Abstract
Lysozymes are known as ubiquitously distributed immune effectors with hydrolytic activity against peptidoglycan, the major bacterial cell wall polymer, to trigger cell lysis. In the present study, the full-length cDNA sequence of a novel sea urchin Strongylocentrotus purpuratus invertebrate-type lysozyme (sp-iLys) was synthesized according to the codon usage bias of Pichia pastoris and was cloned into a constitutive expression plasmid pPIC9K. The resulting plasmid, pPIC9K-sp-iLys, was integrated into the genome of P. pastoris strain GS115. The bioactive recombinant sp-iLys was successfully secreted into the culture broth by positive transformants. The highest lytic activity of 960 U/mL of culture supernatant was reached in fed-batch fermentation. Using chitin affinity chromatography and gel-filtration chromatography, recombinant sp-iLys was produced with a yield of 94.5 mg/L and purity of > 99%. Recombinant sp-iLys reached its peak lytic activity of 8560 U/mg at pH 6.0 and 30 °C and showed antimicrobial activities against Gram-negative bacteria (Vibrio vulnificus, Vibrio parahemolyticus, and Aeromonas hydrophila) and Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis). In addition, recombinant sp-iLys displayed isopeptidase activity which reached the peak at pH 7.5 and 37 °C with the presence of 0.05 M Na+. In conclusion, this report describes the heterologous expression of recombinant sp-iLys in P. pastoris on a preparative-scale, which possesses lytic activity and isopeptidase activity. This suggests that sp-iLys might play an important role in the innate immunity of S. purpuratus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lysozymes are ubiquitous hydrolases existing in numerous phylogenetically diverse organisms, including animals, plants, fungi, bacteria, and bacteriophages. These enzymes play important roles in protecting the host against pathogenic infections by hydrolyzing the 1, 4-β-linkages in the bacterial cell wall peptidoglycan, resulting in bacteriolysis [1, 2]. According to differences in the structures and biological functions, animal lysozymes are categorized into four types: chicken-type (c-type), goose-type (g-type), invertebrate-type (i-type), and chalaropsis-type [3,4,5,6,7]. In 1975, i-type lysozyme was firstly identified from the starfish Asterias rubens by comparing its N-terminal amino acid sequence with other known lysozymes [8]. A salivary gland protein known as destabilase (also called isopeptidase) secreted by the medicinal leech Hirudo medicinalis was proven to be an i-type lysozyme [9]. Nowadays, i-type lysozymes have been recognized as a unique lysozyme family widely distributed in invertebrates [10,11,12,13] and important immune effectors to resist bacterial pathogen invasion in the innate immune system of invertebrates [14, 15].
Sea urchins are ubiquitously distributed in the world’s oceans, constituting an important part of subtidal marine communities, and are an important fisheries resource. Over the past decades, sea urchins have been used as model organisms in developmental biology. Recently, the full-length cDNA encoding a novel sea urchin Strongylocentrotus purpuratus i-type lysozyme (sp-iLys) has been identified, which is composed of 151 amino acid residues. The publication of the genome sequence reveals the complexity of immune response genes in S. purpuratus [16,17,18,19,20]. Despite this, it must rely on the innate immunity, first of all, to protect itself from invading pathogens that coexist in its environment. It is proposed that sp-iLys may function as an important immune effector in the innate immunity of S. purpuratus. However, till now, little is known about its enzymatic properties.
Pichia pastoris has been successfully used as a host system to express heterologous proteins, mainly to produce biopharmaceuticals and industrial enzymes [21, 22]. As a single-celled eukaryote, it is easy to manipulate as Escherichia coli and possesses many advantages similar to those of higher eukaryotic systems [23]. Under the control of the strong inducible alcoholoxidase 1 (AOX1) promoter, P. pastoris has expressed various heterologous proteins at high levels both intracellularly and in a secretory mode. In an example of this high-level extracellular expression of heterologous proteins, native proteins were secreted at very low levels, which significantly simplified the downstream processes.
In the present study, an efficient strategy was established for the heterologous expression of recombinant sp-iLys in P. pastoris on a preparative-scale through a combination of codon modification, strain screening, and glycerol/methanol fed-batch fermentation. The recombinant sp-iLys obtained showed lytic activity and isopeptidase activity, suggesting that sp-iLys is a novel bifunctional invertebrate-type lysozyme and has a great potential as an antimicrobial agent to treat infectious diseases.
Materials and Methods
Strains, Plasmids, Media, and Reagents
E. coli TOP10F′, P. pastoris GS115, and plasmid pPIC9 and pPIC9K were purchased from Invitrogen (Carlsbad, CA, USA). Micrococcus luteus was purchased from China General Microbiological Culture Collection Center (Beijing, China). Luria-Bertani (LB) medium was prepared for the cultivation of E. coli. For yeast culture, yeast extract peptone dextrose medium (YPD), buffered glycerol-complex medium (BMGY), buffered methanol-complex medium (BMMY), minimal dextrose medium (MD), fermentation basal salts medium, and PTM1 trace salt solution were prepared as specified in the Pichia Expression Kit (Invitrogen). Solid media was prepared by adding 1.5% (w/v) agar. Taq DNA polymerase, restriction endonucleases, and T4 DNA ligase were purchased from NEB (Ipswich, MA, USA). All other chemicals used were of analytical grade unless otherwise stated.
Bioinformatics Analysis
The sp-iLys cDNA sequence (accession number: XP_788380) was derived from a genomic sequence (NW_011996389.1) annotated using the gene prediction method Gnomon. The identities between sp-iLys and other i-type lysozymes were analyzed using the online Basic Local Alignment Search Tool Program (BLASTP) (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The ClustalX 2.0 program (http://www.ebi.ac.uk/tools/clustalw2) was applied to produce multiple alignments of amino acid sequences. The molecular weight (MW) was calculated using the online software (http://web.expasy.org/compute_pi/).
Construction of Expression Plasmid pPIC9K-sp-iLys
The full-length sp-iLys cDNA was artificially synthesized by Sangon Biotech (Shanghai, China). The vector sequence was rebuilt through polymerase chain reaction (PCR) with two primers, sp-iLys-F (5′- CCGCTCGAGAAAAGAACTGAGAAGTACCATGTCGCCTTG-3′) and sp-iLys-R (5’-CGGAATTCTCACTTCAAACATTCCTTGACTCTAG-3′), flanked by an Xho I site on the 5′ end and an EcoR I site on the 3′ end, respectively. After digestion with Xho I and EcoR I, the PCR product was ligated into pPIC9. The pPIC9-sp-iLys plasmid was transformed into TOP10F′ and selected on LB plates containing ampicillin. Positive E. coli transformants were identified by PCR with 5’-AOX1 and 3’-AOX1 primers. After digestion with BamH I and EcoR I, the PCR product was ligated into pPIC9K (Fig. 1). Then, the pPIC9K-sp-iLys plasmid was transformed into TOP10F′. The transformants were selected by ampicillin resistance. Finally, the pPIC9K-sp-iLys plasmids were isolated, sequenced, and used to transform P. pastoris GS115 cells.
Multiple alignments of sp-iLys and other i-type lysozymes. Residues involved in lysozyme activity are marked with red downward arrows and residue that is responsible for isopeptidase activity is marked with blue downward arrow. Cysteine residues involved in intra-molecular disulfide bond(s) in sp-iLys are highlighted in black. sp-iLys is highlighted with a red box. ce Caenorhabditis elegans (NP_500206); hm Hirudo medicinalis (AAA96144); ar Asterias rubens (AAR29291); sj Stichopus japonicas (ABK34500); tj Tapes japonica (BAC15553); ea. Eisenia andrei (ABC68610). The percentage identity (I%) and similarity (S%) sp-iLys with other i-type lysozymes are shown at the C-terminus
Transformation and Screening of Transformants
Five micrograms of Sal I-linearized pPIC9K-sp-iLys were transformed into competent P. pastoris GS115 cells using the electroporation method. After transformation, the cell suspension was spread onto MD plates for 2–3 days to select positive transformants. Multi-copy selection of P. pastoris transformants was performed on YPD plates containing 1.0–4.0 mg/mL Geneticin. Some positive transformants with high resistance to Geneticin were confirmed by PCR.
Shake Flask Experiments
The selected P. pastoris transformants were inoculated into 100 mL of BMGY medium and grown at 30 °C for 24 h. The cells were harvested by centrifuging the culture broth at 5000×g for 10 min. The cell pellet was resuspended in 100 mL of BMMY medium and cultivated at a certain temperature. The expression of recombinant sp-iLys was induced with a certain concentration of methanol (100%) every 24 h for 72 h. The culture broth was sampled every 12 h for analysis of wet cell weight (WCW) and lytic activity and for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Fed-Batch Fermentation
A three-phase fed-batch fermentation protocol was carried out as described previously [24]. The selected transformant was inoculated into 100 mL of BMGY medium and cultivated at 30 °C. After the optical density (OD) at 600 nm reached 5–6, the culture broth was transferred into 3 L of basal salts medium in a 5-L bioreactor (NC-Bio, Shanghai, China). The important parameters obtained from prior optimization experiments were used. After glycerol exhaustion, 50% (w/v) glycerol was supplemented at a rate of 20 mL/L/h for a period of 5 h. After the depletion of glycerol, pure methanol was added at a rate of 4 ml/L/h for 2–3 h. When the dissolved oxygen (DO) level was stabilized, the methanol feeding rate was increased to maintain the concentration of methanol at 1.5%. The culture broth was sampled every 12 h for further analysis of WCW and lytic activity.
Purification of Recombinant sp-iLys
The culture supernatant was collected by centrifugation at 5000×g for 30 min and concentrated by ultrafiltration with a Pellicon cassette (Millipore, Billerica, MA, USA) with a 5-kDa-cutoff membrane. Subsequently, chitin affinity matrix was added to the supernatant at a ratio of 1:20 (v/v). The mixture was gently stirred for 10 min and precipitated for 5 min. After three washes with 0.02 M phosphate-buffered saline (PBS, pH 7.4), the matrix was packed into an opened column and further washed with PBS. Bound recombinant sp-iLys was eluted with 0.02 M acetic acid solution. The pooled fraction was loaded onto a Sephadex G-75 column and washed with PBS. The eluate was concentrated and desalted with a 3-kDa-cutoff centrifugal filter unit (Millipore) and lyophilized.
Reverse-Phase High-Performance Liquid Chromatography
Reverse-phase high-performance liquid chromatography (RP-HPLC) was carried out on a C18 column (3.5 μm, 2.1 × 150 mm, Waters) installed in the Waters 600E-2487 system (Milford, MA, USA). Protein was eluted with a linear gradient of 5–95% acetonitrile with 0.1% trifluoroacetic acid in 60 min at a flow rate of 1 mL/min and detected at 215 nm.
Lytic Activity Assay
The turbidimetric assay was performed to measure lytic activity of recombinant sp-iLys. A standard bacterial species, M. luteus, was used as the substrate and bacteria suspension with an OD of approximately 0.8 at 450 nm was prepared in PBS (pH 6.2). Hen egg white lysozyme (HEWL) was used as a positive control. A 50-μL portion of recombinant sp-iLys (100 μg/mL) or HEWL (100 μg/mL) was mixed individually with 250 μL of bacteria suspension and the decrement of OD at 450 nm was recorded. To evaluate the effect of pH, bacteria suspensions with a pH range of 4.0–10 were used, which was prepared with sodium acetate-acetic acid (pH 4.0–5.5), Na2HPO4-NaH2PO4 (pH 6.0–8.5), and boric acid-NaOH (pH 9.0–10). To measure the effect of temperature, bacteria suspensions and protein solutions were placed in a water bath at different temperatures (5–60 °C) for 5 min and then mixed. To assess the thermal stability of the enzyme, protein solutions were placed into a water bath at different temperatures (40–100 °C) for 30 min and after cooling down, added to bacteria suspensions at room temperature. The formula (OD0min − OD1min)/OD0min was used to calculate lytic activity of the enzyme. All analyses were performed in triplicate.
Antimicrobial Activity Assay
Antimicrobial activity of recombinant sp-iLys against six bacterial species, including four Gram-negative species (Vibrio vulnificus, Vibrio parahemolyticus, Aeromonas hydrophila, and E. coli) and three Gram-positive species (M. luteus, Staphylococcus aureus, and Bacillus subtilis), was measured using a modified turbidimetric assay. Briefly, each bacterium was grown in LB medium, pelleted by centrifugation, and resuspended in PBS (OD at 450 nm of approximately 0.8). Fifty microliters of recombinant sp-iLys solution (100 μg/mL) were mixed individually with 250 μL of bacteria suspension. The decrease in OD at 450 nm was recorded after 30 min. HEWL and bovine serum albumin (BSA) were used as controls. All assays were performed in triplicate. The formula (OD0min − OD30min)/OD0min was used to calculate antimicrobial activity of the enzyme.
Isopeptidase Activity Assay
This assay was carried out as described previously [25]. Isopeptidase activity of sp-iLys was determined using L-γ-glutamine-p-nitroanilide (L-γ-Glu-pNA, Sigma-Aldrich) as the substrate and the formation of p-nitroanilide (pNA) was examined by measuring the OD at 405 nm. BSA was used as a control. Fifty microliters of recombinant sp-iLys solutions with different concentrations were mixed with 100 μL of substrate solution containing 1.75 mM L-γ-Glu-pNA and 0.01 M NaCl in 0.05 M 3-(N-morpholino) propane sulfonic acid buffer (pH 7.0) and incubated for 72 h at 37 °C. Different concentrations of the enzyme (0–300 μg/mL) and NaCl (0–0.3 M) and different pH buffers (4.0–10) were used to analyze the optimum conditions for isopeptidase activity and thermal stability of recombinant sp-iLys.
Statistical Analysis
Statistical analysis was carried out using the Student’s t test available in SPSS 21.0 statistical software (SPSS, Inc., Chicago, IL, USA). Values were shown as mean ± SD.
Results
Sequence Characterization
The sp-iLys cDNA sequence was predicted from a genomic sequence and cloned from the male gonad cDNA library through PCR, and then sequenced to confirm its accuracy. The results of BLASTP analysis showed that sp-iLys shared the highest identity (56.8%) with the Stichopus japonicas i-type lysozyme. It also shared 56.1% identity with the Asterias rubens i-type lysozyme and 39.7% identity with H. medicinalis destabilase. Multiple alignments of sp-iLys with five other i-type lysozymes revealed ten conserved cysteine residues which were the essential residues for structure stability of the lysozymes by forming disulfide bonds, implying they share similar molecular structures. Moreover, two conserved residues (Glu44 and Asp57) responsible for lytic activity were highly conserved in all i-type lysozymes, suggesting that sp-iLys and other i-type lysozymes may have lytic activity. In addition, two other conserved residues (Ser97 and His127) responsible for the isopeptidase activity of i-type lysozymes were also found in all i-type lysozymes. The results suggested that sp-iLys, together with other i-type lysozymes, may have potential isopeptidase activity.
Construction of the Expression Plasmid
To achieve secretory expression of sp-iLys with the natural N-terminus, the optimized sp-iLys cDNA was introduced in-frame with the Kex2 signal cleavage site, downstream of the α-factor signal sequence in pPIC9K, which is under the regulation of the AOX1 promoter. Enzyme digestion of pPIC9K-sp-iLys with BamH I and EcoR I produced a single band of the expected 713 base pair (bp) band containing a 393-bp inserted target fragment and an expression vector band. This indicates that the sp-iLys gene was correctly oriented in pPIC9K. DNA sequencing of pPIC9K-sp-iLys confirmed the accuracy of the reading frame of sp-iLys.
Screening of Positive Transformants
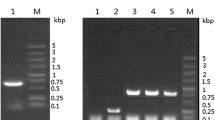
After linearization with Sal I, pPIC9K-sp-iLys was transformed into P. pastoris GS115 competent cells by electroporation. Eighteen highly Geneticin-resistant clones were obtained for a flask-scale expression test, among which six were resistant to 4.0 mg/mL Geneticin and the others were resistant to 2.0 mg/mL Geneticin. After genomic DNA was isolated from P. pastoris recombinants and controls, the integration of the recombinant plasmid was confirmed by PCR with 5′ and 3’ AOX1 sequencing primers. The selected transformants were cultured in shake flasks to measure recombinant sp-iLys expression. SDS-PAGE analysis of the culture supernatants revealed the expression of recombinant sp-iLys, which was indicated by a protein band of approximately 14.76 kDa (Fig. 2a). Lytic activity was detected in the culture supernatants of all the transformants (Fig. 2b). The transformant with the highest lytic activity in its culture supernatant was selected for subsequent experiments. No apparent lytic activity was detected in the supernatant of the transformant with the empty pPIC9K vector.
Screening and analysis of the positive transformants. a SDS-PAGE analysis of recombinant sp-iLys expressed in culture supernatant. Lane M: MW marker; Lanes 1–5: culture supernatants from the positive transformants. For each sample, 10 μL of culture supernatant was loaded. b Profile of recombinant sp-iLys activity secreted by selected highly Geneticin-resistant P. pastoris transformants in shake flasks
Expression of Recombinant sp-iLys in a 5-L Bioreactor
To achieve high-yield production of sp-iLys, the glycerol batch phase was first used after inoculation and continued for 24 h. This resulted in an increase in DO because of carbon source limitation. Subsequently, the glycerol fed-batch phase was started and continued for 6 h. This caused an increase in WCW to 103 g/L. After that, the methanol fed-batch phase was initiated to induce the expression of recombinant protein. There was no apparent lytic activity in the culture supernatant before this phase. At the end of the fermentation run, the WCW and the lytic activity reached final values of 232 g/L and 960 U/mL, respectively (Fig. 3a). SDS-PAGE analysis of the culture supernatants revealed a gradually increasing expression of recombinant sp-iLys (Fig. 3b).
Purification of Recombinant sp-iLys
The culture supernatant was obtained by centrifugation and concentrated using membrane ultrafiltration. Subsequently, the concentrated supernatant was directly incubated with chitin affinity matrix in batch to attach recombinant sp-iLys. The target protein was eluted with acetic acid solution, which yielded only one peak (Fig. 4a), and further purified via Sephadex G-75 gel-filtration chromatography to remove trace impurities. Finally, 94.5 mg of purified recombinant sp-iLys per liter of fermentation supernatant was obtained. SDS-PAGE of the eluates showed a molecular weight of about 14.76 kDa which is identical to the theoretical value (Fig. 4b). The purity of the purified recombinant sp-iLys was > 99%, as determined by RP-HPLC (Fig. 4c).
Purification of recombinant sp-iLys by the two-step chromatrography procedure. a Elution profile of chitin affinity chromatography. b SDS-PAGE of fractions collected through Sephadex G-75 chromatography. Lane M: MW marker; Lanes 1–3: purified recombinant sp-iLys from different fractions. c RP-HPLC analysis of purified protein. A single peak at 22 min retention time indicates the purity of recombinant sp-iLys (> 99%)
Lytic Activity of Recombinant sp-iLys
The optimum pH for lytic activity of recombinant sp-iLys was around 6.0 (Fig. 5a). The optimum temperature for lytic activity was 30 °C. Within the pH range of 15–40 °C, it retained greater than 80% of lytic activity and was more active than HEWL at temperatures below 30 °C (Fig. 5b). Dissimilarly, HEWL demonstrated the highest activity at pH 8.0 and 40 °C. Under the optimum conditions, the specific activity of purified recombinant sp-iLys reached 8560 U/mg. After incubation at 80 °C for 30 min, 34 and 75% of residual activity were recorded for recombinant sp-iLys and HEWL, respectively, suggesting that recombinant sp-iLys was less thermostable than HEWL (Fig. 5c).
Antimicrobial Activity of Recombinant sp-iLys
Both recombinant sp-iLys and HEWL displayed antimicrobial activities against V. vulnificus, V. parahemolyticus, A. hydrophila, S. aureus, and B. subtilis. However, V. vulnificus and V. parahemolyticus were more susceptible to recombinant sp-iLys than HEWL (P < 0.05). In addition, neither sp-iLys nor HEWL showed significant antimicrobial activities against E. coli. BSA did not show significant antimicrobial activity against any bacterial species examined (Fig. 6).
Isopeptidase Activity of Recombinant sp-iLys
There was a significant increase in the production of pNA with the concentration of recombinant sp-iLys increasing in reaction mixtures. The maximum value was observed at the concentration of 100 μg/mL protein which was used for subsequent experiments (Fig. 7a). After incubation of 72 h, recombinant sp-iLys reached a peak activity with the presence of 0.05 M Na+ (Fig. 7b). In addition, the optimum pH and the optimum temperature for isopeptidase activity were observed at pH 7.5 and 35 °C, respectively. Hence, isopeptidase activity of recombinant sp-iLys displayed protein and salt concentration-dependence and was also affected by pH and temperature.
Discussion
During the past several decades, lysozymes have been intensively studied. The major function of lysozymes is host defense, as they act as an antimicrobial and immune-modulating agent involved in immune defense against different pathogen infections [1]. Interestingly, i-type lysozymes have been characterized only in invertebrates even though other lysozyme types such as c-type and g-type lysozymes have been identified in both vertebrates and invertebrates. The phylogenetic position of sea urchins and their importance in studies of embryonic development motivated sequencing the genome of S. purpuratus [16, 26]. The genome sequence went onto show the existence of a gene encoding a novel i-type lysozyme, sp-iLys, which was further identified from the male sea urchin gonad cDNA library. It also demonstrated that the immune response genes of S. purpuratus incorporate an elaborate repertoire of innate pathogen recognition genes [13,14,15,16,17]. However, S. purpuratus need rely on the innate immunity, first of all, to protect itself from invading pathogens that coexist in its environment. Reportedly, some crustacean species, such as Penaeus monodon and Litopenaeus vannamei, contain two i-type lysozymes [24, 27, 28], while sp-iLys seems to be the only one for S. purpuratus, suggesting an important role in the innate immunity system of this organism.
As shown by the bioinformatics analysis, sp-iLys shares the highest identity with the S. japonicas i-type lysozyme [29], supporting that it is a new kind of invertebrate lysozyme. It is noteworthy that sp-iLys also has higher identity with H. medicinalis destabilase and contains a destabilase domain. Ser97 and His127 in sp-iLys correspond to the two essential residues which have been suggested to be responsible for isopeptidase activity of H. medicinalis destabilase and some mollusk i-type lysozymes [30, 31]. In this study, recombinant sp-iLys displayed the highest isopeptidase activity at pH 7.5 and the highest lytic activity at pH 6.0. This finding indicates that these two activities are performed independently by different catalytic sites. One site containing Glu44 and Asp57 is responsible for lytic activity of sp-iLys and another site containing Ser97 and His127 is for isopeptidase activity of sp-iLys. However, some i-type lysozymes possess only one of the activities. For instance, the Crassostrea virginica i-type lysozyme exhibited only lytic activity [32]. Interestingly, despite no apparent lytic activity, some i-type lysozymes, such as the P. monodon i-type lysozyme, showed antimicrobial activity against some bacterial species [27, 32, 33].
To characterize enzymatic properties of sp-iLys, we attempted to express the unprocessed form of the target protein using the methylotrophic yeast P. pastoris as an expression system. This system has advantages over the prokaryotic version, such as stable genomic integration and exact posttranslational modification, in addition to efficient secretory expression and high density cultivation [22, 34, 35]. Secretory expression of bioactive recombinant sp-iLys was achieved using the Saccharomyces cerevisiae α-factor signal peptide. Optimization of the codon and screening of multiple inserts were carried out to improve the enzyme activity expressed in the culture supernatant. To produce the desired heterologous protein more efficiently, bioreactors are preferentially used in the fermentation protocol, as all of the culture parameters can be controlled automatically and a very high P. pastoris density can be achieved [22]. In this study, we employed fed-batch fermentation in a 5-L bioreactor to enhance the biomass and the production of recombinant sp-iLys. This method contained a glycerol batch phase and a fed-batch phase for biomass growth to maximize the cell density, and a methanol fed-batch phase in which methanol was used both for the induction of the target protein and as the carbon source for P. pastoris. Finally, the biomass and the lytic activity of recombinant sp-iLys in the fermentation supernatant increased 4.9- and 17.6- fold, respectively, compared with the shake flask cultivation.
Many purification methods have been employed in the isolation of c-type lysozymes from various sources [36,37,38,39,40]. Affinity chromatography is an attractive method because of its high efficiency and many kinds of affinity matrix have been developed [41,42,43,44]. Up to now, a few kinds of affinity chromatography matrices, such as cellulose matrix, polyacrylamide cryogel, magnetic chitosan bead, and dye-affinity beads, have been developed to purify lysozyme from hen egg white, the transgenic rice seed, and many other bioreactor systems [45]. The sp-iLys conserves several key molecular features, including retention of putative substrate-binding residues and two essential catalytic residues. Because of containing β-1, 4-linked N-acetylglucosamine, chitin can be modified appropriately to use for affinity purification of sp-iLys. Here, chitin affinity matrix, prepared by our laboratory, was successfully used to purify recombinant sp-iLys from the fermentation supernatant. We obtained approximately 94.5 mg of recombinant sp-iLys from 1 L of fermentation supernatant with purity of > 99%. By combining chitin affinity chromatography and gel-filtration chromatography, the developed procedure is low-cost, highly efficient, and is easy to be manipulated. More importantly, the final recombinant protein product has high purity and proper biological activity.
Lytic activities of i-type lysozymes have been found to be different [15, 33, 46]. To elucidate the role of sp-iLys, we conducted a series of experiments to determine lytic activity of recombinant protein. Using M. luteus as an initial substrate, recombinant sp-iLys showed a peak lytic activity of 8560 U/mg under the optimum conditions (pH 6.0 and 30 °C) which vary among different i-type lysoyzmes. In addition, recombinant sp-iLys displayed a wide spectrum of antimicrobial activities against Gram-negative and Gram-positive bacteria, especially the saltwater pathogens, Vibrio species. It has been previously demonstrated that i-type lysozymes harboring a “destabilase domain” can exhibit isopeptidase activity [47]. In this reaction, lysozymes can hydrolyze the bonds between glutamine γ-carboxamide and the lysine ε-amino group in fibrin, which is involved in blood coagulation [48]. Meanwhile, isopeptide bonds have been identified in the peptidoglycan of bacterial cell wall. These isopeptide bonds are located between D-glutamic acid and lysine residues in peptides, which connect each straight chain of 1, 4-β-linked N-acetylmuramic acid and N-acetylglucosamine in an annular fashion and are suspected of being targets of isopeptidases. It is possible that lytic activity and isopeptidase activity of sp-iLys both contribute to its antimicrobial activity, which causes the hydrolysis of peptidoglycan in bacterial cell wall and the subsequent destruction of bacteria [29]. Owing to an antibacterial mechanism completely dissimilar to conventional antibiotics and incompletely similar to other types of lysozymes, sp-iLys has great potential as an antimicrobial agent to treat infectious diseases caused by multidrug-resistant microorganisms [49]. The amino acid sequence comparison showed a high number of conserved cysteine residues in the mature peptide of sp-iLys, which were also identified in many other i-type lysozymes [10, 50]. The predictable four disulfide bonds have been suggested to be responsible for the high thermal stability of recombinant sp-iLys and probably more important for maintaining the stability of sp-iLys in high osmolarity seawater [10].
In conclusion, the heterologous expression of bioactive recombinant sp-iLys was achieved through a combined utilization of codon optimization, expression condition optimization, high density fed-batch fermentation, and affinity purification. The purified recombinant sp-iLys underwent a series of analysis confirming its enzymatic properties and exhibited lytic activity and isopeptidase activity. This suggests that sp-iLys is a novel bifunctional invertebrate-type lysozyme and has a great potential as an antimicrobial agent to treat infectious diseases.
Abbreviations
- sp-iLys:
-
Strongylocentrotus purpuratus invertebrate-type lysozyme
- AOX1 :
-
Alcoholoxidase 1
- YPD:
-
Yeast extract peptone dextrose
- BMGY:
-
Buffered glycerol-complex medium
- BMMY:
-
Buffered methanol-complex medium
- MD:
-
Minimal dextrose
- PCR:
-
Polymerase chain reaction
- WCW:
-
Wet cell weight
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- OD:
-
Optical density
- DO:
-
Dissolved oxygen
- PBS:
-
Phosphate-buffered saline
- RP-HPLC:
-
Reverse-phase high performance liquid chromatography
- HEWL:
-
Hen egg white lysozyme
- L-γ-Glu-pNA:
-
L-γ-glutamine-p-nitroanilide
- pNA:
-
p-nitroanilide
- MOPS:
-
3-(N-morpholino) propanesulfonic acid
References
Jollès, P., & Jollès, J. (1984). What’s new in lysozyme research? Always a model system, today as yesterday. Molecular and Cellular Biochemistry, 63(2), 165–189.
Qasba, P. K., & Kumar, S. (1997). Molecular divergence of lysozymes and alpha-lactalbumin. Critical Reviews in Biochemistry and Molecular Biology, 32(4), 255–306.
Bachali, S., Bailly, X., Jolles, J., Jolles, P., & Deutsch, J. S. (2004). The lysozyme of the starfish Asterias rubens. A paradygmatic type i lysozyme. European Journal of Biochemistry, 271(2), 237–242.
Hikima, S., Hikima, J., Rojtinnakorn, J., Hirono, I., & Aoki, T. (2003). Characterization and function of kuruma shrimp lysozyme possessing lytic activity against Vibrio species. Gene, 316, 187–195.
Jollès, J., Fiala-Médioni, A., & Jollès, P. (1996). The ruminant digestion model using bacteria already employed early in evolution by symbiotic molluscs. Journal of Molecular Evolution, 43(5), 523–527.
Canfield, R. E., & McMurry, S. (1967). Purification and characterization of a lysozyme from goose egg white. Biochemical and Biophysical Research Communications, 26(1), 38–42.
Van Herreweghe, J. M., & Michiels, C. W. (2012). Invertebrate lysozymes: diversity and distribution, molecular mechanism and in vivo function. Journal of Biosciences, 37(2), 327–348.
Jolles, J., & Jolles, P. (1975). The lysozyme from Asterias rubens. European Journal of Biochemistry, 54(1), 19–23.
Zavalova, L. L., Baskova, I. P., Lukyanov, S. A., Sass, A. V., Snezhkov, E. V., Akopov, S. B., Artamonova, I. I., Archipova, V. S., Nesmeyanov, V. A., Kozlov, D. G., Benevolensky, S. V., Kiseleva, V. I., Poverenny, A. M., & Sverdlov, E. D. (2000). Destabilase from the medicinal leech is a representative of a novel family of lysozymes. Biochimica et Biophysica Acta, 1478(1), 69–77.
Ito, Y., Yoshikawa, A., Hotani, T., Fukuda, S., Sugimura, K., & Imoto, T. (1999). Amino acid sequences of lysozymes newly purified from invertebrates imply wide distribution of a novel class in the lysozyme family. European Journal of Biochemistry, 259(1-2), 456–461.
Bachali, S., Jager, M., Hassanin, A., Schoentgen, F., Jolles, P., Fiala-Medioni, A., et al. (2002). Phylogenetic analysis of invertebrate lysozymes and the evolution of lysozyme function. Journal of Molecular Evolution, 54(5), 652–664.
Goto, T., Abe, Y., Kakuta, Y., Takeshita, K., Imoto, T., & Ueda, T. (2007). Crystal structure of Tapes japonica lysozyme with substrate analogue: structural basis of the catalytic mechanism and manifestation of its chitinase activity accompanied by quaternary structural change. Journal of Biological Chemistry, 282(37), 27459–27467.
Bathige, S. D., Umasuthan, N., Kasthuri, S. R., Whang, I., Lim, B. S., Nam, B. H., et al. (2013). A bifunctional invertebrate-type lysozyme from the disk abalone, Haliotis discus discus: genome organization, transcriptional profiling and biological activities of recombinant protein. Developmental and Comparative Immunology, 41(2), 282–294.
Loker, E. S., Adema, C. M., Zhang, S. M., & Kepler, T. B. (2004). Invertebrate immune systems-not homogeneous, not simple, not well understood. Immunological Reviews, 198(1), 10–24.
Callewaert, L., & Michiels, C. W. (2010). Lysozymes in the animal kingdom. Journal of Biosciences, 35(1), 127–160.
Sodergren, E., Weinstock, G. M., Davidson, E. H., Cameron, R. A., Gibbs, R. A., Angerer, R. C., Angerer, L. M., Arnone, M. I., Burgess, D. R., Burke, R. D., Coffman, J. A., Dean, M., Elphick, M. R., Ettensohn, C. A., Foltz, K. R., Hamdoun, A., Hynes, R. O., Klein, W. H., Marzluff, W., Mcclay, D. R., Morris, R. L., Mushegian, A., Rast, J. P., Smith, L. C., Thorndyke, M. C., Vacquier, V. D., Wessel, G. M., Wray, G., Zhang, L., Elsik, C. G., Ermolaeva, O., Hlavina, W., Hofmann, G., Kitts, P., Landrum, M. J., Mackey, A. J., Maglott, D., Panopoulou, G., Poustka, A. J., Pruitt, K., Sapojnikov, V., Song, X., Souvorov, A., Solovyev, V., Wei, Z., Whittaker, C. A., Worley, K., Durbin, K. J., Shen, Y., Fedrigo, O., Garfield, D., Haygood, R., Primus, A., Satija, R., Severson, T., Gonzalez-Garay, M. L., Jackson, A. R., Milosavljevic, A., Tong, M., Killian, C. E., Livingston, B. T., Wilt, F. H., Adams, N., Belle, R., Carbonneau, S., Cheung, R., Cormier, P., Cosson, B., Croce, J., Fernandez-Guerra, A., Geneviere, A.-M., Goel, M., Kelkar, H., Morales, J., Mulner-Lorillon, O., Robertson, A. J., Goldstone, J. V., Cole, B., Epel, D., Gold, B., Hahn, M. E., Howard-Ashby, M., Scally, M., Stegeman, J. J., Allgood, E. L., Cool, J., Judkins, K. M., Mccafferty, S. S., Musante, A. M., Obar, R. A., Rawson, A. P., Rossetti, B. J., Gibbons, I. R., Hoffman, M. P., Leone, A., Istrail, S., Materna, S. C., Samanta, M. P., Stolc, V., Tongprasit, W., Tu, Q., Bergeron, K.-F., Brandhorst, B. P., Whittle, J., Berney, K., Bottjer, D. J., Calestani, C., Peterson, K., Chow, E., Yuan, Q. A., Elhaik, E., Graur, D., Reese, J. T., Bosdet, I., Heesun, S., Marra, M. A., Schein, J., Anderson, M. K., Brockton, V., Buckley, K. M., Cohen, A. H., Fugmann, S. D., Hibino, T., Loza-Coll, M., Majeske, A. J., Messier, C., Nair, S. V., Pancer, Z., Terwilliger, D. P., Agca, C., Arboleda, E., Chen, N., Churcher, A. M., Hallbook, F., Humphrey, G. W., Idris, M. M., Kiyama, T., Liang, S., Mellott, D., Mu, X., Murray, G., Olinski, R. P., Raible, F., Rowe, M., Taylor, J. S., Tessmar-Raible, K., Wang, D., Wilson, K. H., Yaguchi, S., Gaasterland, T., Galindo, B. E., Gunaratne, H. J., Juliano, C., Kinukawa, M., Moy, G. W., Neill, A. T., Nomura, M., Raisch, M., Reade, A., Roux, M. M., Song, J. L., Su, Y.-H., Townley, I. K., Voronina, E., Wong, J. L., Amore, G., Branno, M., Brown, E. R., Cavalieri, V., Duboc, V., Duloquin, L., Flytzanis, C., Gache, C., Lapraz, F., Lepage, T., Locascio, A., Martinez, P., Matassi, G., Matranga, V., Range, R., Rizzo, F., Rottinger, E., Beane, W., Bradham, C., Byrum, C., Glenn, T., Hussain, S., Manning, G., Miranda, E., Thomason, R., Walton, K., Wikramanayke, A., Wu, S.-Y., Xu, R., Brown, C. T., Chen, L., Gray, R. F., Lee, P. Y., Nam, J., Oliveri, P., Smith, J., Muzny, D., Bell, S., Chacko, J., Cree, A., Curry, S., Davis, C., Dinh, H., Dugan-Rocha, S., Fowler, J., Gill, R., Hamilton, C., Hernandez, J., Hines, S., Hume, J., Jackson, L., Jolivet, A., Kovar, C., Lee, S., Lewis, L., Miner, G., Morgan, M., Nazareth, L. V., Okwuonu, G., Parker, D., Pu, L.-L., Thorn, R., & Wright, R. (2006). The genome of the sea urchin Strongylocentrotus purpuratus. Science, 314(5801), 941–952.
Hibino, T., Loza-Coll, M., Messier, C., Majeske, A. J., Cohen, A. H., Terwilliger, D. P., Buckley, K. M., Brockton, V., Nair, S. V., Berney, K., Fugmann, S. D., Anderson, M. K., Pancer, Z., Cameron, R. A., Smith, L. C., & Rast, J. P. (2006). The immune gene repertoire encoded in the purple sea urchin genome. Developmental Biology, 300(1), 349–365.
Rast, J. P., Smith, L. C., Loza-Coll, M., Hibino, T., & Litman, G. W. (2006). Genomic insights into the immune system of the sea urchin. Science, 314(5801), 952–956.
Rast, J. P., & Messier-Solek, C. (2008). Marine invertebrate genome sequences and our evolving understanding of animal immunity. Biological Bulletin, 214(3), 274–283.
Smith, L. C. (2012). Innate immune complexity in the purple sea urchin: diversity of the Sp185/333 system. Frontiers in Immunology, 3, 70.
Cregg, J. M., Vedvick, T. S., & Raschke, W. C. (1993). Recent advances in the expression of foreign genes in Pichia pastoris. Biotechnology, 11(8), 905–910.
Macauley-Patrick, S., Fazenda, M. L., McNeil, B., & Harvey, L. M. (2005). Heterologous protein production using the Pichia pastoris expression system. Yeast, 22(4), 249–270.
Daly, R., & Hearn, M. T. (2005). Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. Journal of Molecular Recognition, 18(2), 119–138.
Supungul, P., Rimphanitchayakit, V., Aoki, T., Hirono, I., & Tassanakajon, A. (2010). Molecular characterization and expression analysis of a c-type and two novel muramidase-deficient i-type lysozymes from Penaeus monodon. Fish & Shellfish Immunology, 28(3), 490–498.
Narmadha, G., & Yenugu, S. (2016). In silico and biochemical characterization of lysozyme-like proteins in the rat. PLoS One, 11(9), e0161909.
Tu, Q., Cameron, R. A., Worley, K. C., Gibbs, R. A., & Davidson, E. H. (2012). Gene structure in the sea urchin Strongylocentrotus purpuratus based on transcriptome analysis. Genome Research, 22, 2079–2087.
Peregrino-Uriarte, A. B., Muhlia-Almazan, A. T., Arvizu-Flores, A. A., Gomez-Anduro, G., Gollas-Galvan, T., Yepiz-Plascencia, G., & Sotelo-Mundo, R. R. (2012). Shrimp invertebrate lysozyme i-lyz: gene structure, molecular model and response of c and i lysozymes to lipopolysaccharide (LPS). Fish & Shellfish Immunology, 32(1), 230–236.
Chen, T., Ren, C., Wang, Y., Luo, P., Jiang, X., Huang, W., Chen, C., & Hu, C. (2016). Molecular cloning, inducible expression and antibacterial analysis of a novel i-type lysozyme (lyz-i2) in Pacific white shrimp, Litopenaeus vannamei. Fish & Shellfish Immunology, 54, 197–203.
Cong, L., Yang, X., Wang, X., Tada, M., Lu, M., Liu, H., et al. (2009). Characterization of an i-type lysozyme gene from the sea cucumber Stichopus japonicus, and enzymatic and nonenzymatic antimicrobial activities of its recombinant protein. Journal of Bioscience and Bioengineering, 107(6), 583–588.
Takeshita, K., Hashimoto, Y., Ueda, T., & Imoto, T. (2003). A small chimerically bifunctional monomeric protein: Tapes japonica lysozyme. Cellular and Molecular Life Sciences, 60(9), 1944–1951.
Yue, X., Liu, B., & Xue, Q. (2011). An i-type lysozyme from the Asiatic hard clam Meretrix meretrix potentially functioning in host immunity. Fish & Shellfish Immunology, 30(2), 550–558.
Xue, Q. G., Itoh, N., Schey, K. L., Li, Y. L., Cooper, R. K., & La Peyre, J. F. (2007). A new lysozyme from the eastern oyster (Crassostrea virginica) indicates adaptive evolution of i-type lysozymes. Cellular and Molecular Life Sciences, 64(1), 82–95.
Zhang, H. W., Sun, C., Sun, S. S., Zhao, X. F., & Wang, J. X. (2010). Functional analysis of two invertebrate-type lysozymes from red swamp crayfish, Procambarus clarkii. Fish & Shellfish Immunology, 29(6), 1066–1072.
Jahic, M., Veide, A., Charoenrat, T., Teeri, T., & Enfors, S. O. (2006). Process technology for production and recovery of heterologous proteins with Pichia pastoris. Biotechnology Progress, 22(6), 1465–1473.
Gu, L., Zhang, J., Liu, B., Du, G., & Chen, J. (2015). High-level extracellular production of glucose oxidase by recombinant Pichia pastoris using a combined strategy. Applied Biochemistry and Biotechnology, 175, 1429–1447.
Thammasirirak, S., Torikata, T., Takami, K., Murata, K., & Araki, T. (2001). Purification and characterization of goose type lysozyme from cassowary (Casuarius casuarius) egg white. Bioscience Biotechnology and Biochemistry, 65(3), 584–592.
Kale, S., & Lali, A. (2011). Characterization of superporous cellulose matrix for high-throughput adsorptive purification of lysozyme. Biotechnology Progress, 27(4), 1078–1090.
Wang, X., Dong, S., & Bai, Q. (2014). Preparation of lysozyme molecularly imprinted polymers and purification of lysozyme from egg white. Biomedical Chromatography, 28(6), 907–912.
Bayramoglu, G., Tekinay, T., Ozalp, V. C., & Arica, M. Y. (2015). Fibrous polymer grafted magnetic chitosan beads with strong poly (cation-exchange) groups for single step purification of lysozyme. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 990, 84–95.
Wu, H., Cao, D., Liu, T., Zhao, J., Hu, X., & Li, N. (2015). Purification and characterization of recombinant human lysozyme from eggs of transgenic chickens. PLoS One, 10, e0146032.
Roy, I., Rao, M. V., & Gupta, M. N. (2003). Purification of lysozyme from other hen’s-egg-white proteins using metal-affinity precipitation. Biotechnology and Applied Biochemistry, 37(1), 9–14.
Boto, R. E., Anyanwu, U., Sousa, F., Almeida, P., & Queiroz, J. A. (2009). Thiacarbocyanine as ligand in dye-affinity chromatography for protein purification. II. Dynamic binding capacity using lysozyme as a model. Biomedical Chromatography, 23(9), 987–993.
Lamppa, J. W., Tanyos, S. A., & Griswold, K. E. (2013). Engineering Escherichia coli for soluble expression and single step purification of active human lysozyme. Journal of Biotechnoloy, 164(1), 1–8.
Mól, P. C., Veríssimo, L. A., Eller, M. R., Minim, V. P., & Minim, L. A. (2017). Development of an affinity cryogel for one step purification of lysozyme from chicken egg white. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 1044–1045, 17–23.
Başar, N., Uzun, L., Güner, A., & Denizli, A. (2007). Lysozyme purification with dye-affinity beads under magnetic field. International Journal of Biological Macromolecules, 41(3), 234–242.
Mai, W. J., & Hu, C. Q. (2009). Molecular cloning, characterization, expression and antibacterial analysis of a lysozyme homologue from Fenneropenaeus merguiensis. Molecular Biology Reports, 36(6), 1587–1595.
Zavalova, L., Lukyanov, S., Baskova, I., Snezhkov, E., Akopov, S., Berezhnoy, S., Bogdanova, E., Barsova, E., & Sverdlov, E. D. (1996). Genes from the medicinal leech (Hirudo medicinalis) coding for unusual enzymes that specifically cleave endoepsilon (gamma-Glu)-Lys isopeptide bonds and help to dissolve blood clots. Molecular Genetics and Genomics, 253(1-2), 20–25.
Karthik, V., Kamalakannan, V., Thomas, A., Sudheer, N. S., Singh, I. S., & Narayanan, R. B. (2014). Functional characterization of a c-type lysozyme from Indian shrimp Fenneropenaeus indicus. Probiotics and Antimicrobial Proteins, 6(2), 114–121.
Jones, M. E., Draghi, D. C., Thornsberry, C., Karlowsky, J. A., Sahm, D. F., & Wenzel, R. P. (2004). Emerging resistance among bacterial pathogens in the intensive care unit—a European and North American Surveillance study (2000–2002). Annals of Clinical Microbiology and Antimicrobials, 3(1), 14–24.
Matsumoto, T., Nakamura, A. M., & Takahashi, K. G. (2006). Cloning of cDNAs and hybridization analysis of lysozymes from two oyster species, Crassostrea gigas and Ostrea edulis. Comparative Biochemistry and Physiology B-Biochemistry & Molecular biology, 145(3-4), 325–330.
Acknowledgments
This work was supported by the Shanghai Municipal Commission of Health and Family Planning (Grant Number: 201740161) and the Natural Science Foundation of Shanghai (Grant Number: 15ZR1421800).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Electronic supplementary material
Fig. S1
Identification of pPIC9K-sp-iLys by digestion with restriction enzymes. Lane M: DNA marker; Lane 1: pPIC9K-sp-iLys digested with BamH I and EcoR I (GIF 313 kb)
Fig. S2
Identification of positive P. pastoris transformants by PCR. Lane M: DNA marker; Lane 1: genomic DNA of P. pastoris GS115; Lane 2: empty pPIC9K vector; Lane 3: positive control; Lanes 4 and 6–10: positive transformants; Lane 5: negative clone (GIF 6.26 mb)
Rights and permissions
About this article
Cite this article
Huang, P., Shi, J., Sun, Q. et al. Engineering Pichia pastoris for Efficient Production of a Novel Bifunctional Strongylocentrotus purpuratus Invertebrate-Type Lysozyme. Appl Biochem Biotechnol 186, 459–475 (2018). https://doi.org/10.1007/s12010-018-2753-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2753-z