.0 (SPSS, Chicago, IL). ANOVA was used to confirm the significance of the factors. Significant differences among the means were compared by Duncan's multiple range tests (P < 0.05).
Results
Plant Growth Parameters
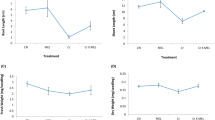
Compared to the control, Cr stress significantly decreased shoot and root lengths and shoot dry weight of the two genotypes, causing 33%, 20%, and 42% reduction for Xiushui 113, and 33%, 26%, and 49% reduction for Dan K5, respectively (Table 1). However, Cr stress had little effect on root dry weight of both genotypes. Without Cr addition, addition of exogenous GSH showed little effect on length and dry weight of both shoots and roots relative to the control. However, GSH addition alleviated the reduction of these growth parameters caused by Cr stress. Hence, the treatment of Cr + GSH had greater values in shoot and root lengths, and shoot dry weight, than the treatment of Cr alone for the two rice genotypes (Table 1). It may be suggested that the Cr-induced growth inhibition was alleviated by GSH application. Moreover, the alleviation of Cr toxicity by exogenous GSH was genotype dependent, with Dan K5 being more affected than Xiushui 113.
Chromium Concentration and Translocation in Plants
Addition of Cr stress (100 μM) in culture solution increased Cr concentrations in both shoots and roots, with roots having much higher Cr concentration than shoots (Fig. 1a and b). Under Cr stress, GSH addition markedly reduced shoot Cr concentration of the two genotypes. On the contrary, root Cr concentration was significantly higher for the plants exposed to exogenous GSH than the plants under Cr stress alone. In addition, higher level of GSH (100 μM) significantly alleviated Cr accumulation in roots (Fig. 1a and b). The translocation factor (TF, ratio of [Cr]shoot/[Cr]root) of Cr was reduced significantly in the GSH treatment, and the reduction was larger for Dan K5 than for Xiushui 113 (Fig. 1c).
Effects of exogenous GSH addition on Cr concentration in shoots (a) and roots (b) and Cr translocation factor (c) of two rice genotypes under non-stress or Cr stress. GSH and chromium were simultaneously added to 25-day-old seedlings for 20 days. Values are means ± SE of three replicates. Bars with different letters within a parameter are significantly different at P < 0.05 according to Duncan's test
Photosynthetic Pigment Content
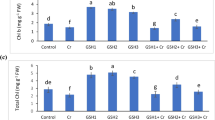
As shown in Table 2, Cr stress significantly reduced the contents of chlorophyll b (Chlb), total chlorophyll (Chla + b) of two rice genotypes, and carotenoids (Car) of Dan K5 and had little effect on chlorophyll a (Chla) content. Addition of GSH significantly mitigated the reduction of Chlb, Chla + b, and Car contents caused by Cr stress for Dan K5. However, the alleviatory effect of GSH on Chlb, Chla + b, and Car contents in Xiushui 113 was not significant (Table 2).
Lipid Peroxidation (MDA)
Cr stress significantly increased MDA content in leaves and roots of the two genotypes (Fig. 2). In the absent of Cr, 100 μM GSH treatment caused little changes of MDA contents in leaves and roots of the two genotypes. However, addition of 50 μM GSH significantly reduced leaf MDA content in the two genotypes and root MDA content in Dan K5, but increased root MDA content in Xiushui 113. Under Cr stress, exogenous application of GSH significantly reduced MDA contents in both leaves and roots, and the reduction varied greatly with GSH level (Fig. 2).
Effects of exogenous GSH addition on MDA contents in leaves (a) and roots (b) of two rice genotypes under non-stress or Cr stress. GSH and chromium were simultaneously added to 25-day-old seedlings for 20 days. Values are means ± SE of three replicates. Bars with different letters within a parameter are significantly different at P < 0.05 according to Duncan's test
Antioxidant Enzyme Activity
Chromium stress significantly increased leaf SOD activity in Dan K5 and decreased root SOD activity in the two genotypes (Fig. 3a and b). For the control without Cr addition, addition of 100 μM GSH significantly decreased leaf SOD activity in the two genotypes and root SOD activity in Dan K5, while addition of 50 μM GSH had no significant effect on SOD activity (Fig. 3a and b). Under Cr stress, addition of GSH significantly increased leaf SOD activity of the two genotypes, although low GSH treatment (50 μM) did not dramatically change root SOD activity relative to Cr treatment alone.
Effects of exogenous GSH addition on the activities of SOD (a and b), POD (c and d), CAT (e and f), APX (g and h), GR (i and j), and GPX (k and l) in leaves (left) and roots (right) for two rice genotypes under non-stress or Cr stress. Values are means ± SE of three replicates. Bars with different letters within a parameter are significantly different at P < 0.05 according to Duncan's test
Chromium stress significantly decreased leaf and root POD activities in the two genotypes (Fig. 3c and d). Addition of GSH in the solution had little effect on leaf POD activity but decreased root POD activity in comparison with the control. Under Cr stress, the reduction of leaf POD activity in Dan K5 was significantly alleviated by application of 50 μM GSH, while for Xiushui 113, there was no such alleviatory effect. The reduction of root POD activity in Cr-stressed plants was significantly alleviated by addition of 50 μM GSH in both genotypes. Both leaf and root CAT activities were significantly increased under Cr stress, (Fig. 3e and f). For the treatment without Cr addition, there was no significant difference between the control and two GSH levels in leaf and root CAT activities of both genotypes. Under Cr stress, CAT activities in both leaf and root were significantly increased by the exogenous application of 100 μM GSH.
Leaf APX activity of the two genotypes was not significantly affected by Cr stress, while root APX activity was significantly decreased under Cr stress (Fig. 3g and h). Without Cr addition, there was no significant difference between the control and GSH treatments in leaf and root APX activities of both genotypes, except leaf APX activity of Dan K5 under the treatment GSH 100. Under Cr stress, APX activities in both leaf and root were significantly increased by two GSH levels.
Cr stress significantly increased leaf GR activity in Dan K5 but decreased root GR activity in the two genotypes (Fig. 3i and j). Without Cr addition, exogenous GSH had little effect on GR activities in leaves and roots of both genotypes, except leaf GR activity of Xiushi 113, which showed a significant decrease in the two GSH levels. Under Cr stress, GR activities in both leaves and roots were significantly increased due to addition of GSH, in particular 100 μM GSH.
Leaf GPX activity of the two genotypes was not significantly affected by Cr stress, while root GPX activity was significantly increased under Cr stress (Fig. 3k and l). Without Cr addition, GSH had little effect on leaf and root GPX activities of Xiushui 113, whereas application of 100 μM GSH increased that of Dan K5, compared with the control. Under Cr stress, leaf GPX activities of Xiushui 113 was not affected by GSH addition, but that of Dan K5 was significantly increased in the treatment of 100 μM GSH. Root GPX activities of the two genotypes were significantly increased in the lower GSH level, but reduced in the higher GSH level.
Discussion
High Cr concentration reduced the elongation of root and stem [32], reduced the dry weight of root and shoot [33], and altered the production of chlorophyll and soluble protein [10, 34, 35]. Similar results were obtained in the present study. However, the inhibition of plant growth and reduction of chlorophyll content were significantly alleviated by exogenous application of 100 μM GSH (Tables 1 and 2). The present results indicate that GSH alleviates the inhibitory effect of Cr stress on plant growth and some physiological processes.
MDA is a product of cell membrane lipid peroxidation, and its content in vivo can indicate the extent of oxidative stress in plants and cell membrane homeostasis [36]. Chromium stress initiates the process of lipid peroxidation and increases the amount of MDA in many plant species [10, 37]. The present results showed that Cr stress caused a dramatic increase in MDA content in rice leaves and roots, indicating an occurrence of high oxidative stress (Fig. 2). To fight against oxidative stress, plants have evolved a complex antioxidant defense system. Actually, induction and activation of antioxidant enzymes like SOD and CAT are considered as an important mechanism of metal detoxification and tolerance in plants [38]. SOD is the first line of defense against ROS-mediated toxicity and responsible for the scavenging of toxic O2− in plant cells [39]. POD, CAT, and the ascorbate–glutathione cycle (APX and GR) play a crucial role in scavenging H2O2 [40]. In the present study, excessive Cr induced a significant enhancement in leaf SOD and GR activities in Dan K5 and leaf CAT activity in both genotypes, and root CAT and GPX activities (Fig. 3a, e, and i), suggesting the importance of these antioxidative enzymes in fighting against oxidative stress. Furthermore, the activities of SOD, POD, APX, and GR in rice roots were dramatically reduced when the plants were exposed to Cr stress (Fig. 3b, d, h, and j), suggesting that rice roots suffer from greater oxidative stress than leaves under Cr stress, and the modulation of SOD, POD, APX, and GR in response to Cr stress is limited due to the Cr-induced root damage. As an important antioxidant, GSH plays a prominent role in tolerance to heavy metals. We observed the protection of exogenous GSH against oxidative stress caused by high Cr level in this study. The activities of antioxidant enzymes in both leaves and roots were significantly increased, and MDA content in plants was significantly decreased in GSH-treated plants compared to those in Cr-stressed plants. These results demonstrate that GSH enhances the antioxidant capacity of rice plants, thus alleviating Cr toxicity.
In general, the uptake of Cr(VI) is an active and metabolically driven processes in plants [7]. Thus, Cr(VI) is readily taken up by plants. However, there is a distinct restriction in its translocation from roots to shoots. The present results showed that most Cr absorbed in rice plants was retained in roots (Fig. 1a and b). The poor translocation of Cr from roots to shoots could be attributed to Cr compartmentalization in the vacuoles of the root cells, thus rendering it non-toxic to plants [41]. In comparison with the treatment of Cr alone, addition of GSH markedly decreased Cr concentration in shoots and increased Cr concentration in roots, irrespective of the two rice genotypes (Fig. 1a and b). Furthermore, the translocation factor of Cr from roots to shoots was also reduced significantly in the GSH-treated plants (Fig. 1c). Glutathione is a precursor of phytochelatin (PC) synthesis in plants, which could be activated by heavy metal ions [42]. A range of metal ions, such as Cd, Cu, Hg, Pb, and Cr, have been reported to be effective to induce the biosynthesis of PC in plant cells [42, 43]. Phytochelatins have a high affinity for heavy metal to form PC–heavy metal complexes. The complexes are then transported into the vacuole of plant cells, so that the metal ions the complexes carry are stored safely away from the proteins of the cytosol [15]. It has been well documented that PCs play an important role in Cd and arsenate detoxification [42, 43]. Apart from being a substrate of PC synthesis, GSH can also detoxify heavy metal through forming a glutathione–metal complex. Lima et al. [44] found that GSH acts as a Cd2+ chelator in Rhizobium leguminosarum strains, and its presence is believed to be very important in intracellular cadmium detoxification. Indeed, some previous studies have demonstrated that dichromate also can react with GSH at the sulfhydryl group, forming an unstable glutathione–CrO −3 complex [45]. Therefore, it can be hypothesized that GSH can reduce the uptake and translocation of chromium by means of synthesizing phytochelatins and chelating metals.
Genotypic variations exist in the responses to heavy metal stress in terms of plant growth, metal uptake, photosynthesis, and enzyme activity [46, 47]. In the present study, the two rice genotypes, Xiushui 113 and Dan K5, showed a distinct difference in the response to Cr stress and GSH addition. Dan K5 had much more reduction in plant height and shoot dry weight than Xiushui 113 under Cr stress, while the effect of GSH in alleviating growth inhibition of rice plants was much larger for Dan K5 than for Xiushui 113 (Table 1). Although the reduction of Chlb, Chl(a + b), and Car contents by Cr stress was greater in Dan K5 than in Xiushui 113, the effect of GSH in alleviating the reduction of the photosynthetic pigment content was more pronounced in Dan K5 than in Xiushui 113 (Table 2). It could be concluded that the effect of GSH addition on alleviating Cr stress is genotype dependent.
References
Sinha S, Saxena R, Singh S (2005) Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: role of antioxidants and antioxidant enzymes. Chemosphere 58:595–604
Zayed AM, Terry N (2003) Chromium in the environment: factors affecting biological remediation. Plant Soil 249:139–156
Kotas J, Stasicka Z (2000) Chromium occurrence in the environment on methods of its speciation. Environ Pollut 107:263–283
Dixit V, Pandey V, Shyam R (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv. Azad) root mitochondria. Plant Cell Environ 25:687–690
Panda SK (2003) Heavy metal phytotoxicity induces oxidative stress in Taxithelium sp. Cur Sci 84:631–633
Panda SK (2007) Chromium-mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. J Plant Physiol 164:1419–1428
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Inter 31:739–753
Bai TH, Li Y, Ma FW, Shu HR, Han MY (2009) Exogenous salicylic acid alleviates growth inhibition and oxidative stress induced by hypoxia stress in Malus robusta Rehd. J Plant Growth Regul 28:358–366
Panda SK, Khan MH (2003) Antioxidant efficiency in rice (Oryza saliva L.) leaves under heavy metal toxicity. Biol Plant 30:23–29
Choudhury S, Panda SK (2005) Toxic effect, oxidative stress and ultrastructural changes in moss Taxitheelium nepalense (Schwaegr.) Broth. under lead and chromium toxicity. Water Air Soil Pollut 167:73–90
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios L (2006) Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM 9 (Malus domestica Borkh). Environ Exp Bot 56:54–62
Ogawa K (2005) Glutahione-associated regulation of plant growth and stress responses. Antioxid Redox Sign 7:973–981
Salt DE, Rauser WE (1995) MgATP-dependent of phytochelatins across the tonoplast of oat roots. Plant Physiol 107:1293–1301
Wu FB, Chen F, Wei K, Zhang GP (2004) Effect of cadmium on free amino acid, glutathione and ascorbic acid concentrations in two barley genotypes (Hordeum vulgare L.) differing in cadmium tolerance. Chemosphere 57:447–454
Srivalli S, Khanna-Chopra R (2008) Role of glutathione in abiotic stress tolerance. In: Khan NA, Singh S, Umar S (eds) Sulfur assimilation and abiotic stress in plants. Springer, Berlin, pp 207–225
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Zhu YL, Pilon-Smiths EA, Jouanin L, Terry N (1999) Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol 109:1141–1149
Zhu YL, Pilon-Smits EA, Tarun A, Weber SU, Jouanin L, Terry N (1999) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing g-glutamylcysteine synthetase. Plant Physiol 121:1169–1177
Xiang C, Werner B, Christensen E, Oliver DC (2001) The biological function of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol 126:564–574
Bogs J, Bourbouloux A, Cagnac O, Wachter W, Rausch T, Delrot S (2003) Functional characterization and expression analysis of a glutathione transporter, BjGT1, from Brassica juncea: evidence for regulation by heavy metal exposure. Plant Cell Environ 26:1703–1711
Zeng FR, Mao Y, Cheng WD, Wu FB, Zhang GP (2008) Genotypic and environmental variation in chromium, cadmium and lead concentrations in rice. Environ Pollut 153:309–314
Yoshida S, Forna DA, Cock HJ, Gomez KA (1976) Laboratory manual for physiological studies of rice. International Rice Research Institute, Los Baños, pp 62–63
Miller RO (1998) Nitric-perchloric acid wet digestion in an open vessel. In: Kalra YP (ed) Handbook of reference methods for plant analysis. CRC Press, Taylor & Francis Group, Boca Raton, pp 57–62
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Putter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis: II. Academic, New York, pp 685–690
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Garcia-Limones C, Hervas A, Navas-Cortes JA, Jimenez-Diaz RM, Tena M (2002) Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. Ciceris. Physiol Mol Plant Pathol 61:325–337
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Mei B, Puryear JD, Newton RJ (2002) Assessment of Cr tolerance and accumulation in selected plant species. Plant Soil 247:223–231
Shanker AK (2003) Physiological, biochemical and molecular aspects of chromium toxicity and tolerance in selected crops and tree species. PhD thesis, Tamil Nadu Agricultural University. Coimbatore, India
Sinha S, Saxena R, Singh S (2002) Comparative studies on accumulation of Cr from metal solution and tannery effluent under repeated metal exposure by aquatic plants: its toxic effects. Environ Monitor Assess 80:17–31
Panda SK, Choudhury S (2005) Changes in nitrate reductase activity and oxidative stress response in the moss Polytrichum commune subjected to chromium, copper and zinc toxicity. Braz J Plant Physiol 17:191–197
Panda SK, Choudhury S (2005) Chromium stress in plants. Braz J Plant Physiol 17:95–102
Panda SK, Chaodhury I, Khan MH (2003) Heavy metal phytotoxicity induces lipid peroxidation and affect antioxidants in wheat leaves. Biol Planta 46:289–294
Shanker AK, Sudhagar R, Pathmanabhan G (2001) Growth, phytochelatin SH and antioxidative response of sunflower as affected by chromium speciation. 2nd International Congress of Plant Physiology on sustainable plant productivity under changing environment. New Delhi, India
Fridovich I (1986) Biological effects of superoxide radical. Arch Biochem Biophys 247:1–11
Matés JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Shanker AK, Djanaguiraman M, Sudhagar R, Chandrashekar CN, Pathmanabhan G (2004) Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L) R Wilczek, cv CO 4) roots. Plant Sci 166:1035–1043
Cobbett C (2000) Phytochelatins and their role in heavy metal detoxification. Plant Physiol 123:825–832
Diwan H, Khan I, Ahmad A, Iqbal M (2010) Induction of phytochelatins and antioxidant defence system in Brassica juncea and Vigna radiata in response to chromium treatments. Plant Growth Regul 61:97–107
Lima AIG, Corticeiro SC, Figueira E (2006) Glutathione-mediated cadmium sequestration in Rhizobium leguminosarum. Enzyme Microb Technol 39:763–769
Brauer SL, Wetterhahn KE (1991) Chromium (VI) forms thiolate complex with glutathione. J Am Chem Soc 113:3001–3007
Wu FB, Zhang GP, Peter D (2003) Four barley genotypes respond differently to cadmium:lipid peroxidation and activities of antioxidant capacity. Environ Exp Bot 50:67–77
Diwan H, Ahmad A, Iqbal M (2008) Genotypic variation in the phytoremediation potential of Indian mustard for chromium. Environ Manage 41:734–741
Acknowledgments
The project was supported by the Zhejiang Bureau of Science and Technology (2009C12050).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
►Exogenous application of GSH alleviated the reduction of plant growth and chlorophyll contents in Cr-stressed rice plants.
►Exogenous application of GSH reduced MDA accumulation and enhanced the antioxidant capacity in Cr-stressed rice plants.
►Exogenous application of GSH decreased Cr uptake and root-to-shoot transport in Cr-stressed rice plants.
Rights and permissions
About this article
Cite this article
Zeng, F., Qiu, B., Wu, X. et al. Glutathione-Mediated Alleviation of Chromium Toxicity in Rice Plants. Biol Trace Elem Res 148, 255–263 (2012). https://doi.org/10.1007/s12011-012-9362-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9362-4