Abstract
In the present work, the response of tobacco (Nicotiana tabaccum L.) wild-type SR1 and transgenic CAT1AS plants (with a basal reduced CAT activity) was evaluated after exposure to the herbicide paraquat (PQ). Superoxide anion (O .−2 ) formation was inhibited at 3 or 21 h of exposure, but H2O2 production and ion leakage increased significantly, both in SR1 or CAT1AS leaf discs. NADPH oxidase activity was constitutively 57% lower in non-treated transgenic leaves than in SR1 leaves and was greatly reduced both at 3 or 21 h of PQ treatment. Superoxide dismutase (SOD) activity was significantly reduced by PQ after 21 h, showing a decrease from 70% to 55%, whereas catalase (CAT) activity decreased an average of 50% after 3 h of treatment, and of 90% after 21 h, in SR1 and CAT1AS, respectively. Concomitantly, total CAT protein content was shown to be reduced in non-treated CAT1AS plants compared to control SR1 leaf discs at both exposure times. PQ decreased CAT expression in SR1 or CAT1AS plants at 3 and 21 h of treatment. The mechanisms underlying PQ-induced cell death were possibly not related exclusively to ROS formation and oxidative stress in tobacco wild-type or transgenic plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) are generated in plants during photosynthesis and respiration, and their homeostasis is regulated by the equilibrium between synthesis and detoxification. Plants can cope with ROS by enzymatic and non-enzymatic antioxidant systems.
The major sites of the main ROS (O .−2 and H2O2) formation during abiotic stress is still a matter of debate; however, NADPH oxidase, peroxidases, and lipooxygenase have been put forward as putative sources of ROS production during the oxidative burst [1–3].
Catalase (CAT, EC 1.11.1.6) is a tetrameric iron-porphyrin protein that catalyzes the decomposition of H2O2 into water and dioxygen. In plants, it is located in microbodies such as peroxisomes and glyoxysomes and is involved in detoxification of H2O2 generated by various environmental stresses like high light [4, 5], temperature [6], and metals [7, 8], as well as H2O2 produced under physiological conditions. CAT1 is the dominant CAT isoform in leaves, where it serves in the removal of photorespiratory-derived H2O2 [9], and CAT1AS plants (CAT-deficient plants) were originally produced to study the role of CAT in plants [10]. Under photorespiratory conditions created by high light exposure, CAT-deficient plants showed no visible disorders (except for a reduced growth compared to WT plants), and in elevated light rapidly accumulated H2O2, developed white necrotic lesions on the leaves, stimulated salicylic acid, and ethylene production, and induced the expression of acidic and basic pathogenesis-related proteins with a timing and magnitude similar to the hypersensitive response against pathogens [9, 11, 12].
The active component of the herbicide paraquat, methyl viologen (MV; 1,10-dimethyl-4,4′-bipyridinium dichloride), exerts its phytotoxic effects on plants by transferring electrons from photosystem I to molecular oxygen, resulting in the accumulation of superoxide radicals in chloroplasts [13]. Both its herbicidal and toxicological properties depend on the transformation of bipyridyl residues to monocation radicals that result from an enzymatic one-electron reduction in the presence of NADPH. The radical reacts with oxygen rendering superoxide anion and regenerating the paraquat cation, thus closing a redox cycle. Thus, MV toxicity results from deleterious reactions of cellular components with ROS which can trigger harmful reactions such as DNA alterations, enzyme inactivation, protein degradation, and lipid peroxidation, affecting key components of plant cell metabolism [1, 5, 14]. It has been reported that paraquat can readily penetrate the leaf cuticle, quickly decreasing photosynthetic activity, rupturing membranes, and causing loss of turgor in treated leaves within hours [15, 16].
Esterases (EC 3.1.1.x) represent a diverse group of hydrolases catalyzing the cleavage and formation of carboxyl ester bonds. They are involved in the formation of the cell wall [17], degradation of xenobiotics [18], and signaling [19]. Esterases have been used as markers of viability in combination with other dyes for a long time. Esterase activity depends on cell viability but is independent of membrane integrity [20]. Their connection with development has made them a suitable marker of development in plants.
In the present work, we compared paraquat-induced damage in wild-type and CAT-deficient plants, with the aim to elucidate the importance of the ROS detoxifying system in avoiding PQ toxicity.
Materials and Methods
Plant Growth Conditions and Treatments
Nicotiana tabacum var. Petit Havana SR1-wild-type and N. tabacum CAT1AS (a transgenic line that expresses only 10–30% of wild-type CAT activity in the leaves and only 40% in the roots due to the antisense expression of the cat1 gene) derived from N. tabacum Petit Havana SR1 [12] were used for the experiment. The seeds of the transgenic tobacco line were kindly provided by Dr. F. Van Breusegem (Gent University, Belgium). Seeds were germinated and grown as described previously [12]. All treatments were performed in a controlled environmental chamber with a relative humidity of 70% and temperature of 24/21°C for day/night period, with a light intensity of 120 mmol m−2 s−1. Unless otherwise indicated, experiments were performed using the fourth or fifth leaf (counting from the bottom) of 6–8-week-old plants. Leaf discs (8 mm diameter) were cut with a cork-borer, put in glass flasks containing 25 ml of the treatment solution (distilled water or 100 μM PQ), and incubated in a rotary shaker for 3 or 21 h, under continuous illumination. After that, leaf discs were washed with distilled water, dried with adsorbent paper, and used for analysis.
In Situ O .−2 Localization
To estimate O .−2 content, leaf discs from SR1 or CAT1AS were immersed in a 0.05% solution of nitroblue tetrazolium (NBT) in 50 mM potassium phosphate buffer (pH 6.4), vacuum-infiltrated for 3 min, and illuminated 2 h until appearance of dark spots. Leaf discs were bleached by immersing in boiling ethanol. Before staining with NBT, leaves were immersed for 2 h in 20 μM diphenylene iodonium (DPI, a NADPH oxidase inhibitor), infiltrated, and then treated with PQ. Inhibition of an oxidative burst by DPI is indicative of the involvement of a NADPH oxidase in the process [21].
In Situ H2O2 Localization
Leaf discs from SR1 and CAT1AS plants were exposed to PQ to evaluate “in situ” H2O2 production by an endogenous peroxidase-dependent staining procedure using 3,3′-diaminobenzidine (DAB) [22]. Leaf segments were immersed in a 1 mg ml−1 DAB solution, pH 3.8, vacuum-infiltrated for 3 min, incubated at room temperature for 2 h in light until appearance of brown spots to evidence H2O2 formation. Leaves were bleached in boiling ethanol. Ascorbic acid was used as antioxidant to confirm that brown spots correspond to H2O2 formation.
Cell Death Detection: Evans Blue Staining, Electrolyte Leakage, and Esterase Activity
To determine changes in viability of cells by PQ treatment, leaf discs were infiltrated with a 0.25% (w/v) aqueous solution of Evans Blue [23] during 15 min at room temperature and then washed twice for 15 min with distilled water to remove the dye in excess. Finally, leaf discs were incubated in distilled water overnight. For quantitative assessment, blue precipitates were quantified by solubilization with 1% (w/v) SDS in 50% (v/v) methanol at 50°C for 1 h, and the absorbance was measured at 595 nm.
Cell death was also estimated by measuring ion leakage from leaf discs according to the method described by Shou et al. [24]. Conductivity of treatment solutions was measured at time zero (T0) and following 3 or 21 h of incubation (T1). Then, leaf discs were boiled in the solution at 100°C for 1 h, and the conductivity was measured again (T2), expressing the results as relative conductivity [(T1 − T0)/(T2 − T0)] × 100.
Extracts for determination of esterase (EA, EC 3.1.1.x) were prepared using 0.150 g FW of leaf discs in 1.0 ml of 250 mM potassium phosphate buffer pH 8.7 containing 1 mM DTT and 1 g PVP. The homogenates were centrifuged at 10,000×g for 15 min. After centrifugation, 150 μl of supernatant (S) was collected and mixed with 2,850 μl of newly prepared phosphate buffer containing 0.0125% FDA (solution in dry acetone). The mixture was incubated at 35°C for 15 min and rapidly placed on ice to stop the reaction. The absorbance was measured at 490 nm as described by Steward et al. [25]. A control containing phosphate buffer instead of supernatant was performed to take into account FDA self-degradation. The amount of acetone did not exceed 1% in the reaction mixture.
Thiobarbituric Acid Reactive Substances Determination
Lipid peroxidation was determined as the amount of thiobarbituric acid reactive substances (TBARS) as described by Heath and Packer [26], using 300 mg FW homogenized in 3 ml of 20% (w/v) trichloroacetic acid (TCA). To 1 ml of the aliquot of the supernatant, 1 ml of 20% (w/v) TCA containing 0.5% (w/v) TBA and 100 μl 4% (w/v) BHT in ethanol were added. The mixture was heated at 95°C for 25 min and then quickly cooled on ice. The contents were centrifuged at 3,000 rpm for 3 min, and the absorbance was measured at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The concentration of TBARS was calculated using an extinction coefficient of 155 mM−1 cm−1.
Subcellular Fractionation and Assay of NADPH Oxidation
Samples (1 g FW) were homogenized in 3.5 ml of HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-KOH buffer (pH 7.8), containing 250 mM sucrose, 0.5 mM PMSF, 1.0 mM ethylenediaminetetraacetic acid (EDTA), 1.0 mM DTT, and 0.5 mM MgCl2 (reaction buffer). To obtain microsomes, the homogenates were centrifuged successively at 600×g for 15 min, at 2,000×g for 20 min, and at 140,000×g for 1 h. From this last centrifugation, pellet (microsomal fraction) and a supernatant (cytosolic fraction) were obtained. The final pellet was resuspended in 500 μL of the reaction buffer and was used to determine the NADPH oxidation rate, following the method used by Van Gestelen et al. [27], monitoring NBT reduction by NADPH at 530 nm for 3 min. NADPH oxidation activity was calculated by taking the difference between the apparent reaction rates with or without SOD (75 U/ml) in the reaction mixture, using an extinction coefficient of 12.8 mM−1 cm−1.
Enzyme Preparations and Assays
Extracts for determination of ascorbate peroxidase (APOX, EC 1.11.1.11) and guaiacol peroxidase (GPOX, EC 1.11.1.7) were prepared using 0.2 g FW of leaf discs in 1.5 ml of 50 mM potassium phosphate buffer pH 7.8 containing 1 mM EDTA and 1 g PVP. APOX activity was measured immediately in fresh extracts as described by Nakano and Asada [28]. GPOX activity was determined following the increase in absorbance at 470 nm due to the formation of tetraguaiacol (ε 26.6 mM−1 cm−1)[29]. Homogenates for superoxide dismutase (SOD, EC 1.15.1.1) and CAT activities were prepared under ice-cold conditions from 0.3 to 0.6 g FW of leaf discs homogenized in 3 or 2 ml of extraction buffer (containing 50 mM phosphate buffer (pH 7.8), 0.5 mM EDTA, 1 g polyvinylpyrrolidone (PVP), and 0.5% (v/v) Triton X-100) at 4°C for SOD and CAT, respectively. Total SOD activity was assayed as described by Becana et al. [30]. CAT activity was determined in the homogenates by measuring the decrease in absorbance at 240 nm (Hitachi U-2000, Hitachi Ltd, Tokyo, Japan) in a reaction medium containing 50 mM potassium phosphate buffer (pH 7.2) and 2 mM H2O2. The pseudo-first order reaction constant (k′ = k × [CAT]) of the decrease in H2O2 absorption was determined, and CAT content in picomoles per gram FW was calculated using k = 4.7, 107 M−1 s−1 [31].
Western Blot Analysis of CAT
Soluble protein extracts were prepared from leaf discs, and 25 μg of total protein were electrophoresed on 12% SDS-PAGE in a Mini PROTEAN III equipment (Bio-Rad), as described by Laemmli [32]. Following electrophoresis at 4–8°C, proteins were transferred to a nitrocellulose membrane (Amersham Biosciences). To immunodetect CAT, the membrane was incubated at 4–8°C overnight with a polyclonal antibody raised in rabbit against a cottonseed CAT (kindly provided by Dr Trelease from Arizona State University). Bands were detected with an anti-rabbit IgG peroxidase conjugated secondary antibody (Sigma), and 3,3′-diaminobenzidine (DAB) was used as substrate for staining procedure. Membranes were photographed with a Fotodyne equipment and analyzed with GelPro software.
Determination of Protein Concentration
Protein concentration for all samples was determined by the method of Bradford [33] using bovine serum albumin as a standard.
Statistics
All data presented are the mean values of two or three independent set of experiments. Each value was presented as means ± standard errors of the mean (SE), with a minimum of three replicates. Statistical analysis was carried out by one-way ANOVA using the Tukey test to evaluate whether the means were significantly different, taking one asterisk *p < 0.05 and three asterisks ***p < 0.001 as significant.
Results
Measurement of Hydrogen Peroxide and Superoxide Anion Accumulation by NBT and DAB Staining
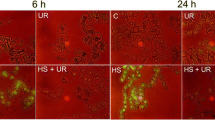
Superoxide anion was detected by the blue formazan precipitation produced after the reaction with nitroblue tetrazolium. At 3 or 21 h, non-treated SR1 or CAT1AS leaf discs showed similar O .−2 -dependent formazan deposition that was almost completely reversed when diphenyleneiodonium (DPI), an inhibitor of flavin-containing oxidases like NADPH oxidase, was used (Fig. 1). In PQ-treated leaves, except for a slight formazan formation in CAT1AS leaf discs at 3 h, almost no NBT staining was observed in any of the exposure times, revealing a complete inhibition of O .−2 formation, either in SR1 or CAT1AS plants.
Accumulation of O .−2 in leaf discs of tobacco SR1 or CAT1AS plants. Control and PQ-treated leaf discs were vacuum-infiltrated for 3 min with 0.05% nitroblue tetrazolium (NBT) and incubated 2 h under illumination as described in “Materials and Methods”. O .−2 formation was revealed by the appearance of blue spots characteristics of formazan deposition. DPI, an inhibitor of flavin-containing oxidases like NADPH oxidase, was used to confirm the involvement of a NADPH oxidase in O .−2 formation
H2O2 production was visualized by staining leaf segments with 3,3′-diaminobenzidine (DAB), a histochemical reagent that polymerizes and turns brown in the presence of H2O2 (Fig. 2). An evident accumulation of H2O2 was observed in leaf discs under PQ treatment, DAB staining being moderately more intense in SR1 than in CAT1AS leaf discs at 3 h of treatment. At 21 h, H2O2 content increased compared to 3 h of treatment in both SR1 and CAT1AS leaf discs (Fig. 2). A slight staining was observed in wild-type or transgenic non-treated discs. H2O2 accumulation was avoided to different extents by infiltrating leaf discs with ascorbate previously to DAB staining, which demonstrated the specificity of this reaction for H2O2 (Fig. 2).
Accumulation of H2O2 in leaf discs of tobacco SR1 or CAT1AS plants. Control and PQ-treated leaf discs were vacuum-infiltrated for 3 min with 1 mg ml−1 3,3′-diaminobenzidine (DAB) and incubated 2 h under illumination as described in “Materials and Methods”. H2O2 was revealed by the appearance of brown spots due to DAB polymerization. Ascorbic acid was used as antioxidant to confirm that brown spots correspond to H2O2 formation
NADPH Oxidase Activity
The effect of PQ on the generation of O .−2 through NADPH oxidase activity was measured using microsomal fractions of tobacco leaf discs using the NADPH-dependent NBT reduction reaction. NADPH oxidase activity in non-treated transgenic plants was constitutively 57% lower than in control wild-type plants. At 3 h, PQ reduced the enzyme activity to 13% and 18% of the control in SR1 and CAT1AS leaf discs, respectively, whereas at 21 h, NADPH oxidase activity decreased to 59% and 13% of the controls in SR1 and CAT1AS, respectively (Fig. 3).
NADPH-dependent superoxide production in SR1 or CAT1AS tobacco leaf discs. Leaf discs were exposed to 100 μM PQ for 3 or 21 h. The NADPH-dependent oxidase activity was performed indirectly monitoring NBT reduction at 530 nm according to the description in “Materials and Methods”. Values are the means of three different experiments with three replicated measurements, and bars indicate S.E. Asterisk indicates significant differences (p < 0.05) according to Tukey's multiple range test
Antioxidant Enzymes Activity
Superoxide dismutase activity, thought to be an important source of superoxide anion-derived H2O2 in plant cells, did not show changes at 3 h but was significantly reduced at 21 h, in SR1 and CAT1AS leaf discs exposed to PQ, reaching a range of decay from 70% to 55%, respectively (p < 0.01) (Table 1).
Analysis of CAT clearly showed that the enzyme activity in non-treated CAT1AS plants was only 15% of that in SR1 plants, thus confirming that the transgenic cultivar conserved the original described protein reduction. PQ treatment reduced CAT activity in a time-dependent manner in SR1 and CAT1AS leaves compared to non-treated leaves, from an average of 50% at 3 h of treatment to 10% at 21 h in both cultivars (Table 1).
The herbicide markedly decreased CAT protein content at 21 h in SR1 leaf discs, but it almost did not affect the protein content in CAT1AS plants with respect to the non-treated transgenic leaves. The hardly detectable expression of CAT protein in the SR1 plants at 21 h was in accordance with the enzyme activity by this time. Almost no differences were observed either in SR1 or CAT1AS leaf discs at 3 h of PQ exposure (Fig. 4).
Catalase protein expression in leaf discs of tobacco SR1 or CAT1AS plants determined by western blot protein and densitometry analysis. Leaf discs were exposed to 100 μM PQ for 3 or 21 h. CAT expression was revealed with a polyclonal antibody raised in rabbit against a cottonseed CAT as described in “Materials and Methods”. The experiment was repeated three times and a representative image is presented
APOX activity was almost completely abolished by PQ at 3 and 21 h of exposure (p < 0.001). The total activity of GPOX was also reduced in response to PQ treatment, to 75% and 37% of the controls, in SR1 and CAT1AS, respectively, at 3 h of treatment. Almost no GPOX activity was detected at 21 h of PQ treatment either in SR1 or CAT1AS leaf discs (p < 0.001) (Table 1).
Evidence of Oxidative Damage and Cell Death: Thiobarbituric Acid Reactive Substances Content, Electrolyte Leakage, Evans Blue Staining, and Esterase Activity
Paraquat-induced lipid peroxidation determined by TBARS content was observed only in wild-type SR1 leaf discs at 21 h of exposure (Fig. 5), where the content of TBARS increased by 52% with respect to non-treated plants.
Thiobarbituric acid reactive substances (TBARS) content of tobacco SR1 or CAT1AS plants. Lipid peroxidation was measured in control and PQ-treated leaf discs as the amount of thiobarbituric acid reactive substances according to the description in “Materials and Methods”. Values are the means of two different experiments with three replicated measurements, and bars indicate S.E. Asterisk indicates significant differences (p < 0.05) according to Tukey's multiple range test
Ion leakage is considered a cell death marker and can be measured by changes in the conductivity of leaf discs. No symptoms of damage were observed after 3 h of PQ treatment in both SR1 and CAT1AS leaf discs (Fig. 6). Electrolyte leakage significantly increased in leaf discs under 21 h of PQ treatment in SR1 or transgenic plants with increments of 300% and 219%, respectively (Fig. 6). Ascorbic acid almost completely reversed the PQ-induced leakage of electrolytes, suggesting that H2O2 is playing a key role in PQ-induced membrane damage (data not shown).
Cell death measured as electrolyte leakage in leaf discs of tobacco SR1 or CAT1AS plants, expressed as relative conductivity. Leaf discs were exposed to 100 μM PQ, 10 mM, or 50 mM H2O2, for 3 or 21 h as described in “Materials and Methods”. Values are the means of two different experiments with three replicated measurements, and bars indicate S.E. Asterisk indicates significant differences (*p < 0.05, ***p < 0.001) according to Tukey's multiple range test
Cell death in tobacco leaf discs exposed to PQ was determined using the Evans blue staining (a dye that specifically stains dead cells). Surprisingly, no eluted staining was observed in wild-type or transgenic leaf discs exposed for up to 21 h to PQ (Fig. 7).
Cell death estimated as Evans blue staining in leaf discs of tobacco SR1 or CAT1AS plants, expressed as percentage of the controls. Leaf discs were exposed to 100 μM PQ for 3 or 21 h as described in “Materials and Methods”. Values are the means of two different experiments with three replicated measurements, and bars indicate S.E
Esterase activity was measured as another cell death factor because this method is dependent not only on cell viability but also on the metabolic state of the cell [20]. Esterase activity was comparable in non-treated wild-type or transgenic leaf discs at both exposure times. PQ treatment inhibited the enzyme activity to half of that observed in controls at 3 h and an average of 72% at 21 h, in both cultivars (Fig. 8).
Cell viability measured as esterase activity in leaf discs of tobacco SR1 or CAT1AS plants. Leaf discs were exposed to 100 μM PQ for 3 or 21 h as described in “Materials and Methods”. Values are the means of two different experiments with three replicated measurements, and bars indicate S.E. Asterisks indicate significant differences (***p < 0.001) according to Tukey's multiple range test
Discussion
It is well known that PQ causes superoxide-dependent cell death [34] and has been used together with aminotriazole (a CAT inhibitor that leads to H2O2 accumulation and subsequent cell death [35]) to study ROS involvement in the cell death processes in Arabidopsis plants [36]. Considerable controversy about the mechanisms involved in PQ toxicity in animals is well documented [37]. Peixoto et al. [38] demonstrated that H2O2 formation is not the major factor of PQ toxicity at mitochondrial level in plants and animals and showed that PQ toxicity depends on fatty acid composition of membranes related to peroxidability. Hence, the main objective of this work was to investigate whether CAT-deficient plants had an increased susceptibility to PQ exposure compared to the wild-type SR1 plants.
In either wild-type or transgenic leaves exposed for 3 or 21 h to PQ, NBT staining was completely abolished, and this reduction was related to a PQ-direct inhibition of superoxide generation dependent on a NADPH oxidase. This result was rather surprising considering that PQ is an herbicide which alters the normal electron transport in chloroplasts and consequently, increased ROS formation in plants. In spite of the diminished superoxide anion production, DAB deposition due to H2O2 accumulation was clearly evident. This accumulation could be the consequence of PQ transfer of 1 e− to O2 to generate O .−2 in illuminated cell chloroplasts, which is then converted to H2O2 either spontaneously or enzymatically via superoxide dismutase and by PQ inhibition of H2O2-detoxifying enzymes activities (CAT, APOX, and GPOX). ROS could also have been generated by peroxidases [39] as well as amino oxidases in the apoplast and xanthine oxidase [40, 41]. In addition, the increase of catalytic Fe in PQ-treated plants postulated by Iturbe-Ormaetxe et al. [42] could be strengthening PQ toxicity by the generation of hydroxyl radicals [43].
In PQ-treated tobacco leaves, the accumulation of H2O2 was strongly correlated to an increase in electrolyte leakage and a decrease in esterases activity, both are markers of cell damage or cell death. However, PQ-treated leaves showed no Evans positive staining and TBARS only increased in SR1 leaves treated with PQ. Montillet et al. [44] demonstrated that a massive H2O2 production is necessary to provoke an intense ROS-mediated lipid peroxidation despite that lipid peroxidation does not always accompany cell death processes in CAT1AS tobacco plants.
The lack of correlation of Evans blue staining with the other cell death markers in tobacco leaves could be a consequence of the severe injury of membranes that occurred in leaves under PQ treatment, which in turn could not retain the blue stain inside the cells, giving an underestimated result [25].
The avoidance in the increase in electrolyte leakage mediated by ascorbic acid or CAT, either in the wild-type SR1 or in the transgenic CAT1AS leaf discs (data not shown), clearly suggested the involvement of H2O2 in the cell death process, in accordance with the results of Dat et al. [5] who showed that changes in H2O2 homeostasis induced cell death in tobacco CAT1AS plants exposed to high light intensities.
CAT deficiency reduces the H2O2-removing capacity of plant cells and, consequently, may lead to a higher steady-state of the other H2O2 detoxifying enzymes, as APOX and GPOX [45]. Willekens et al. [9] demonstrated that tobacco transgenic CAT1AS plants activated alternate enzymatic mechanisms for H2O2 scavenging to compensate for the shortage of CAT after being transferred to high illumination (300 mmol/m2/s PPFR) for 48 h, showing a persistent increase in both glutathione peroxidase and ascorbate peroxidase expression but without avoiding necrosis in CAT1AS plants. A similar result was obtained in tobacco SR1 or CAT1AS control leaf discs, where APOX activity was higher in the transgenic cultivar compared to SR1 at 21 h of incubation under light (Table 1). However, PQ treatment inhibited APOX activity in both cultivars contributing to the strong toxicity of the herbicide observed by the electrolyte leakage or cell death at 21 h of treatment. This result is supported by the data of Murgia et al. [46] that highlighted the role of APOX in the fine modulation of H2O2 levels.
The overall results obtained in the present work suggested that cell damage was mediated by an increased level of H2O2 in PQ-treated tobacco plants, though to different extents depending on the cultivar and time. In our system, H2O2 increased independently of the inhibition of NADPH oxidase activity, which suggests other ways of H2O2 formation. In chloroplasts under normal physiological conditions, SOD and APOX activities are closely balanced, i.e., the excess of superoxide radicals is eliminated by SOD, generating H2O2 that is scavenged through APOX activity. The increase observed in H2O2 could not be detoxified in tobacco leaves in this way due to the strong decay in both GPOX and APOX activities. Some experimental evidence has suggested that APOX is more labile than SOD under oxidative stress conditions generated by MV in plants like spinach [47] or tobacco [48]. In the presence of PQ, chloroplasts start to accumulate H2O2 within minutes [28], and APOX is the first enzyme to become inactivated after short light exposure [47]. In accordance, in SR1 or CAT1AS PQ-treated leaves, SOD was shown to be more resistant to damage than APOX (Table 1).
The results obtained in the present work suggested that the mechanisms underlying PQ-induced cell death were possibly not related exclusively to ROS formation and oxidative stress in tobacco wild-type or transgenic plants, and these facts are currently under investigation.
References
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340
Van Breusegem F, Bailey-Serres J, Mittler R (2008) Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol 47:978–984
Bechtold U, Karpinski S, Mullineaux PM (2005) The influence of the light environment and photosynthesis on oxidative signalling responses in plant–biotrophic pathogen interactions. Plant Cell Environ 28:1037–1045
Dat JF, Pellinen R, Beeckman T, Van De Cotte B, Langebartels C, Kangasjärvi J, Inzé D, Van Breusegem F (2003) Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J 33:621–632
Scandalios JG, Acevedo A, Ruzsa S (2000) Catalase gene expression in response to chronic high temperature stress in maize. Plant Sci 156:103–110
Groppa MD, Tomaro ML, Benavides MP (2007) Polyamines and heavy metal stress: the antioxidant behavior of spermine in cadmium- and copper-treated wheat leaves. Biometals 20:185–195
Azpilicueta CE, Benavides MP, Tomaro ML, Gallego SM (2007) Mechanism of CATA3 induction by cadmium in sunflower leaves. Plant Physiol Biochem 45:589–595
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu C, Inzé D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16:4806–4816
Willekens H, Inzé D, Van Montagu M, Van Camp W (1995) Catalases in plants. Mol Breed 1:207–228
Chamnongpol S, Willekens H, Langebartels C, Van Montagu M, Inzé D, Van Camp W (1996) Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis related expression under high light. Plant J 10:491–503
Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, Van Montagu M, Inzé D, Van Camp W (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic plants. Proc Natl Acad Sci USA 95:5818–5823
Timbrell JA (1996) Introduction to toxicology. Taylor and Francis, London
Suntres ZE (2002) Role of antioxidants in paraquat toxicity. Toxicology 180:65–77
Benavides MP, Gallego SM, Comba ME, Tomaro ML (2000) Relationship between polyamines and paraquat toxicity in sunflower leaf discs. Plant Growth Regul 31:215–224
Qian H, Chen W, Sun L, Jin Y, Liu W, Fu Z (2009) Inhibitory effects of paraquat on photosynthesis and the response to oxidative stress in Chlorella vulgaris. Ecotoxicology 18:537–543
Willats WG, Orfila C, Limberg G, Buchholt HC, van Alebeek GJ, Voragen AG, Marcus SE, Christensen TM, Mikkelsen JD, Murray BS, Knox JP (2001) Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J Biol Chem 276:19404–19413
Cummins I, Burnet M, Edwards R (2001) Biochemical characterization of esterases active in hydrolyzing xenobiotics in weath and competing weeds. Physiol Plant 113:477–485
Stuhlfelder C, Lottspeich F, Mueller MJ (2002) Purification and partial amino acid sequences of an esterase from tomato. Phytochemistry 60:233–240
Steward N, Martin R, Engasser JM, Goergen JL (1999) A new methodology for plant cell viability assessment using intracellular esterase activity. Plant Cell Rep 19:171–176
Frahry G, Schopfer P (1998) Inhibition of O2-reducing activity of horseradish peroxidase by diphenyleneiodonium. Phytochemistry 48:223–227
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J 11:1187–1194
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tiss Org Cult 39:7–12
Shou H, Bordallo P, Fan JB, Yeakley JM, Bibikova M, Sheen J, Wang K (2004) Expression of an active tobacco mitogen-activated protein kinase enhances freezing tolerance in transgenic maize. Proc Natl Acad Sci USA 101:3298–3303
Steward N, Martin R, Engasser JM, Goergen JL (1999) Determination of growth and lysis kinetics in plant cell suspension cultures from the measurement of esterase release. Biotech Bioeng 66:114–121
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Van Gestelen P, Asard H, Caubergs RJ (1997) Solubilization and separation of a plant plasma membrane NADPH-O2-synthase from other NAD(P)H oxidoreductases. Plant Physiol 115:543–550
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach choroplast. Plant Cell Physiol 22:867–880
Maehly AC, Chance B (1954) The assay of catalase and peroxidase. Meth Biochem Anal 1:357–424
Becana M, Aparicio-Tejo P, Irigoyen JJ, Sánchez-Díaz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol 82:1169–1171
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Laemmli UK (1970) Clivage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Bradford MM (1956) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Vranová E, Atichartpongkul S, Villarroel R, Van Montagu M, Inzé D, Van Camp W (2002) Comprehensive analysis of gene expression in Nicotiana tabacum leaves acclimated to oxidative stress. Proc Natl Acad Sci USA 99:10870–10875
Gechev TS, Gadjev I, Van Breusegem F, Inzé D et al (2002) Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cell Mol Life Sci 59:708–714
Gechev TS, Ferwerda MA, Mehterov N et al (2008) Arabidopsis AAL-toxin-resistant mutant atr1 shows enhanced tolerance to programmed cell death induced by reactive oxygen species. Biochem Biophys Res Commun 375:539–544
Kornbrust DJ, Mavis RD (1980) The effect of paraquat on microsomal lipid peroxidation in vitro and in vivo. Toxicol Appl Pharmacol 53:323–332
Peixoto F, Vicente J, Madeira VMC (2004) A comparative study of plant and animal mitochondria exposed to paraquat reveals that hydrogen peroxide is not related to the observed toxicity. Toxicol In Vitro 18:733–739
Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies DR (2006) Peroxidase dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 47:851–863
Allan AC, Fluhr R (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9:1559–1572
De Biasi MG, Astolfi S, Acampora A, Zuchi S, Fonzo V, Santangelo E, Caccia R, Badiani M, Soressi JP (2003) A H2O2-forming peroxidase rather than a NAD(P)H-dependent O −2 synthase may be the major player in cell death responses controlled by the PtoFen complex following fenthion treatment. Func Plant Biol 30:409–417
Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M (1998) Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol 116:173–181
Babbs CF, Pham JA, Coolbaugh RC (1989) Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol 90:1267–1270
Montillet JL, Chamnongpol S, Rustéucci C, Dat J, van de Cotte B, Agnel JP, Battesti C, Inzé D, Van Breusegem F, Triantaphylidés C (2005) Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol 138:1516–1526
Vicente JA, Peixoto F, Lopes ML, Madeira VM (2001) Differential sensitivities of plant and animal mitochondria to the herbicide paraquat. J Biochem Mol Toxicol 15:322–330
Murgia I, Tarantino D, Vannini C, Bracale M, Carravieri S, Soave C (2004) Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J 38:940–953
Mano J, Ohno C, Domae Y, Asada K (2001) Chloroplastic ascorbate peroxidase is the primary target of methylviologen-induced photooxidative stress in spinach leaves: its relative relevance to monodehydroascorbate radical detected with in vivo ESR. BBA Bioenerg 1504:275–287
Miyake C, Shinzaki Y, Nishioka M, Horiguchi S, Tomizawa KI (2006) Photoinactivation of ascorbate peroxidase in isolated tobacco chloroplasts: Galdieria partita apx maintains the electron flux through the water–water cycle in transplastomic tobacco plants. Plant Cell Physiol 47:200–210
Acknowledgements
This work was supported by the University of Buenos Aires (Grant B044). Benavides MP and Groppa MD are researchers of the Consejo Nacional de Investigaciones Científicas y Técnicas (IQUIFIB-CONICET) and MF Iannone and EP Rosales have fellowships from CONICET. We thank Dr. Frank van Breusegem, Ghent University, Belgium, for providing SR1 and CAT1AS tobacco seeds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iannone, M.F., Rosales, E.P., Groppa, M.D. et al. Reactive Oxygen Species Formation and Cell Death in Catalase-Deficient Tobacco Leaf Discs Exposed to Paraquat. Biol Trace Elem Res 146, 246–255 (2012). https://doi.org/10.1007/s12011-011-9244-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9244-1