Abstract
Divalent metal transporter 1 (DMT1) is likely responsible for the release of iron from endosomes to the cytoplasm in placental syncytiotrophoblasts (STB). To determine the localization and the regulation of DMT1 expression by iron directly in placenta, the expression of DMT1 in human term placental tissues and BeWo cells (human placental choriocarcinoma cell line) was detected and the change in expression in response to different iron treatments on BeWo cells was observed. DMT1 was shown to be most prominent near the maternal side in human term placenta and predominantly in the cytoplasm of BeWo cells. BeWo cells were treated with desferrioxamine (DFO) and human holotransferrin (hTf-2Fe) and it was found that both DMT1 mRNA and protein increased significantly with DFO treatment and decreased with hTf-2Fe treatment. Further, DMT1 mRNA responded more significantly to treatments if it possessed an iron-responsive element than mRNA without this element. This study indicated that DMT1 is likely involved in endosomal iron transport in placental STB and placental DMT1 + IRE expression was primarily regulated by the IRE/IRP mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Divalent metal transport 1 (DMT1) is an iron transport protein that is also called natural resistance associated macrophage protein 2 (Nramp2) or divalent cation transporter 1 (DCT1). DMT1 is a transmembrane divalent metal transfer protein that was first reported in 1997 [1, 2] and belongs to solute carrier family 11 member 2 (SLC11A2). The 3′-untranslated regions (UTRs) of DMT1 mRNA may or may not possess an iron regular element (IRE); therefore, DMT1 can be divided into two types, DMT1 + IRE and DMT1-IRE [3]. DMT1 distributes widely in the mammalian body and has been detected in most tissues and cell types [4–6]. DMT1 plays a vital role in iron homoeostasis although it also can transport other metal ions. It has been proved that DMT1 plays a major role in the uptake of dietary ferrous iron from the intestinal lumen [7–9]. DMT1 knockout mice demonstrate impaired intestinal absorption and severe microcytic anemia characteristic [10].
DMT1 also participates in iron reabsorption of kidney [11], macrophage iron recycling [12], and the transport of non-transferrin (Tf) bound iron (NTBI) in hepatocytes [13]. In addition, DMT1 can mediate iron transport across endosomal membranes into the cytoplasm after transferrin receptor (TfR)-mediated endocytosis. This function of DMT1 is more ubiquitous because iron uptake in a TfR-Tf way exists in most cell types throughout the body. This function was supported by the findings that DMT1 localizes in endosomes and lysosomes [14, 15], co-localizes with TfR1 [16], transports iron optimally at pH 5.5 [17], and can uptake iron into endosomes in a TfR-Tf manner, but the iron released from transferrin in endosomes cannot be transported into the cytoplasm thereafter in DMT1-mutant rats (Begrade rat) [18].
The placenta is the only link between mother and fetus during pregnancy. A mother provides all iron needed for the growth and development of the fetus across the placenta against a concentration gradient. Although the detailed molecular mechanism for this process is unclear, some processes of placental iron transfer have been studied. Studies have confirmed that placental iron uptake proceeds via the TfR1-Tf pathway, followed by endocytosis, acidification of the vesicle, release of the iron into the cytosol, and transfer across the basolateral membrane. DMT1, ferritin (Ft), and ferroportin 1 (FPN1) have been suggested to be involved in this process [19].
A previous study found that both DMT1 + IRE and DMT1-IRE exist in the placenta and the expression of placental DMT1 increases during iron deficiency in rats [20]. Therefore, DMT1 seems to be the best candidate responsible for the release of iron from the endosome to the cytoplasm in placental syncytiotrophoblast (STB) [21]. However, whether DMT1 is involved in the placenta is not completely understood until now [22]. There are inconsistent reports on the localization of DMT1 in the placenta that makes it difficult to speculate on its role in placental iron transport. On the other hand, a previous study indicated that placental DMT1 expression may be regulated by iron. BeWo cells are a human placental cell line that originate from a choriocarcinoma and has been widely used as an in vitro model for the placenta [23]. Therefore, to further test the localization and the regulation of DMT1 expression by iron directly in placenta, DMT1 protein was detected in human term placental tissues and BeWo cells. Additionally, the change of DMT1 expression at the transcription and translation levels in response to iron was determined using the BeWo cells.
Materials and Methods
Immunohistochemical and Immunocytochemical Staining
Human term placenta tissues were obtained from healthy pregnant women delivering at The First Affiliated Hospital of the College of Medicine, Xi'an Jiaotong University. Informed consent was obtained from each participating pregnant woman before their delivery. Placenta tissues were embedded in paraffin and 5-μm sections were deparaffinized and hydrated. For DMT1 antigen retrieval, sections were boiled in 0.01 M citric acid buffer (pH 6.0) for 15 min. BeWo cells were seeded onto coverslips coated with poly-l-lysine in 24-well plates. Cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.25% v/v Triton X100, and blocked for 20 min with normal goat serum. Primary antibody incubations were performed overnight at 4°C using the rabbit polyclonal against DMT1 (Abcam, USA) at a 1:50 dilution. Negative controls included incubations with phosphate-buffered saline without antibody. Diaminobenzidine (DAB) (Sino-American Biotechnology Co., China) was used to visualize treated sections and were acquired using a digital camera.
Cell Culture and Treatment
The BeWo cell line was purchased from the Chinese Center for Type Culture Collection. The cell line was grown in Ham’s F12 (Invitrogen, USA) with 12% fetal calf serum (Hyclone, USA). Cells were cultured in 75 cm2 culture flasks under standard culture conditions of 5% CO2 at 37°C. The medium was renewed every 2 days. Cells were sub-cultured every 4 days. Cells were treated with different concentrations of desferrioxamine (DFO; 0, 30, and 60 μM) or transferrin (hTf-2Fe; 0, 1.25, and 12.5 mM). DFO and human holotransferrin were purchased from Sigma (USA). Cells were harvest at 48 and 72 h after treatment. Lysis buffers for protein (radioimmunoprecipitation assay, RIPA; Sigma, USA) and RNA extraction (TRIzol; Invitrogen, Canada) were added to the culture flasks according to the manufacturer’s instructions.
Real-Time Fluorescence Quantitative PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Canada) according to the manufacturer’s instructions. Total RNA was quantified using a spectrophotometer (ND-1000; NanoDrop, USA). The purity of the samples was assessed by calculating the optical density (OD) ratio (260/280 nm) and by 1% agarose gel electrophoresis. Total RNA (500 ng) was used for cDNA synthesis with RevertAid First Strand cDNA Synthesis Kit (Fermentas, USA) according to the manufacturer’s instructions. The cDNA was subjected to real-time quantitative PCR using an iCycler IQ5 (Bio-Rad, USA) with a SYBR green detection platform. Sequences of the primers to amplify DMT1 + IRE and DMT1-IRE were produced as previously described [24]. The primer sequences used to amplify the β-actin gene were: 5´-TGAAGTACCCCATCGAGCACG-3´ (forward) and 5´-CAAACATGATCTGGGTCATCTTCTC-3´ (reverse).
PCR was performed in a total reaction volume of 25 μL and run in triplicate in microcapillaries. The PCR reaction mixture consisted of 12.5 μL of SYBR Green Realtime PCR Master Mix (TOYOBO, Japan), 1.0 μL of each 10 μM forward and reverse primers, 9.5 μL of H2O, and 1.0 μL of cDNA. The PCR thermal cycling was initiated with denaturation at 95°C for 60 s, 40 cycles at 95°C (15 s), 60°C for 15 s, and 72°C for 45 s. Melting curve analysis and 2% agarose gel shift assay of PCR amplification products were employed to distinguish interference from the primer dimers and non-specific amplification.
Western Blotting
Total cell protein was extracted using RIPA (Sigma, USA) lysis buffer and quantified using the BCA protein assay kit (Thermo, USA). In total, 20 μL of total protein and sample buffer was loaded per lane and electrophoretically separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein bands were transferred to a polyvinylidene fluoride membrane using a semi-dry electrophoresis transfer cell (Bio-Rad, USA). The primary antibody, rabbit polyclonal to DMT1 (1:500; Abcam, USA), was incubated overnight at 4°C. β-actin protein (1:2,000, Santa Cruz, USA) was used as a loading control for SDS-PAGE and Western blot procedures. Goat anti-rabbit IgG conjugated with peroxidase (Thermo, USA) was diluted 1:10,000 and incubated for 2 h at room temperature. Experiments for each group were performed in three replicates. Western blot signals were visualized using SuperSignal West Femto (Thermo, USA) and were quantified by densitometry using the Gel Doc 2000 gel documentation system (Bio-Rad, USA).
Statistical Analysis
For the analysis of PCR results, each sample was normalized to the Ct value for β-actin. The relative quantification was performed with a comparative Ct method through calculation software for the relative expression in real-time PCR [25] which is based on the calculation of 2−ΔΔCt value. The relative quantification of each protein was expressed as a ratio of densitometry values of the DMT1 protein to the β-actin reference protein. The data for relative mRNA and protein expression were presented as means ± S.D. One-way analysis of variance was used to compare the differences among groups. The comparison was conducted among three control groups, among three groups at 48 h, and among three groups at 72 h. The least significant difference test was used to compare between groups. Statistical significance was assumed to be P < 0.05.
Results
Immunolocalization of DMT1 Protein in Human Placenta and BeWo Cells
In human term placental tissues, DMT1 was detected mainly in STB cells and was prominent near to the maternal side but rarely on the fetal side. Scattered staining was also visible in the stroma. In BeWo cells, the staining pattern demonstrated a predominantly cytoplasmic distribution (Fig. 1).
The localization of DMT1 protein expression in human term placenta and BeWo cells (×400). a Negative control image in placental tissues, b DMT1 protein positive staining, c negative control image in BeWo cells, d DMT1 protein positive staining in BeWo cells. Positive staining on the maternal side is indicated by an arrow
Effect of DFO Treatment on the Expression of DMT1 in BeWo Cells
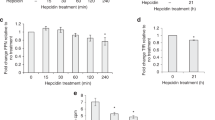
BeWo cells were made iron deficient by incubation with DFO. The expression of DMT1 mRNA and protein increased significantly with prolonged DFO incubation time and increased DFO concentration (Fig. 2). This increase was more significant in DMT1 + IRE mRNA expression than for DMT1-IRE mRNA. In comparison to the control group at 48 h, the expression of DMT1 + IRE mRNA was upregulated 2.36- and 3.13-fold by treatment with 30 and 60 μM DFO, respectively. Further, DMT1 + IRE mRNA was also upregulated 3.07- and 5.21-fold, respectively, in comparison to the control group at 72 h. The expression of DMT1-IRE mRNA was upregulated significantly only at 72 h. In comparison to the control group at 72 h, the expression of DMT1-IRE mRNA was upregulated 3.11- and 3.71-fold with 30 and 60 μM DFO, respectively. The expression of DMT1 protein treated with DFO showed a dose-dependent increase similar to that seen for DMT1 + IRE mRNA expression.
Relative DMT1 expression in BeWo cells treated with DFO. a The relative expression of DMT1 + IRE mRNA as compared with the control group at 0 h, b relative expression of DMT1-IRE mRNA as compared with the control group at 0 h, c representative bands of DMT1 (~60 kDa) and β-actin (~42 kDa) protein expression for groups with different culture time and DFO concentration, d relative expression of DMT1 protein. The DMT1 antibody used here did not distinguish the isoforms of DMT1. Values represent mean ± S.D. of each group (n = 3). *P < 0.05 versus the control group at the same time
Effect of hTf-2Fe Treatment on the Expression of DMT1 in BeWo Cells
The expression of DMT1 mRNA and protein decreased with prolonged hTf-2Fe incubation time and increased hTf-2Fe concentration (Fig. 3). The decrease observed was more significant for DMT1-IRE mRNA expression than that in DMT1 + IRE mRNA. The expression of DMT1 + IRE mRNA was downregulated significantly only with treatment 12.5 mM hTf-2Fe at 72 h by 2.66-fold in comparison to the control group at 72 h. The expression of DMT1-IRE mRNA was downregulated significantly at 12.5 mM hTf-2Fe after 48 h and at 1.25 and 12.5 mM hTf-2Fe after 72 h.
Relative expression of DMT1 expression in BeWo cells treated with hTf-2Fe. a The relative expression of DMT1 + IRE mRNA as compared with the control group at 0 h, b relative expression of DMT1-IRE mRNA as compared with the control group at 0 h, c representative bands of DMT1 (~60 kDa) and β-actin (~42 kDa) protein expression for groups with different culture time and hTf-2Fe concentration, d relative expression of DMT1 protein. The DMT1 antibody used here did not distinguish the isoforms of DMT1. Values represent mean ± S.D. of each group (n = 3). *P < 0.05 versus the control group at the same time
In comparison to the control group at 48 h, the expression of DMT1-IRE mRNA was downregulated by the factor 2.17 with hTf-2Fe 12.5 mM. The expression of DMT1-IRE mRNA was downregulated 2.76- and 6.43-fold with 1.25 and 12.5 mM hTf-2Fe, respectively, in comparison to the control group at 72 h. The expression of DMT1 protein was downregulated significantly only at 12.5 mM hTf-2Fe after 72 h, similar to DMT1 + IRE mRNA expression.
Discussion
The distribution of DMT1 in placenta is uncertain. A previous study found that DMT1 was localized in both cytoplasm and at the junction of the fetal membrane and fetal vessels, while TfR was localized predominantly to the maternal side of the STB membrane [26]. The two proteins were demonstrated to not be co-localized on the apical membrane and minimal areas of overlap in the cytoplasm. It has also been shown that the two types of DMT1(+IRE and -IRE) localize to the plasma membrane and cytoplasm of STB [27] along with diffuse staining in cytotrophoblasts and other stromal cells of villous core regions. Bastin et al. [28] showed discontinuous staining of DMT1 on both STB and stromal cells, and perhaps colocalization of DMT1 and TfR1 on STB. The current study demonstrated that DMT1 protein was prominent near to the maternal side in human term placenta and a predominantly cytoplasmic distribution in BeWo cells. This finding indicates that DMT1 may mediate placental endosomal iron transport.
Like many other iron transporters, DMT1 + IRE contains an IRE at the 3′-termini of the mRNA; therefore, the expression of DMT1 may be regulated post-transcriptionally by intracellular iron levels in an IRE/IRP manner [29]. Under conditions of iron depletion, IRP binds to IRE at the 3′-UTR of DMT1 + IRE mRNA and increases mRNA stability and, thus, DMT1 expression. There is compelling evidence that the major regulation of DMT1 in the small intestine is via the IRE/IRP system. However, in liver, the regulation of DMT1 protein expression appears to be inconsistent with the IRE/IRP mechanism [30]. In addition, DMT1 + IRE was regulated by iron in CaCo-2 cells but not regulated by iron in human 293 and HeLa cells [31]. DMT1 mRNA expression was also dramatically enhanced in the duodenum, but to a lesser extent in kidney, liver, brain, heart, lung, and testis following diet-induced iron deficiency, suggesting that the duodenum is more sensitive to iron than other areas of the body [32]. Besides iron status, cytokines [33], TNF-α [34], development [35], and divalent metals [36, 37] may also be involved in the regulation of DMT1 expression. Therefore, the IRE/IRP regulation mechanism cannot explain the complex expression and regulation of DMT1 in a tissue-specific manner [38]. It is likely that there are other mechanisms for the regulation of DMT1 in different cell types and tissues. However, we know little about the specific regulation mechanism for DMT1 expression until now.
In this study, we found that DMT1 + IRE mRNA expression was upregulated significantly with DFO treatment in a dose-responsive manner, consistent with other studies [20]. The expression of DMT1 + IRE mRNA was downregulated by hTf-2Fe treatment although this was only significant at 12.5 mM hTf-2Fe for 72 h. This indicates that DMT1 + IRE mRNA expression in the placenta is regulated mainly by the IRE/IRP mechanism. A study indicated an enhanced cellular iron release on hTf-2Fe supplementation [39]. Therefore, only hTf-2Fe supplementation for a long period at a high dose elevates the cellular iron concentration significantly and, thereby, downregulates the expression of DMT1 + IRE mRNA.
In this study, we unexpectedly found that the expression of DMT1-IRE mRNA also changed upon iron treatment, especially with hTf-2Fe supplementation. However, it seems that iron does not influence DMT1-IRE expression since it lacks the IRE element. In addition, a study by Gambling et al. [20] also found that DMT1-IRE mRNA expression was unchanged in a rat model of iron deficiency. Given these inconsistencies, we recognize that there is likely to be a more complex regulation mechanism responsible for DMT1-IRE expression that is able to compensate more efficiently in vivo than in vitro. Therefore, the response to outside stimulation may be adjusted to a minimum level in vivo. On the other hand, another study found that DMT1-IRE mRNA expression can be regulated by iron to some extent, although it is far less sensitive than DMT1 + IRE mRNA [40]. This may suggest why the DMT1-IRE mRNA expression showed a significant increase over time and with a high dose of DFO, as found in the current study.
DMT1-IRE mRNA was found to be more sensitive to hTf-2Fe than DFO; however, we could not explain this finding. Previously, a study on airway epithelial cells found that there was a greater amount of mRNA for the DMT1-IRE than DMT1 + IRE and there was greater response of DMT1-IRE to iron and negligible change in the DMT1 + IRE isoform [41]. Further investigation is needed to determine whether DMT1-IRE is more sensitive to iron repletion than DMT1 + IRE. Certainly, since DMT1-IRE lacks an IRE, identifying the mechanism controlling the response to iron may yield particularly intriguing results.
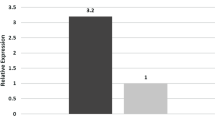
Increased DMT1 protein expression was observed with increasing concentration of DFO and is consistent with other studies [20]. It is likely that DMT1 protein expression was upregulated with enhanced cellular iron transport in the case of iron deficiency and indicates that DMT1 may be involved in the transport of iron in the placenta. The antibody for the DMT1 protein detection used in this study cannot distinguish the types of DMT1 + IRE and DMT1-IRE, but the expression of DMT1 protein correlated with DMT1 + IRE mRNA expression but not DMT1-IRE in this study. This may be due to the predominant expression of DMT1 + IRE mRNA in the placenta. According to the Ct value of DMT1 + IRE mRNA (19.24) and DMT1-IRE mRNA (28.91) in this study, the expression of DMT1 + IRE mRNA is higher than that of DMT1-IRE mRNA in BeWo cells.
In summary, the localization of DMT1 protein in the placenta and the change of DMT1 expression in BeWo cells with iron treatment suggest that DMT1 is involved in placental intercellular iron transport. Furthermore, this may play a role in endosomal iron transport although alternative pathways of iron transfer [42] could not be excluded by the current study.
References
Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC (1997) Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet 16:383–386
Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA (1997) Cloning and characterization of a mammalian proton-coupled metal-iron transporter. Nature 388:482–488
Lam-Yuk-Tseunq S, Gros P (2006) Distinct targeting and recycling properties of two isoforms of the iron transporter DMT1 (NRAMP2, Slc11A2). Biochem 45:2294–2301
Canonne-Hergaux F, Levy JE, Fleming MD, Montross LK, Andrews NC, Gros P (2001) Expression of the DMT1 (NRAMP2/DCT1) iron transporter in mice with genetic iron overload disorders. Blood 97:1138–1140
Roth JA, Horbinski C, Feng L, Dolan KG, Higgins D, Garrick MD (2000) Differential localization of divalent metal transporter 1 with and without iron response element in rat PC12 and sympathetic neuronal cells. J Neurosci 20:7595–7601
Ferguson CJ, Wareing M, Ward DT, Green R, Smith CP, Riccard D (2001) Cellular localization of divalent metal transporter DMT-1 in rat kidney. Am J Physiol Renal Physiol 280:F803–F814
Frazer DM, Wilkins SJ, Becker EM, Murphy TL, Vulpe CD, McKie AT, Anderson GJ (2003) A rapid decrease in the expression of DMT1 and Dcytb but not Ireg1 or hephaestin explains the mucosal block phenomenon of iron absorption. Gut 52:340–346
Mims MP, Prchal JT (2005) Divalent metal transporter 1. Hematology 10:339–345
Fleming RE, Migas MC, Zhou XY, Jiang J, Britton RS, Brunt EM, Tomatsu S, Waheed A, Bacon BR, Sly WS (1999) Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1. Proc Natl Acad Sci USA 96:3143–3148
Priwitzerova M, Nie G, Sheftel AD, Pospisilova D, Divoky V, Ponka P (2005) Functional consequences of the human DMT1 (SLC11A2) mutation on protein expression and iron uptake. Blood 106:3985–3987
Smith CP, Thévenod F (2009) Iron transport and the kidney. Biochimica et Biophysica Acta 1790:724–730
Soe-Lin S, Apte SS, Mikhael MR, Kayembe LK, Nie G, Ponka P (2010) Both Nramp1 and DMT1 are necessary for efficient macrophage iron recycling. Exp Hematol 38:609–617
Shindo M, Torimoto Y, Saito H, Motomura W, Ikuta K, Sato K, Fujimoto Y, Kohgo Y (2006) Functional role of DMT1 in transferrin-independent iron uptake by human hepatocyte and hepatocellular carcinoma cell, HLF. Hepatol Res 35:152–162
Tabuchi M, Yoshimori T, Yamaguchi K, Yoshida T, Kishi F (2000) Human Nramp2 /DMT1, which mediates iron transport across endosoma membranes, is localized to late endosomes and lysosomes in HEp-2 cells. J Biol Chem 275:22220–22228
Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, Gros P (1999) The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J Exp Med 189:831–841
Su MA, Trenor CC, Fleming JC, Fleming MD, Andrews NC (1998) The G185R mutation disrupts function of the iron transporter Nramp2. Blood 92:2157–2163
Touret N, Furuya W, Forbes J, Gros P, Grinstein S (2003) Dynamic traffic through the recycling compartment couples the metal transporter Nramp2 (DMT1) with the transferrrin receptor. J Biol Chem 278:25548–25557
Fleming MD, Romano MA, Su MA, Garrick LM, Andrews NC (1998) Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA 95:1148–1153
Mok H, Mendoza M, Prchal JT, Balogh P, Schumacher A (2004) Dysregulation of ferroportin 1 interferes with spleen organogenesis in polycythaemia mice. Development 131:4871–4881
Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N, Joory KD, Srai SKS, Mcadle HJ (2001) Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J 356:883–889
Gruper Y, Bar J, Bacharach E, Ehrlich R (2005) Transferrin receptor co-localizes and interacts with the hemochromatosis factor (HFE) and the divalent metal transporter-1 (DMT1) in trophoblast cells. J Cell Physiol 204:901–912
McArdle HJ, Andersen HS, Jones H, Gambling L (2008) Copper and iron transport across the placenta: regulation and interactions. J Neuroendocrinol 20:427–431
Heaton SJ, Eady JJ, Parker ML, Gotts KL, Dainty JR, Fairweather-Tait SJ, McArdle HJ, Srai KS, Elliott RM (2008) The use of BeWo cells as an in vitro model for placental iron transport. Am J Physiol Cell Physiol 295:C1445–C1453
Leong WI, Bowlus CL, Tallkvist J, Lönnerdal B (2003) DMT1 and FPN1 expression during infancy: developmental regulation of iron absorption. Am J Physiol Gastrointest Liver Physiol 285:1153–1161
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Georgieff MK, Wobken JD, Welle J, Burdo JR, Connor JR (2000) Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta. Placenta 21:799–804
Chong WS, Kwan PC, Chan LY, Chiu PY, Cheung TK, Lau TK (2005) Expression of divalent metal transporter 1 (DMT1) isoforms in first trimester human placenta and embryonic tissues. Hum Reprod 20:3532–3538
Bastin J, Drakesmith H, Rees M, Sargent I, Townsend A (2006) Localisation of proteins of iron metabolism in the human placenta and liver. Brit J Haematol 134:532–543
Han O, Fleet JC, Wood RJ (1999) Reciprocal regulation of HFE and NNamp2 gene expression by iron in human intestinal cells. J Nutr 129:98–104
Trinder D, Oates PS, Thomas C, Sadleir J, Morgan EH (2000) Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut 46:270–276
Hubert N, Hentze MW (2002) Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc Natl Acad Sci USA 99:12345–12350
Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA (2001) Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett 509:309–316
Ludwiczek S, Aigner E, Theurl I (2003) Cytokine-mediated regulation of iron transport in human monocytic cells. Blood 101:4148–4154
Paradkar PN, Roth JA (2006) Nitric oxide transcriptionally down-regulates specific isoforms of divalent metal transporter (DMT1) via NF-κB. J Neurochem 96:1768–1777
Williams K, Wilson MA, Bressler J (2000) Regulation and developmental expression of the divalent metal-ion transporter in the rat brain. Cell Mol Biol 46:563–571
Han O, Wessling-Resnick M (2002) Copper repletion enhances apical iron uptake and transepithelial iron transport by CaCo-2 cells. Am J Physiol Gastrointest Liver Physiol 282:G527–G533
Yamaji S, Tennant J, Tandy S, Williams M, Srai SKS, Sharp P (2001) Zinc regulates the function and expression of the iron transporters DMT1 and IREG1 in human intestinal Caco-2 cells. FEBS Lett 507:137–141
Zoller H, Theurl I, Koch R, Kaser A, Weiss G (2002) Mechanisms of iron mediated regulation of the duodenal iron transporters divalent metal transporter 1 and ferroportin 1. Blood Cell Mol Dis 29:488–497
Kroos MJ, Starreveld JS, Verrijt CEH, van Eijk HG, van Dijk JP (1996) Regulation of transferring receptor synthesis by human cytotrophoblast cells in culture. Eur J Obstet Gyn R B 65:231–234
Anderson GJ, Frazer DM, McKie AT, Vulpe CD (2002) The ceruloplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cell Mol Dis 29:367–375
Wang X, Garrick MD, Yang F, Dailey LA, Piantadosi CA, Ghio AJ (2005) TNF, IFN-γ, and endotoxin increase expression of DMT1 in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 289:L24–L33
Gunshin H, Fujiwara Y, Custodio AO, DriRenzi C, Robine S, Andrews NC (2005) Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensible in placenta and liver. J Clin Invest 115:1258–1266
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 30800909).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, YQ., Bai, B., Cao, XX. et al. Divalent Metal Transporter 1 Expression and Regulation in Human Placenta. Biol Trace Elem Res 146, 6–12 (2012). https://doi.org/10.1007/s12011-011-9214-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9214-7