Abstract
Schizochytrium limacinum SR21 is an important strain for industrial production of docosahexaenoic acid (DHA), which is an important omega-3 fatty acid used in the nutraceutical and food industry. However, the high cost of carbon sources has limited its further application in the market with much larger volume, such as animal feed for aquaculture, poultry, and livestock. To seek low-cost carbon source, acetic acid is tested in the present study. The effect of different factors, including initial carbon source concentration, pH, aeration rate, and nitrogen sources, on biomass, lipid, and DHA production were tested. With optimized culture conditions, the biomass concentration of 146 g/L, total fatty acids (TFAs) of 82.3 g/L, and DHA content of 23.0 g/L were achieved with a pH-auxostat fed-batch cultivation. These results suggested that acetic acid is a promising feedstock for the low-cost production of DHA.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Docosahexaenoic acid (DHA) is an important nutraceutical and dietary supplement, as it helps in the prevention of cardiovascular disease, depression, certain cancers, as well as maintenance of the brain and learning functions [1,2,3]. Marine fish oil is a major source of DHA but has sustainability problems due to the exhaustion of ocean resources, as well as the risks of contamination with hazardous environmental pollutants [4]. To solve this problem, bioprocess to produce DHA directly from microalgae fermentation has been developed and played a major role for DHA production in recent years [5]. However, DHA produced from microalgae fermentation still has a higher production cost than fish oil by far, which limits its application in high-value market such as infant formula or functional foods [6], but not in the low-value market, such as animal feed for aquaculture, poultry, and livestock, to provide meat, eggs, and milk enriched with DHA. As a matter of fact, the market volume of animal feed is much larger than the infant formula and functional foods [7]. To meet this demand, further reduction of DHA production cost from fermentation process is required.
Carbon source is the major part of industrial fermentation, and its market price significantly affects the production cost [8]. As the major carbon source for current industrial production of DHA, glucose has a market price around $500/ton [9]. This is acceptable for DHA production for high value market, but not for animal feed. To seek carbon sources with lower cost, a variety of organic waste has been investigated for DHA production, including crude glycerol [10], fructose corn syrup [11], food waste [12], and sweet sorghum juice [13]. However, these feedstocks have a limited abundance, and the developed processes with them often resulted in much lower productivity than that with glucose and showed limited potential for industrial application.
Acetic acid can be utilized as a carbon source by oleaginous microorganisms to produce lipids [14]. It may be produced from organic waste as volatile fatty acid (VFAs) [15, 16] through dark fermentation and various anaerobic bioprocesses, which is efficient and profitable from economic and environmental point of view [17, 18]. The organic waste is expected to reach 2.25 billion tons per year by 2025, out of which food waste accounts for one-third [19]. Fei et al. [9] calculated that 1 ton of VFAs produced from food wastes would cost 30 US dollar (USD), which is much lower than glucose. Depending on the type of substrate and fermentation processes, acetic acid accounts for 43~69% of these VFAs liquid stream [20]. Also, with acetyl-CoA synthetase, acetic acid can be directly converted into acetyl-CoA, a central intermediate for the biosynthesis of lipids [21]. Thus, it has shorter metabolic pathways [17] and has high conversion efficiency to lipids as compared to glucose and glycerol [22]. This would provide an abundant cheap carbon source to produce DHA as animal feed. Although previous effort has been made for this purpose with Crypthecodinium cohnii, it resulted in a long ferementation process of 400 h. This showed limited potential for industrial application, since it is difficult to prevent comtamination for so long time in practical operation for an industrial scale fermentor [23].
In this study, acetic acid was investigated as low-cost carbon source for DHA production with S. limacinum SR21, which is an important strain for industrial production DHA [7] [24]. The effect of different factors on biomass, TFAs, and DHA production was investigated, including initial carbon source, pH, aeration rate, and nitrogen source. pH-auxostat fed-batch culture mode was used, and high biomass concentration and DHA production were obtained with the optimized process. These results showed that acetic acid is a good carbon source for DHA production with S. limacinum SR21 at a low cost.
Materials and Methods
Strain and Medium
The strain S. limacinum SR21 (ATCC MYA-1381) was used in this study. The cells stored at − 80 °C in 20% (v/v) glycerol were transferred into 50 mL medium for inoculum growth and incubated at 25 °C in an orbital shaker at 170 rpm. The seed culture medium consisted of (g/L) glucose 5.0, yeast extract 1.0, peptone 1.0, NaCl 18, KCl 0.6, MgSO4·7H2O 2.44, NaNO3 1.0, CaCl2 · 6H2O 0.45, Tris buffer 2.0, KH2PO4 0.05, PΙΙ metal solution 10.0 mL/L, iron solution 1.0 mL/L, VB12 solution 1.0 mL/L, and NH4Cl 0.267 g/L. The medium pH was adjusted up to 7.3 before autoclave at 121 °C for 20 min.
Fed-Batch Culture
The pH-auxostat fed-batch fermentations were performed with acetic acid as a carbon source in 5-L laboratory bioreactor (BIOTECH-5BG-7000A). The culture was inoculated according to the initial fermentation medium with 10% (v/v) of inoculum. Two types of mediums were used in fed-batch culture.
Initial Culture Medium
The experiment was started with an initial culture volume of 1.5 L contained (g/L) sodium acetate 10.0, yeast extract 2.8, corn steep solids 0.5, CH3COONH4 0.5, NaNO3 0.5, KH2PO4 1.0, KCl 0.6, NaCl 9.0, MgSO4·7H2O 2.44, CaCl2 · 6H2O 0.9, Tris buffer 1.0, PΙΙ metal solution 20.0 mL/L, iron solution 5.0 m/L, and VB12 2.0 mL/L. The temperature of bioreactor was maintained at 30 °C.
Feeding Medium
The feeding medium contained (g/L) KH2PO4 6.0, KCl 6.0,MgSO4 · 7H2O 24.4, CaCl2 · 6H2O 9.0, PII metal solution 200 mL/L, the chelated iron solution 50 mL/L, and VB12 20 mL/L, with an optimized source of mixed organic and inorganic nitrogen sources as described below in “Feeding Medium.” Before autoclaving at 121 °C for 20 min, the medium pH was adjusted to 7.3 using 3 M NaOH solution. The medium with acetic acid (100% w/w) was feed automatically through a pH-control system.
pH Optimization
To study the effect of pH on microalgae growth with acetic acids as a carbon source, pH optimization was conducted and used for subsequent experiments. Three pH values of 6.0, 6.5, and 7.0 were studied and compared to find out optimal pH. All other culture conditions and methods were identical.
Optimization of Mixed Organic and Inorganic Nitrogen Concentrations
To obtain a high production of biomass, TFAs, and DHA, two different nitrogen sources were used and results were compared: (N1) yeast extract 100 g/L, corn steep solid 40 g/L; (N2) CH3COONH4 80 g/L, NaNO3 20 g/L. The optimal concentration of mixed organic and inorganic nitrogen sources was identified and used for subsequent experiments. All other culture conditions and methods were identical.
Aeration Strategy Optimization
To study the effect of aeration rate on microalgae growth with acetic acid as a carbon source, two-stage aeration strategies were studied in the 5-L bioreactor. Two types of aeration strategies: (1) 1.5 vvm in growth stage and 1.0 vvm in the lipogenic stage, respectively, and (2) 1.0 vvm in growth stage and 0.5 vvm in a lipogenic stage were compared. The agitation speed of 300 rpm was used at the start of the fermentation.
Determination of Cell Density, Dry Cell Weight, and Total Fatty Acid Analysis
The cell density of culture was determined by diluting fermentation broth thirty times with distilled water to disperse the cells as single cells and then counted with a hemocytometer. Dry biomass was determined by centrifuging 5 mL broth at 10,000×g for 8 min. The supernatant was stored at − 20 °C for further analysis. The cell pellet was rinsed three times with pure water and dried at 104 °C for 4 h. For fatty acid analysis, harvested biomass was centrifuged, washed, and then lyophilized at − 50 °C for 48 h in a freeze dryer. The method for FAME analysis was the same as described by Chi et al. [10]. In short, 10–20 mg freeze-dried biomass and 1.0 mg of heptadecanoic acid (C17: 0), as the internal standard, were transferred to a 50-mL glass tube along with a 5-mL mixture of CH3Cl, CH3OH, and H2SO4 (3.26:1.63:0.1 v/v/v) and heated in a water bath at 90 °C for 50 min. One milliliter distilled water was added to each tube and mixed through the vortex for 40 s to form two phases. The lower FAMEs containing phase was transferred to a tube for drying containing anhydrous sodium sulfate. Dried samples were analyzed by GC machine (Shimadzu GC) with a capillary column (PEG-20w; 0.25 mm × 30 m, 0.1 μm thickness) and flame ionization detector (FID). The temperature of injector and detector was set at 220 °C and 260 °C, respectively. Nitrogen was applied as a carrier gas with a flow rate of 14.4 mL/min and pressure at 500 kPa. Air pressure was 59 kPa. The hydrogen gas pressure was 80 kPa, and the tail gas purge flow was 3 mL/min. The injected sample volume was 1 μL, and the split ratio was 20:1. The quantity of fatty acids was identified from the comparison with a peak area of the internal standards of heptadecanoic acid (C17:0). The DHA percentage in fatty acids was identified by area normalization and the internal standard method. The GC chromatography figure for fatty acids is given in Fig. S2 in supporting information (SI).
Determination of Acetic Acid and Nitrogen Concentration
The residual concentration of acetic acid in filtered aliquots was determined by HPLC fitted with an Aminex HPX-87H column. The mobile phase consisted of 5 mM sulfuric acid, with a flow rate of 0.7 mL/min and 70 kPa. For acetic acid analysis, the culture broth was 2.5 times diluted and mixed with 1 mL chloroform for protein removal. The mixture was centrifuged at 1000×g for 12 min. The upper phase was filtered and used for acetic acid analysis. Nitrogen concentration was determined through the Kjeldahl method in accordance with GB 5009.5-2016.
Results and Discussion
Effect of Initial Carbon Source on Cell Density, Biomass, and DHA Production
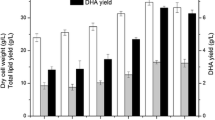
S. limacinum SR21 was grown in a series of pH-auxostat fed-batch cultures with different concentrations of sodium acetate as initial carbon ranging from 8.0 to 16.0 g/L. The sodium acetate of 10.0 g/L as the initial medium carbon source resulted in better performance than other concentrations (Fig. S1 in supporting information SI). So, this concentration was used in high-density culture, and the cell density, dry cell weight, total lipid, and DHA content in this culture were further compared with glucose (60 g/L) as an initial carbon source (Fig. 1a–d).
The effect of initial carbon sources sodium acetate (10 g/L) and glucose (60 g/L) on a, Cells density, b Dry cell weight, c DHA (% w/w), and d TFA (% w/w) in pH-auxostat culture of S. limacinum SR21 with acetic acid as carbon source. The data presented are the mean and standard error of three triplicate fermentations
As shown in Fig. 1a, the cell density was 19, 84, 457, and 751 million cells/mL and 44, 344, 498, and 610 million cells/mL at 12, 24 36, and 48 h respectively in cell division phase with sodium acetate and glucose as initial carbon source. In a culture with sodium acetate as an initial carbon source, culture cells first enter into the lag phase to adapt itself to the environment and then enter into the exponential phase. This is because inoculum was grown in glucose-containing seed culture media; therefore, the utilization of acetate by cells required inductions of some key enzymes for growth that was not active in the glucose-grown seed culture [25]. In contrast, a lag phase was not detected during the growth of S. limacinum SR21 using glucose as an initial carbon source. The culture cells quickly adapt to the environment and directly enter into the exponential phase. At the first 36 h, cell density was higher than that of sodium acetate-grown cells, because here cells faced the same carbon source in the fermenter as seed culture medium, resulted in rapid cell division and depletion of glucose. As a consequence of the faster growth rate, pH of culture medium rises. As the pH drift from the threshold level, fresh acetic acid was added by auxostat system to bring back the pH to a set point. Cell adaptation to acetic acid was also observed here, just as explained above in the case of sodium acetate as an initial carbon source, which in turn slows down cell proliferation in the cell division stage that lasts for 48 h, followed by cell enlargement and lipid accumulation in the later growth stage. On the whole, at the end of the cell division phase, culture with sodium acetate results in higher cell density than glucose, and this laid a favorable foundation in the later growth stage for high biomass and lipid accumulation.
Regarding biomass concentration, a positive correlation was observed between cell density and dry cell weight (Fig. 1b). The highest dry cell weight for S. limacinum SR21 cultivated with sodium acetate as initial carbon source was 146 g/L vs 124 g/L for glucose as initial carbon source after 144 h. At the same time, both TFA and DHA contents for the culture with sodium acetate as the initial carbon source were substantially greater than that of glucose (Fig. 1 d and c), and the final TFA and DHA yields (g/L) with sodium acetate were 19% and 26% higher than glucose. These results indicate that sodium acetate in initial culture media can be efficiently utilized by S. limacinum SR21 during the initial stage of cultivation for high cell density culture.
Effect of Different Aeration Rates on Cell Growth and DHA Production
There were significant differences between the two aeration strategies: 1.5 vvm and 1.0 vvm vs 1.0 vvm and 0.5 vvm, in terms of acetic acid assimilation, as well as biomass, lipids and DHA production (Fig. 2). As shown in Fig. 2a, the acetic acid consumption rate was increased with the enhanced of aeration rate, and it resulted in a higher total amount of feed acetic acid, which reflects a high acetic acid consumption rate and assimilation capacity of culture cells. Guo et al. [26] have also reported that substrate consumption capacity was high under the condition of high oxygen supply and was insubstantial in cultures with low oxygen supply. A marked difference was observed in the growth pattern of Schizochytrium limincaum SR21 due to a high acetic acid consumption rate. Because cell growth has a direct relation to substrate utilization, which was considerably affected by the different aeration rates. As shown in Fig. 2b, the highest dry cell weight of 141.4 g/L was achieved with aeration strategy of 1.5 and 1.0 vvm and a lower dry cell weight of 122 g/L was observed with that of 1.0 and 0.5 vvm. These results suggest that high oxygen supply leads to more carbon assimilation for cell respiration and energy metabolism for the rapid growth of biomass [11].
The time course of fermentation profile of S. limacinum SR21 in pH-auxostat fed-batch culture with the aeration rates at 1.5 and 1.0 vvm and 1.0 and 0.5 vvm, respectively: a Consumed acetic acid (g/L), b Dry cell weight (g/L), c DHA content (% w/w), and d total lipid (TFA) (% w/w). The data presented are the mean and standard error of three replicate fermentation
In terms of lipid accumulations, oxygen supply, especially in the lipogenic phase, greatly influenced TFA and DHA productivity. The polyketide synthase (PKS) is a major pathway for PUFA synthesis in Schizichytrium sp. which relies on limiting oxygen supply [27]. As shown in Fig. 2 c and d, the highest TFA of 54.5% (w/w) and DHA content of 14.6% (w/w) were achieved with aeration strategy of 1.5 to 1.0 vvm. Compared with this, 1.0 to 0.5 vvm resulted in TFA of 50.0% (w/w) and DHA content of 12.5% (w/w). The decreased TFA and DHA productivity at lower aeration rate (1.0 to 0.5 vvm) should be due to the disturbance of the inlet flow of acetic acid into fermenter by shifting the aeration rate from 1.0 to 0.5 vvm (data not shown). The rate of a rapid increase in culture pH often reflects high metabolic activity and acts as a growth-dependent parameter in pH-auxostate fed-batch culture [25]. It is important to notice that the rate of substrate addition is dependent on the assimilation rate of cultured cells and the buffering capacity of growth medium. So aeration shift to 0.5 vvm directly affects the pH drift to a set point value, which in turn directly affects nutritional balance inside the fermenter.
Cell growth was restrained when the oxygen supply level falls under a “critical” value, since an efficient supply of oxygen is acquired for acetic acid metabolism [23]. However, shifting of aeration rate from 1.5 to 1.0 vvm in the lipogenic phase has no adverse effect on the growth performance and inlet flow of acetic acid into the fermenter. The high volume of acetic acid feeding was observed at 1.0 vvm as compared with 0.5 vvm (data not shown). This demonstrated that the cells are still in the active metabolic mode and consumed more carbon substrate for energy and physical maintenance, which leads to high TFA and DHA contents in the lipid accumulation stage. Ratledge [28] also reported that an efficient supply of carbon source is important at the last stage of growth for lipid accumulation. Moreover, increased level of acetic acid would provide more acetyl-CoA and NADPH which are the main precursors of DHA synthesis in Schizochytrium sp. [29]. Thus, based on the current study, we suggest that aeration rate from 1.5 to 1.0 vvm could be effectively applied to get high TFA and DHA productivity in fed-batch culture with acetic acid as a carbon source.
Effect of Culture pH on Biomass and DHA Production
As known in various literature, culture pH shows a strong effect on the growth performance of oleaginous cells when acetic acid was used as a sole carbon source that can directly affect the acidic form of culture solution [30]. Figure 3 a–c demonstrate the effect of different culture pHs on dry cell weight, TFA, and DHA production. The highest biomass, total fatty acids, and DHA content of 139.1 g/L, 54.0% (w/w), and 14.7%(w/w) were observed at pH 7.00. It can be seen from Fig. 3a–c that S. limacinum SR21 gave a better performance in all growth parameters at pH 7.00, as compared with pH 6.50 and 6.00. When pH was changed from 7.00 to 6.00, the dry cell weight was found to be reduced by 27% (Fig. 3a), TFA concentration by 40% (Fig. 3c), and DHA concentration by 50% (Fig. 3b), respectively, which shows strong inhibition under pH 6.0.
Acid dissociated constant (pKa) for acetic acid is 4.75. According to the Henderson Hasselbalch equation, about 90% of acetic acid is dissociated into acetate anion at pH value 6.0. So adverse effects of acetic acid at pH 6.0 on cells are presumably caused by the undissociated form of the acid molecule, which may cause intracellular acidification via dissociation into protons and the corresponding anions after entering the near-neutral cytoplasm [31]. As a result, floating layer was observed after centrifugation at the end of fermentation due to cell rupture or lysis. Moreover, microscopic analysis conforms burst cells and small lipid bodies near rupture cells (data not shown). This cell lysis causes a significant loss in biomass, total lipids, and DHA production. However, at pH 7.00, acetic acid dissociates completely into acetate anion. Therefore, higher pH resulted in a lower inhibition of acetic acid molecule, and this may be the reason why a higher pH of 7.00 resulted in better growth performance than pH 6.00. A similar trend was reported by Beligon et al. [32] for high biomass and lipid production from C. curvatures at culture pH 7.00 compared with 6.00 with acetate as a carbon source. Different from Schizochytrium, C. cohnii [25] had the best performance at pH 6.5 with acetic acids as a carbon source. The difference in these results may be due to strain performance at different culture pH.
Effect of Nitrogen Sources on Biomass and DHA Production
Microalgae biomass and product composition have been considered to be greatly influenced by the type and amount of nitrogen sources [26]. Therefore, the examination of suitable nitrogen sources for high biomass, lipid, and DHA production is an important step towards optimization process. Figure 4 a and b demonstrate the effect of mixed nitrogen sources on dry cell weight, lipid, and DHA production. Organic nitrogen source–rich media (N1) accelerates the substrate consumption rate, which in turn results in a higher dry cell weight of 144 g/L, TFA of 53.5% (w/w), and DHA of 15.0% (w/w), in comparison with inorganic nitrogen source–rich media (N2) which led to dry biomass of 129 g/L, TFA of 51.0% (w/w), and DHA of 13.3% (w/w) production.(Fig. 4a, b). Compared with the N1 medium, N2 medium resulted in a reduction of dry cell weight by 10.0%, TFA by 12.7%, and DHA production by 18.5%, respectively. These results suggest that ammonium-based nitrogen sources are less efficient for high cell density culture with acetic acid as a carbon source.
The time course of fermentation profile of S. limacinum SR21 in pH-auxostat fed-batch cultivations with different nitrogen sources. a The effect of organic-rich nitrogen sources (N1) on dry cell weight(g/L), TFA (% w/w), and DHA(% w/w). b The effect of inorganic rich nitrogen sources (N2) on dry cell weight (g/L), TFA(% w/w), and DHA(% w/w). c The effect of organic-rich nitrogen sources (N1) on acetic acid concentration in fermenter. d The effect of inorganic rich nitrogen sources (N2) on acetic acid concentration in fermenter. The data presented are the mean and standard error of triplicate fermentation
Sun et al. [33] reported that the ammonium-based nitrogen source enhanced DHA production in Schizochytrium sp. with glucose as the carbon source. However, it resulted in lower biomass and DHA production when acetic acid is used as a carbon source in this study. The difference may be attributed to the use of different carbon sources in the fermentation medium. The increasing amount of organic nitrogen sources like yeast extract and corn steep solids in feeding media promotes cell growth and lipid accumulation. Organic nitrogen sources contain important growth factors such as amino acids, vitamins, nucleotides, fatty acids, and related components that facilitated cell growth and various catalytic reactions. Moreover, the presence of some important amino acids like arginine and lysine in the yeast extract may increase the acid tolerance of the oleaginous microorganism [34]. This may be the reason why organic nitrogen sources resulted in better biomass, TFA, and DHA production in pH-auxostat with acetic acid.
It is interesting to highlight that the type of nitrogen source not only affects growth performance but also affects acetic acid feeding in pH-auxostat culture. A rapid increase in pH to the setpoint value was observed with organic nitrogen-rich feeding media (N1), as compared with inorganic nitrogen-rich media (N2), which directly affects the residual concentration of acetic acid in the fermenter, as shown in the Fig. 4 c and d. This might be explained by the fact that ammonium-based nitrogen source assimilation could produce acidic substances that act as an alkaline pH regulator. So it will compete with acetic acid which is the main carbon source and pH regulator. Therefore, the increased amount of ammonium nitrogen source in feeding media may cause an inadequate level of acetic acid supply to the fermenter. In conrast, comsumption of organic nitrogen source (yeast extract) does not change pH. Therefore, organic nitrogen sources resulted in better growth performance than ammonium-rich nitrogen sources in pH-auxostat culture with acetic acid as carbon source.
Table 1 summarizes the results of the literature using different carbon sources in comparison with the results obtained in this study with acetic acid. In the current study, high biomass of 146 g/L and DHA productivity of 160 mg/L/h were obtained, which is quite higher than the listed literature reports with glucose and glycerol as a carbon source. As mentioned above, VFAs are considered to be less expensive than both glucose and glycerol, which can be produced from organic waste at a low cost. Also, acetic acid has a shorter metabolic pathway and high conversion efficiency to lipids as compared to glucose and glycerol [16]. Moreover, the pH-auxostat cultivation showed easy operability, where carbon flow is controlled by itself without any additional operational cost.
Conclusion
With pH-auxostat fed-batch culture, 146 g/L biomass concentration, 82.3 g/L TFA, and 23.0 g/L DHA yield were obtained. This study proved the viability of using acetic acid as a cheap feedstock for DHA production, which may significantly reduce the production cost so that it can be used as animal feed.
References
Hussein, J., El-Naggar, M., Badawy, E., El-laithy, N., El-Waseef, M., Hassan, H., & Abdel-Latif, Y. (2020). Homocysteine and asymmetrical dimethylarginine in diabetic rats treated with docosahexaenoic acid–loaded zinc oxide nanoparticles. Applied Biochemistry and Biotechnology, 1–13.
Ramakrishnan, U., Gonzalez-Casanova, I., Schnaas, L., DiGirolamo, A., Quezada, A. D., Pallo, B. C., & Martorell, R. (2016). Prenatal supplementation with DHA improves attention at 5 y of age: a randomized controlled trial. The American Journal of Clinical Nutrition, 104(4), 1075–1082.
Geng, L., Chen, S., Sun, X., Hu, X., Ji, X., Huang, H., & Ren, L. (2019). Fermentation performance and metabolomic analysis of an engineered high-yield PUFA-producing strain of Schizochytrium sp. Bioprocess and Biosystems Engineering, 42(1), 71–81.
Hu, X. C., Ren, L. J., Chen, S. L., Zhang, L., Ji, X. J., & Huang, H. (2015). The roles of different salts and a novel osmotic pressure control strategy for improvement of DHA production by Schizochytrium sp. Bioprocess and Biosystems Engineering, 38(11), 2129–2136.
Kyle, D. J. (2001). The large-scale production and use of a single-cell oil highly enriched in docosahexaenoic acid. American Chemical Society, 92–107.
Lan, J. C. W. (2015). The optimization of docosahexaenoic acid production from waste by Schizochytrium limacinum SR21. Journal of Biotechnology, 208, S33.
Patil, K. P., & Gogate, P. R. (2015). Improved synthesis of docosahexaenoic acid (DHA) using Schizochytrium limacinum SR21 and sustainable media. Journal of Chemical Engineering, 268, 187–196.
Zeng, Y., Ji, X. J., Lian, M., Ren, L. J., Jin, L. J., Ouyang, P. K., & Huang, H. (2011). Development of a temperature shift strategy for efficient docosahexaenoic acid production by a marine fungoid protist, Schizochytrium sp. HX-308. Applied Biochemistry and Biotechnology, 164(3), 249–255.
Gong, G., Zhang, X., & Tan, T. (2019). Simultaneously enhanced intracellular lipogenesis and β-carotene biosynthesis of Rhodotorula glutinis by light exposure with sodium acetate as the substrate. Bioresource Technology, 295, 122274.
Chi, Z., Pyle, D., Wen, Z., Frear, C., & Chen, S. A. (2007). Laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochemistry, 42(11), 1537–1545.
Yu, X. J., Yu, Z. Q., Liu, Y. L., Sun, J., Zheng, J. Y., & Wang, Z. (2015). Utilization of high-fructose corn syrup for biomass production containing high levels of docosahexaenoic acid by a newly isolated Aurantiochytrium sp. YLH70. Applied Biochemistry and Biotechnology, 177(6), 1229–1240.
Fan, K. W., Chen, F., Jones, E. B. G., & Vrijmoed, L. L. P. (2000). Utilization of food processing waste by Thraustochytrids. Fungal Diversity.
Liang, Y., Sarkany, N., Cui, Y., Yesuf, J., Trushenski, J., & Blackburn, J. W. (2010). Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresource Technology, 101(10), 3623–3627.
Song, H. S., Seo, H. M., Jeon, J. M., Moon, Y. M., Hong, J. W., Hong, Y. G., Bhatia, S. K., Ahn, J., Lee, H., & Kim, W. (2018). Enhanced isobutanol production from acetate by combinatorial overexpression of acetyl-CoA synthetase and anaplerotic enzymes in engineered Escherichia coli. Biotechnology and Bioengineering, 115(8), 1971–1978.
Bhatia, S. K., & Yang, Y. H. (2017). Microbial production of volatile fatty acids: current status and future perspectives. Reviews in Environmental Science and Tecnology., 16(2), 327–345.
El-Gammal, M., Abou-Shanab, R., Angelidaki, I., Omar, B., Sveding, P. V., Karakashev, D. B., & Zhang, Y. (2017). High efficient ethanol and VFA production from gas fermentation: effect of acetate, gas and inoculum microbial composition. Biomass and Bioenergy., 105, 32–40.
Gong, Z., Shen, H., Zhou, W., Wang, Y., Yang, X., & Zhao, Z. K. (2015). Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnology Biofuels, 8(1), 189.
Zhao, X., Davis, K., Brown, R., Jarboe, L., & Wen, Z. (2015). Alkaline treatment for detoxification of acetic acid-rich pyrolytic bio-oil for microalgae fermentation: effects of alkaline species and the detoxification mechanisms. Biomass and Bioenergy., 802, 03–212.
Han, W., Wang, X., Ye, L., Huang, J., Tang, J., Li, Y., & Ren, N. (2015). Fermentative hydrogen production using wheat flour hydrolysate by mixed culture. Hydrogen Energy., 40(13), 4474–4480.
Yuan, Q., Sparling, R., & Oleszkiewicz, J. A. (2011). VFA generation from waste activated sludge: effect of temperature and mixing. Chemosphere., 82(4), 603–607.
Perez-Garcia, O., Escalante, FM., de-Bashan, LE., Bashan, YJ. (2011). Heterotrophic cultures of microalgae: metabolism and potential products. Water Research. 45(1), 11–36.
Lian, J., Garcia-Perez, M., Coates, R., Wu, H., & Chen, S. J. (2012). Yeast fermentation of carboxylic acids obtained from pyrolytic aqueous phases for lipid production. Bioresource Technology, 118, 177–186.
Sijtsma, L., Anderson, A. J., & Ratledge, C. (2010). Alternative carbon sources for heterotrophic production of docosahexaenoic acid by the marine alga Crypthecodinium cohnii. In Single cell oils (Second Edition) (pp. 131–149). Elsevier.
Zhu, L., Zhang, X., Ji, L., Song, X., & Kuang, C. (2007). Changes of lipid content and fatty acid composition of Schizochytrium limacinum in response to different temperatures and salinities. Process Biochemistry, 42(2), 210–214.
Ratledge, C., Kanagachandran, K., Anderson, A. J., Grantham, D. J., & Stephenson, J. C. (2001). Production of docosahexaenoic acid by Crypthecodinium cohnii grown in a pH-auxostat culture with acetic acid as principal carbon source. Lipids, 36(11), 1241–1246.
Guo, D.-S., Ji, X.-J., Ren, L.-J., Li, G.-L., Yin, F.-W., & Huang, H. (2016). Development of a real-time bioprocess monitoring method for docosahexaenoic acid production by Schizochytrium sp. Bioresource Technology, 216, 422–427.
Metz, J. G., Kuner, J., Rosenzweig, B., Lippmeier, J. C., Roessler, P., & Zirkle, R. (2009). Biochemical characterization of polyunsaturated fatty acid synthesis in Schizochytrium: release of the products as free fatty acids. Journal of Plant Physiology and Biochemistry., 47(6), 472–478.
Ratledge, C. (2004). Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie, 86(11), 807–815.
Ren, L. J., Huang, H., Xiao, A. H., Lian, M., Jin, L. J., & Ji, X. J. (2009). Enhanced docosahexaenoicacid production by reinforcing acetyl-CoA and NADPH supply in Schizochytriumsp. HX-308. Bioprocess and Biosystems Engineering, 32(6), 837–843.
Casal, M., Paiva, S., Queirós, O., & Soares-Silva, I. (2008). Transport of carboxylic acids in yeasts. FEMS Microbiology Reviews, 32(6), 974–994.
Liu, Z. J., Liu, L. P., Wen, P., Li, N., Zong, M. H., & Wu, H. (2015). Effects of acetic acid and pH on the growth and lipid accumulation of the oleaginous yeast Trichosporon fermentans. Bio Resources., 10(3), 4152–4166.
Béligon, V., Poughon, L., Christophe, G., Lebert, A., Larroche, C., & Fontanille, P. (2015). Improvement and modeling of culture parameters to enhance biomass and lipid production by the oleaginous yeast Cryptococcus curvatus grown on acetate. Bioresource Technology, 192, 582–591.
Sun, L., Ren, L., Zhuang, X., Ji, X., Yan, J., & Huang, H. (2014). Differential effects of nutrient limitations on biochemical constituents and docosahexaenoic acid production of Schizochytrium sp. Bioresource Technology, 159, 199–206.
Álvarez-Ordóñe, A., Fernández, A., Bernardo, A., & López, M. (2010). Arginine and lysine decarboxylases and the acid tolerance response of Salmonella Typhimurium. International Journal of Food Microbiology, 136(3), 278–282.
Zhu, L., Zhang, X., Ren, X., & Zhu, Q. (2008). Effects of culture conditions on growth and docosahexaenoic acid production from Schizochytrium limacinum. Journal of Ocean University of China, 7(1), 83–88.
Huang, T. Y., Lu, W. C., & Chu, I. M. (2012). A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22: 6 proportions in the total fatty acid. Bioresource Technology, 12, 38–14.
Qu, L., Ren, L. J., Sun, G. N., Ji, X. J., Nie, Z. K., & Huang, H. (2013). Batch, fed-batch and repeated fed-batch fermentation processes of the marine thraustochytrid Schizochytrium sp. for producing docosahexaenoic acid. Bioprocess and Biosystems Engineering, 36(12), 1905–1912.
Furlan, V. J. M., Maus, V., Batista, I., & Bandarra, N. M. (2017). Production of docosahexaenoic acid by Aurantiochytrium sp. ATCC PRA-276. Brazilian Journal of Microbiology, 48(2), 359–365.
Sahin, D., Tas, E., & Altindag, U. H. (2018). Enhancement of docosahexaenoic acid (DHA) production from Schizochytrium sp. S31 using different growth medium conditions. AMB Express, 8(1), 7.
Funding
This work is supported by the Open Research Fund Program of Key Laboratory of Cleaner Production and Integrated Resource Utilization of China National Light Industry (CP-2018-YB8) and the Department of Ocean and Fishery of Liaoning Province, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Acetic acid efficiently converted into omega-3 fatty acids by S. limacinum SR21
• High biomass concentration of 146 g/L and DHA production of 23.0 g/L achieved
• Acetic acid resulted in higher biomass concentration and DHA production than glucose
Electronic Supplementary Material
ESM 1
(DOCX 295 kb)
Rights and permissions
About this article
Cite this article
Shafiq, M., Zeb, L., Cui, G. et al. High-Density pH-Auxostat Fed-Batch Culture of Schizochytrium limacinum SR21 with Acetic Acid as a Carbon Source. Appl Biochem Biotechnol 192, 1163–1175 (2020). https://doi.org/10.1007/s12010-020-03396-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03396-6