Abstract
This paper reports the synthesis of a new bioactive complex, 8-hydroxyquinolinato-bis-(salicylato) yttrium (III) (HSAY), whose composition and structure were characterized by elemental analysis, IR spectra, thermogravimetric analysis, and X-ray diffraction. The power–time curves of the compounds HSAY, C7H6O3, C9H7NO, and YCl3·6H2O on the growth metabolism of Schizosaccharomyces pombe (S. pombe) were determined at 32.00°C, respectively. The corresponding thermokinetics parameters, which include the microbial growth rate constant (κ), inhibition ratio (I), and half inhibition concentration (IC50), were also derived. The results showed that the generation time was 168.2 min, and all the compounds HSAY, C7H6O3, C9H7NO, and YCl3·6H2O possessed good bioactivities on the growth metabolism of S. pombe, with the values of IC50 being 0.055, 3.57, 0.057, and 1.35 mmol L−1, respectively. The inhibition ability of these compounds above on the growth of the S. pombe has been observed to decrease in the order HSAY>C9H7NO>YCl3·6H2O>C7H6O3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is known that rare earth ions possess the properties of antibacterial [1], antitumor [2], and antivirus [3–5] agents when coordinated with organic small molecule ligands. Due to their strong affinities for many biological molecules, rare earth ions can effectively participate in many important life processes and activate or inhibit a variety of enzymes or pro-enzymes. For example, yttrium complexes exhibit some attractive bioactivities that are cytotoxic [6, 7] and antiproliferative [8–11]. Interestingly, recent reports showed that salicylic acid and its derivatives have potential anticancer [12, 13] and antibacterial [14] activities. In addition, complexing ligands such as 8-hydroxyquinoline and its derivatives were also reported to have promising bioactivities, including anticancer [15, 16], antibacterial [17, 18], antidyslipidemic and antioxidative properties [19], vasorelaxing properties [20], antivirus [21, 22], and antiplatelet [23] activities. Accordingly, the bioactivities of the product from the reaction of yttrium chloride hexahydrate with salicylic acid and 8-hydroxyquinoline could be expected to be significantly stronger than those of the yttrium ion, salicylic acid, and 8-hydroxyquinoline alone.
Biological microcalorimetry, providing a continuous measurement of heat production, can be employed to directly determine the biological activities of a living system. Heat flux is an expression of overall metabolic flux, and the detection of small changes in heat production to respond to toxic insult will be a sensitive indicator of altered metabolism. Since microcalorimetry is a nondestructive method with high accuracy and automaticity, it is now widely applied in biological research [24] and pharmacological analysis [25].
In this paper, we report the synthesis and characterization of the new bioactive complex, 8-hydroxyquinolinato-bis-(salicylato) yttrium (III) (HSAY). To further study the biological effects of HSAY and to clarify its pharmacological mechanisms, Schizosaccharomyces pombe (S. pombe) was used, as it provides an ideal model for studies in cell morphogenesis [26]. The interactions of HSAY with S. pombe were followed by means of microcalorimetry. The heat output power curve of metabolism of S. pombe was determined by a TAM Air calorimeter [27]. In addition, we analyzed the relationship between the concentration of HSAY and the growth of S. pombe by the thermokinetics model.
Experimental
Materials and Instrument
The microcalorimetric study was performed on a 3116-2/3239 TAM Air calorimeter (Thermometric AB, Sweden). Cell incubation was carried out in a temperature oscillation incubator (BS-1EA, China) and a carbon dioxide cell incubator (WJ-160A-II, China). The FT-IR spectrum was measured on an Avatar 360 FT-IR spectrometer (Thermo Nicalet Corporation, USA). Measurement of thermogravimetry (TG) and differential scanning calorimetry (DSC) curves was carried out on a NETZSCH STA449C thermal analysis instrument (NETZSCH Corporation, Germany). An elemental analyzer (Perkin-Elmer 2400 CHN, USA) was used to measure the C, H, and N contents of the complex. The conductivity monitor (DDS-12A, Shanghai, China) was used to measure the conductance of the complex. X-ray diffraction patterns were recorded on a D8 Advance X-ray diffractometer (Bruker Corporation, Germany).
S. pombe (ACCC 20047) was provided by Agricultural Culture Collection of China. The Edinburgh minimal medium (EMM) culture composition was K2HPO4 3 g, Na2HPO4 2.2 g, NH4Cl 5 g, and glucose 20 g; the yeast extract medium composition was 20 mL and H2O 1,000 mL (natural pH).
The chemicals YCl3·6H2O(s) (>99%), C7H6O3(s) (>99.5%), and C9H7NO(s) (>99.5%) were purchased from Shanghai Reagent Company. YCl3·6H2O(s) was dried in a desiccator containing sulfuric acid (60%) at room temperature. C7H6O3(s) and C9H7NO(s) were dried in a vacuum desiccator containing P4O10 until their mass remained constant.
Synthesis and Characterization of the Complex
Synthesis of the Complex
8-Hydroxyquinolinato-bis-(salicylato) yttrium (III) was prepared according to the literature method [28]. A mass of 0.02 mol of powdered C7H6O3 (s) was dissolved in 40 cm3 of absolute ethyl alcohol (solution A). Sodium salicylate solution (solution B) was formed when an aqueous solution of 10% NaOH (0.02 mol NaOH) was added into the solution A. A mass of 0.01 mol of powdered 8-hydroxyquinoline was dissolved in 40 cm3 of absolute ethyl alcohol (solution C). A mixture solution (solution D) was obtained when the solution B was added into the solution C. A mass of 0.01 mol of powdered YCl3·6H2O was dissolved in 40 cm3 of anhydrous ethyl alcohol (solution E). Primrose yellow crystals were separated out when the solution E was added slowly into the solution D at 37.5°C after 3 h of magnetic stirring. The reaction solution was left to settle down at pH = 6.5 to 7.0 for 12 h. Finally, the primrose yellow solid complex was obtained by vacuum filtration and successively washed with distilled water, absolute ethyl alcohol, and acetone several times. The product was put into a vacuum desiccator at 85°C for 24 h and kept until the mass of the crystals became constant.
Characterization of the Complex
The complex is obtained as a yellow solid. It is soluble in dimethyl formamide (DMF) and dimethyl sulfoxide (DMSO). A few of it can be dissolved in absolute ethyl alcohol but it cannot be dissolved in water, acetone, aether, and benzene. The molar conductance of the complex in DMF is 4.1 × 10−4 S m2 mol−1, indicating that the complex is a nonelectrolyte and exists as a neutral molecule in DMF.
The chemical composition of the synthetic complex was determined by elemental analysis for C, H, and N, by the EDTA titration for Y3+ [29], by mercury salt titration for Cl−, and by TG-DSC curves for H2O. The elemental analysis data [observed/percent (calculated/percent)], C 54.59 (54.46), H 3.08 (3.18), N 2.77 (2.76), and Y 17.47(17.52), showed that the composition of complex was (C7H5O3)2Y(C9H6NO), and its purity was more than 99.8%.
Infrared spectra of the complex (C7H5O3)2Y(C9H6NO), salicylic acid, and 8-hydroxyquinoline were obtained from KBr pellets at room temperature using an IR spectrophotometer. There are five characteristic bands observed for the salicylic acid: \( \nu_{\text{OH}}^{\text{COOH}} \) (intramolecular hydrogen bond, 3,237 cm−1 s), \( \nu_{\text{OH}}^{\text{COOH}} \) (intermolecular hydrogen bond, 2,857 cm−1 s), ν O–H (COOH, 2,598 cm−1 s), ν C–O (COOH, 1,663 cm−1 vs), and δ O–H (phenol, 1,483 cm−1 s). All these bands disappeared after the complex formation except the band due to the angular deformation of the OH group. At the same time, two new absorption bands due to the carboxylate group \( \nu_{\text{as}}^{{{\text{CO}}{{\text{O}}^{{ - }}}}} \) (1,593 cm−1, s) and \( \nu_{\text{s}}^{{{\text{CO}}{{\text{O}}^{{ - }}}}} \) (1,389 cm−1, s), appeared, indicating that the oxygen atoms of the carboxylate group were coordinated to the rare earth ion (Y3+). The values of the splitting for the absorption bands of the valency vibration \( \nu_{\text{as}}^{{{\text{CO}}{{\text{O}}^{{ - }}}}} \) and \( \nu_{\text{s}}^{{{\text{CO}}{{\text{O}}^{{ - }}}}} \) are all \( \Delta \nu \left( {{\nu_{\text{as}}} - {\nu_{\text{s}}}} \right) = 204{\text{c}}{{\text{m}}^{{ - 1}}} \). This Δν is equivalent to the Δν of sodium salicylate. Accordingly, from these results, it can be concluded that the carboxylate group was coordinated to the rare earth ion through two oxygen atoms, as a symmetrical bidentate ligand.
There are four characteristic absorption bands observed for free 8-hydroxyquinoline: ν O−H (3,102 cm−1), ν C=O (1,095 cm−1), ν C=N (1,579 cm−1), and δ O–H (1,224 cm−1). Both of the two characteristic absorption bands ν O−H (3,102 cm−1) and δ O–H (1,224 cm−1) disappeared after formation of the coordinate complex; and meanwhile, ν C=O (1,095 cm−1) shifted towards higher frequency, increasing by 9 to 11 cm−1, which may due to the rare earth ion (Y3+) was coordination with ligands and form a Y–O bond in which the electronegativity of rare earth ion is less than that of hydrogen, while the electronegativity of oxygen is larger. In addition, the original absorption band ν C=N (1,579 cm−1) shifted towards lower frequency, decreasing by 7 to 10 cm−1. From these results, we can conclude that the rare earth ion (Y3+) was coordinated with the hydroxyl oxygen atom and hetero-nitrogen atom in 8-hydroxyquinoline, as a five-membered chelate ring. The chemical structure of the complex is given in Fig. 1.
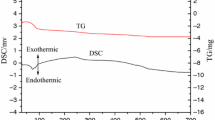
TG and DSC curves of (C7H5O3)2Y(C9H6NO) in the temperature range of room temperature to 1,320°C at a heating rate of 10°C min−1 in flowing N2 are shown in Fig. 2. The thermal decomposition process of (C7H5O3)2Y(C9H6NO) can be divided into three stages. The first stage is from 389°C to 540°C. The TG curve shows that the mass loss corresponding to this temperature range is 25.89%, which roughly coincides with the value of 26.84%, calculated for the loss of 1 mol of (C9H6NO)− from the complex. The second stage ranges from 621°C to 710°C with the mass loss of 33.36%, which corresponds to the loss of 1 mol of (C7H5O3)−. The theoretical mass loss is 34.79%. The third stage degradation temperature is in the range of 710°C to 992°C with the mass loss of 52.60%, which corresponds to the loss of 1 mol of (C7H5O3)−. The theoretical mass loss is 53.35%. On the basis of experimental and calculated results, the thermal decomposition of (C7H5O3)2Y(C9H6NO) can be postulated as follows:
X-ray diffraction measurement was performed on a D8 ADVANCE X-ray diffraction apparatus (Bruker Corporation, Germany) scanning from 5° to 80° using Cu Kα (λ = 1.54187 Å) radiation. The tube voltage was 40 KV, tube current was 40 mA, scanning speed was 0.5 s, and scanning step was 0.02°. The details of the XRD analysis results are shown in Fig. 3. According to the standard card (no. 33-1954) in a spectral database, it was demonstrated that the crystal type of (C7H5O3)2Y(C9H6NO) was similar to that of C5H11NO2. The cell parameters were a = 5.426 nm, b = 22.105 nm, and c = 5.277 nm.
Determination of Bioactivity of HSAY on S. pombe Cells by Biocalorimetry
The microcalorimetric measurements were carried out on a TAM air isothermal microcalorimeter at 32.00°C. Baselines were taken before each measurement and the calorimeter was calibrated electrically. More details of the performance and construction of the instrument are available in [27].
When the system had gained a stable baseline, 5 mL EMM-sterilized culture medium was added into the sterilized sample ampoules. S. pombe was inoculated with an initial density of 1 × 106 cells·mL−1. Samples of HSAY at different concentrations were added to the cell suspension, respectively. Power–time curves for all measurements were performed at 32.00°C. All the microcalorimetric experiments were repeated three times and the results were identical.
Results and Discussion
Thermogenic Curves
The thermogenic curves for growth of S. pombe cells treated by different concentrations of the compounds HSAY, C7H6O3, C9H7NO, and YCl3·6H2O were determined by using the ampoule method at 32.00°C, respectively. All microcalorimetric experiments were repeated three times. The results are illustrated in Fig. 4. From Fig. 4, we can find that the thermogenic curves are similar to those of S. pombe treated by different concentrations of HSAY. As shown as in Fig. 4a, the metabolic process can be divided into four phases: lag phase (AB), activity recovery phase (BC), stationary phase (CD), and decline phase (DE). Figure 4 obviously revealed that all the compounds HSAY, C7H6O3, C9H7NO, and YCl3·6H2O possessed the bidirectional biological effect and Hormesis effect. They stimulated the growth of the S. pombe at low concentration, but inhibited the growth of S. pombe at high concentration.
Thermokinetics
During the lag phase of Fig. 4, the power–time curves obeyed the following equation:
where P t was the heat output power of the S. pombe cell at time t, and k was the growth rate constant of the S. pombe cell at specified conditions, whose size represented growth speed. Using this equation, the growth rate constant k could be calculated and the results are shown in Table 1.
Plot of the growth rate constant (k) against concentration (c) is shown in Fig. 5. It could be seen from Fig. 5 that the growth rate constant (κ) of S. pombe slightly increased with the increasing of the concentration of HSAY among the range of 0.00 to 0.01 mmol L−1, while it decreased with the increasing of the concentration of HSAY among the range of 0.01 to 0.10 mmol L−1, which showed the HSAY possessed the bidirectional biological effect and Hormesis effect that was HSAY stimulated the growth of S. pombe at low concentration, but inhibited the growth of S. pombe at high concentration. The relationship between κ and c HSAY could be described as:
The correlation coefficient R was 0.9862. Thus, the growth rate constant (k) in the range of the above applied amount of HSAY on S. pombe could be clearly inferred through the application of Eq. 2.
The generation time can be calculated as: \( {t_{\text{G}}} = \ln 2/k = 168.2\min \).
The inhibition ratio of the growth metabolism of S. pombe cells by drug was defined as following:
where k 0 was the control rate constant (without any drug inhibition) of S. pombe and k c was the growth rate constant of S. pombe under an inhibitor with a concentration of c. The values of (I) are shown in Table 1. When the inhibition ratio was 50%, the drug concentration was the half inhibition concentration (IC50).
By plotting the inhibition ratio (I) against concentration (c), Fig. 6 was obtained. It could be seen that the inhibition ratio (I) was gradually increased with the increasing of concentration (c) of HSAY, which indicated that the growth of S. pombe was significantly inhibited. Using the Lorentz curve fitting the data of the inhibition ratio (I) of S. pombe with concentration (c) of HSAY, the curve equation was obtained:
The correlation coefficient R was 0.9813. Thus, the inhibition ratio in the range of the above applied amount of HSAY on S. pombe could be clearly inferred through the application of Eq. 4. The half inhibition concentrations (IC50) of HSAY, C7H6O3, C9H7NO, and YCl3·6H2O were found to be 0.055, 3.57, 0.057, and 1.35 mmol L−1, respectively. The inhibition ability of these compounds above on the growth of the S. pombe has been observed to decrease in the order TSAS>C9H7NO>YCl3·6H2O>C7H6O3. The inhibitory action of the complex HSAY was greatly stronger than that of the two ligands (C7H6O3 and C9H7NO) and YCl3·6H2O alone. From the above-mentioned results, a conclusion can be reached that the compound HSAY could be a good inhibitor on the bacteria and the tumor cells.
Thermodynamics
The total thermal effect (Q total) value can be calculated by the area under the power–time curves and shown in Table 1. Where the heat output has been observed to remain almost the same and the mean value can be obtained.
The curve in Fig. 7 shows the plotting of the total thermal effect (Q total) of S. pombe growth against concentration (c). By using the Gauss curve fitting the data of the total thermal effect (Q total) of S. pombe growth with concentration of HSAY (c), the curve equation was obtained:
The correlation coefficient R was 0.9999. Thus, the total thermal effect in the range of the above applied amount of HSAY on S.pombe could be clearly inferred through the application of Eq. 5. From Fig. 7, it could be seen that under low concentration of HSAY, the total thermal effect (Q total) of S. pombe growth augmented with the increasing of the concentration of HSAY, which indicated that HSAY ratherish stimulated the growth of S. pombe cell; while under high concentration, the total thermal effect (Q total) of S. pombe growth decreased with increasing of the concentration of HSAY, which indicated that HSAY significantly inhibited the growth of S. pombe cell.
Conclusions
In this work, a new bioactive complex, 8-hydroxyquinolinato-bis-(salicylato) yttrium (III) (HSAY), was synthesized and characterized. The experimental results indicated that HSAY possessed the bidirectional biological effect and Hormesis effect. HSAY stimulated the growth of the S. pombe at low concentration, but inhibited the growth of the S. pombe at high concentration. The half inhibition concentrations (IC50) of HSAY, C7H6O3, C9H7NO, and YCl3·6H2O were found to be 0.055, 3.57, 0.057, and 1.35 mmol L−1, respectively. The inhibition ability of these compounds on the growth of the S. pombe has been observed to decrease in the order HSAY>C9H7NO>YCl3·6H2O>C7H6O3.
The present study demonstrates that microcalorimetry is a valuable tool for studies of S. pombe because it could provide important thermokinetics and thermodynamic information that cannot be obtained from conventional biological techniques. In conclusion, the present work provides new insights for the biothermochemical studies of HSAY on S. pombe and has theoretical and practical significance for the life science.
References
Yang L, Tao D, Yang X, Li Y, Guo Y (2003) Synthesis, characterization, and antibacterial activities of some rare earth metal complexes of pipemidic acid. Chem Pharm Bull(Tokyo) 51:494–502
Zhou J, Wang LF, Wang JY, Tang N (2001) Synthesis, characterization, antioxidative and antitumor activities of solid quercetin rare earth(III) complexes. J Inorg Biochem 83:41–49
De Gusseme B, Du Laing G, Hennebel T, Renard P, Chidambaram D, Fitts JP, Bruneel E, Van Driessche I, Verbeken K, Boon N, Verstraete W (2010) Virus removal by biogenic cerium. Environ Sci Technol 44:6350–6356
Liu YN, Shi S, Mei WJ, Tan CP, Chen LM, Liu J, Zheng WJ, Ji LN (2008) In vitro and in vivo investigations on the antiviral activity of a series of mixed-valence rare earth borotungstate heteropoly blues. Eur J Med Chem 43:1963–1970
Manolov I, Raleva S, Genova P, Savov A, Froloshka L, Dundarova D, Argirova R (2006) Antihuman immunodeficiency virus type 1 (HIV-1) activity of rare earth metal complexes of 4-hydroxycoumarins in cell culture. Bioinorg Chem Appl 2006:71938
Saturnino C, Napoli M, Paolucci G, Bortoluzzi M, Popolo A, Pinto A, Longo P (2010) Synthesis and cytotoxic activities of group 3 metal complexes having monoanionic tridentate ligands. Eur J Med Chem 45:4169–4174
Hou AX, Liu Y, Wong WK, Xue Z, Qu SS (2003) Interaction of Y3+ and its cationic monoporphyrinate complex with Staphylococcus aureus. Acta Chim Sin 61:1382–1387
Kostova I, Trendafilova N, Momekov G (2008) Theoretical, spectral characterization and antineoplastic activity of new lanthanide complexes. J Trace Elem Med Biol 22:100–111
Kostova I, Kostova R, Momekov G, Trendafilova N, Karaivanova M (2005) Antineoplastic activity of new lanthanide (cerium, lanthanum and neodymium) complex compounds. J Trace Elem Med Biol 18:219–226
Kostova I, Manolov I, Nicolova I, Konstantinov S, Karaivanova M (2001) New lanthanide complexes of 4-methyl-7-hydroxycoumarin and their pharmacological activity. Eur J Med Chem 36:339–347
Zhu WZ, Lin QY, Lu M, Hu RD, Zheng XL, Cheng JP, Wang YY (2009) Synthesis, characterization, DNA-binding and antiproliferative activity of Nd(III) complexes with N-(nitrogen heterocyclic) norcantharidin acylamide acid. J Fluoresc 19:857–866
Stark LA, Reid K, Sansom OJ, Din FV, Guichard S, Mayer I, Jodrell DI, Clarke AR, Dunlop MG (2007) Aspirin activates the NF-kappaB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis 28:968–976
Price CT, Lee IR, Gustafson JE (2000) The effects of salicylate on bacteria. Int J Biochem Cell Biol 32:1029–1043
Shaw AY, Chang CY, Hsu MY, Lu PJ, Yang CN, Chen HL, Lo CW, Shiau CW, Chern MK (2010) Synthesis and structure-activity relationship study of 8-hydroxyquinoline-derived Mannich bases as anticancer agents. Eur J Med Chem 45:2860–2867
Shen AY, Wu SN, Chiu CT (1999) Synthesis and cytotoxicity evaluation of some 8-hydroxyquinoline derivatives. J Pharm Pharmacol 51:543–548
Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE (2005) Anticancer activity of the antibiotic clioquinol. Cancer Res 65:3389–3395
Darby CM, Nathan CF (2010) Killing of non-replicating Mycobacterium tuberculosis by 8-hydroxyquinoline. J Antimicrob Chemother 65:1424–1427
Ibrahim SA, Makhlouf MT, Abdel-Hafez AA, Moharram AM (1986) Some transition metal chelates of 8-hydroxyquinoline-5-sulfonamides as possible drugs. J Inorg Biochem 28:57–65
Kay K (1978) The use of 8-hydroxyquinoline hydrochloride as a dental antibacterial agent. J Periodontol 49:47
Sashidhara KV, Kumar A, Bhatia G, Khan MM, Khanna AK, Saxena JK (2009) Antidyslipidemic and antioxidative activities of 8-hydroxyquinoline derived novel keto-enamine Schiffs bases. Eur J Med Chem 44:1813–1818
Bertini S, Calderone V, Carboni I, Maffei R, Martelli A, Martinelli F, Minutolo Rajabi M, Testai L, Tuccinardi T, Ghidoni R, Macchia M (2010) Synthesis of heterocycle-based analogs of resveratrol and their antitumor and vasorelaxing properties. Bioorg Med Chem 18:6715–6724
Liu YJ, Zhao Y, Zhai X, Feng X, Wang J, Gong P (2008) Synthesis and anti-hepatitis B virus evaluation of novel ethyl 6-hydroxyquinoline-3-carboxylates in vitro. Bioorg Med Chem 16:6522–6527
Zeng XW, Huang N, Xu HY, Yang WB, Yang LM, Qu H, Zheng YT (2010) Anti human immunodeficiency virus type 1 (HIV-1) agents 4. Discovery of 5,5'-(p-phenylenebisazo)-8-hydroxyquinoline sulfonates as new HIV-1 inhibitors in vitro. Chem Pharm Bull (Tokyo) 58:976–979
Montanari MLC, Beezer AE, Montanari CA, Pilo-Veloso D (2000) QSAR based on biological microcalorimetry. J Med Chem 43:3448–3452
Urakami K (2005) Characterization of pharmaceutical polymorphs by isothermal calorimetry. Curr Pharm Biotechnol 6:193–203
Juan Cortes JC, Carnero E, Ishiguro J, Sánchez Y, Durán A, Ribas JC (2005) The novel fission yeast (1,3)beta-d-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and poSmrized growth. J Cell Sci 118(Pt 1):157–174
Li QG, Yang DJ, Li X, Ye LJ, Wei DL, Xiao SX (2008) Thermokinetic studies of action of complexes [RE(Hsal)2-(tch)]-2H2O on growth metabolism of Escherichia Coli. Acta Chim Sin 66:2686–2692
Li QG, Huang Y, Li X, Ye LJ, Xiao SX, Yang DJ, Liu Y (2008) Synthesis, characterization and standard molar enthalpy of formation of Nd(C7H5O3)2 (C9H6NO). J Therm Anal Cal 91:615–620
Xie ZQ (1985) Determination of center lanthanide ions in organic complex salts. J Wuhan Univ 2:117–122
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (no. 20973145), the Hunan Provincial Natural Science Foundation (no. 08JJ3014) of China, and the Hunan Provincial Educational Ministry Foundation of China (no. 06C784). The authors would like to gratefully acknowledge Master Hui-Wen Gu (State Key Laboratory of Chemo/Biosensing & Chemometrics, Hunan University, People’s Republic of China) for his help in reviewing and revising our manuscript. Prof. Qiang-Guo Li would like to thank Dr. Qing-Qi Chen (MedKoo Biosciences, Inc, USA) for his help in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Li, QG., Zhang, H. et al. Synthesis and Bioactive Studies of Complex 8-Hydroxyquinolinato-Bis-(Salicylato) Yttrium (III). Biol Trace Elem Res 147, 366–373 (2012). https://doi.org/10.1007/s12011-011-9297-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9297-1