Abstract
The gene coding for lysozyme in banana prawn (Fenneropenaeus merguiensis) was cloned, sequenced and expressed in pET-32a vector. The deduced amino acid sequence of F. merguiensis lysozyme showed 37–93% similarity with the mouse, human, chicken, and tiger prawn counterparts. The lysozyme was purified to homogeneity and observed as a band of approximately 15 kDa in 12% SDS-PAGE. Semiquantitative RT-PCR analysis demonstrated that mRNA transcripts of lysozyme could be mainly detected in the tissues of hemocytes, gill, gonad and lymphoid organ of unchallenged shrimps, whereas the expression of lysozyme transcripts was increased in all the tested tissues after heat-killed Vibrio alginolyticus challenge. The temporal expression of lysozyme mRNA in hemolymph challenged by Micrococcus luteus and V. alginolyticus was both up-regulated and reached the maximum level at 8 and 16 h post stimulation, respectively, and then dropped back to the original level. Bacteriolytic activity of lysozyme against different bacterial cultures was determined by solid phase as well as turbidimetric assay. Lysis was obtained against Gram positive and Gram negative bacteria with strong inhibition against shrimp pathogens V. alginolyticus and V. parahemolyticus. In addition, the study of inhibition mechanism revealed that the antibacterial activity of lysozyme was a result of bactericidal effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lysozyme (EC.3.2.1.17) is widely distributed among eukaryotes and prokaryotes and takes part in protecting microbial infections or digestion. Lysozyme kills bacteria by hydrolyzing β-1,4-glycosidic linkages between N-acetylglucosamine and N-acetylmuramic acid of the peptidoglycan layer in the bacterial cell wall. Lysozymes are classified into three major types: chicken type (c-type, [4]), goose type (g-type, [32]) and invertebrate type (i-type, [16]). The c-type lysozyme has been found in many organisms including virus, bacteria, plants, insects, reptiles, birds, and mammals.
Lysozymes have been characterized in white and kuruma shrimps [12, 33]. Shrimp lysozymes are reported to defend against bacteria or aid in digestion [28]. Lysozymes involved in antibacterial defense are induced when shrimps are infected [3, 6]. Lysozyme activity has been detected in various tissue and cell types such as gills, heart, hemocytes, hepatopancreas, and muscle [2, 3, 6, 9, 12]. The high numbers of lysozyme mRNA transcripts and high enzymatic activity in hemocytes suggest it is the main sites of lysozyme synthesis and is likely the major contributors of lysozyme to other tissues [3, 6].
The banana prawn, Fenneropenaeus merguiensis has a relatively high market value, and is widely cultured throughout China [7]. However, outbreaks of diseases caused by parasites, bacteria and viruses have caused severe economic losses to the aquaculture industry, and in some cases the mortality can reach as high as 100% [13]. It is well known that some Gram negative Vibrio sp. including Vibrio alginolyticus and Vibrio parahemolyticus are highly pathogenic to penaeid shrimps which can cause significant economic loss in cultured shrimp [10, 20]. Despite the economical importance of the shrimp and severe economic losses caused by diseases, little research has been carried out on the banana prawn immune factors. To our knowledge, only a few immune factors have been so far purified and characterized from banana prawn, and those immune factors were reported to act important roles in pattern recognition and immune defense response against microorganism infection [21, 29]. The present study was designed to identify the lysozyme gene of the banana prawn, the expression of lysozyme in the shrimp tissues and the antibacterial activity of its recombinant protein.
Materials and methods
Shrimps, immune challenge, and hemolymph collection
The banana prawn F. merguiensis was collected from a commercial farm in Yangjiang, Guangdong Province, China. Individuals with a fresh weight of 10 ± 3 g were cultured in filtered aerated seawater at 18–20°C for 2 weeks before processing. For the bacterial challenge experiment, 700 shrimps were employed and kept in fourteen aerated tanks (50 individuals in each tank). A 50 μl of live V. alginolyticus or Micrococcus luteus resuspended in 0.1 mol l−1 PBS (pH 6.4, OD600 = 0.4) was injected into the tail muscles of ten shrimps per tank. Untreated shrimps and shrimps injected with 50 μl of PBS (10 shrimps per tank) were used as the blank and control group, respectively. The injected shrimps were returned to separate treatment tanks (Vibrio tank, Micrococcus tank, blank tank, control tank) and one individual were randomly sampled from each group at the time point of 2, 4, 6, 8, 16, 24, and 32 h post injection. The hemolymph (about 0.5 ml per individual) was collected using a syringe from the tail muscles. The hemolymph from five shrimps were pooled together as one sample, and immediately centrifuged at 800×g, 4°C for 10 min to collect the hemocytes. Three replicates were employed for each time point.
Cloning of c-type lysozyme cDNA
Primers were designed from a conserved region obtained by comparing all known c-type lysozyme sequences, and all primers used in this study are listed in Table 1. Total RNA extracted from hemolymph samples using TRIzol reagent (Invitrogen, USA) was reverse transcribed into cDNA by Powerscript II reverse transcriptase with CDS primer (SMART RACE cDNA Amplification kit, Clontech, USA). The PCR cycling conditions were 94°C for 3 min followed by 30 cycles of 94°C for 30 s, 54°C for 30 s and 72°C for 1 min, and then a final elongation step at 72°C for 5 min. PCR products were cloned into pGEM-T vector (Promega, USA) and sequenced.
To recover the full-length cDNA sequence, 3′ RACE and 5′ RACE were performed by using the gene specific primers and adaptor primers (Table 1). The PCR cycling conditions were 94°C for 3 min followed by 30 cycles of 94°C for 30 s, 64°C for 30 s and 72°C for 1 min, and then a final elongation step at 72°C for 5 min.
Data analysis
The BLAST program from the National Center for Biotechnology Information was used to identify similar sequences. The multiple sequence alignments were performed using the CLUSTALW 1.83 program [35].
The amino acid sequences of lysozymes were obtained from the GenBank, EMBL, DDBJ databases. All Sequences were aligned using the program CLUSTAL W version 1.8 [35]. All alignment illustrations were created with the program GENEDOC, version 2.6.001 [24]. A kind of acid matrix of sequence divergence was carried out using the subroutine provided in the program PAUP version 4.0 [34]. Data was bootstrapped 1,000 times. A phylogenetic tree was constructed by neighbor-joining method (NJ) using PAUP version 4.0 [34], with α-lactalbumins used as the outgroup for analysis.
Tissue specific expression of lysozymes
The mRNA expression of lysozyme in different tissues of healthy and challenged banana prawn was measured by semiquantitative RT-PCR. The V. alginolyticus in logarithmic phase was heated at 65°C for 15 min, and the heat-killed cells were collected and resuspended in PBS to the concentration of OD600 = 0.4. Fifty microliters of the bacterial suspension was injected into the tail muscle of ten banana prawns. The injected banana prawns were returned to seawater and cultured for another 8 h. Total RNA was extracted from different tissues of healthy and challenged banana prawns, respectively, including hemolymph, kidney, gill, hepatopancreas, muscle, gonad and lymphoid organ.
The first-strand cDNA synthesis was carried out based on Promega M-MLV RT usage information using the DNase-treated total RNA as template. Reactions were incubated at 37°C for 1 h, terminated by heating at 95°C for 5 min. Two lysozyme gene specific primers S3 and AS3 (Table 1) were used to amplify a product of 494 bp. A set of β-actin primers (Table 1) served as a control for amount and quality of cDNA. All PCR reactions were performed according to the following protocol: 1 μl of cDNA was mixed with 2 μl dNTPs (2.5 mmol l−1), 0.2 μl Taq polymerase (5 U μl l−1), 1 μl of each gene specific primer (10 μmol l−1), 2.5 μl 10× PCR buffer, 1.5 μl Mg2+ (25 mmol l−1) and 15.8 μl of PCR water. The PCR reactions were performed in a PTC-100 Programmable Thermal Controller (MJ research, USA) with 5 min at 94°C followed by 23 cycles (for β-actin) or 30 cycles (for Lysozyme) of 94°C for 30 s, 58°C for 30 s, 72°C for 45 s and a final extension step at 72°C for 10 min. The PCR products were separated in 2% agarose gel and stained with ethidium bromide. To confirm the specificity of RT-PCR amplification, the RT-PCR products were purified from the gel and submitted for sequencing.
The temporal mRNA expression of lysozyme after bacterial challenge
The expression of banana prawn lysozyme in hemolymph after bacterial challenge was measured by semi-quantitative RT-PCR. The templates for RT-PCR were prepared as described above. The PCR reactions were performed in a PTC-100 programmable thermal controller (MJ research, USA), following the same conditions described above. After amplification, the PCR products were separated in 2% agarose gel and stained with ethidium bromide. Electrophoretic image and the densities of target bands were analyzed using the Quantity one 1D analysis software in the Gel Doc 2000 System (Bio-Rad, USA). The data obtained from RT-PCR analysis were subjected to one-way analysis of variance (one-way ANOVA) followed by an unpaired, two-tailed t-test. Differences were considered significant at P < 0.05.
Production of banana prawn recombinant lysozyme C in E. coli
The PCR amplified c-type lysozyme gene fragment encoding the open reading frame was digested with KpnI and HindIII, ligated into the pET-32a expression vector (Novagen, Germany) linearized with the same enzymes, and transformed into DH5α competent cells. After sequencing the positive clones to ensure in frame insertion, the pET-32a-lysozyme construct was transformed into E. coli BL21 (DE3) strain for protein expression. Expression of lysozyme was induced by adding IPTG to the final concentration of 0.5 mM when the optical density at 600 nm of the cell culture reached 0.6–0.8, and the incubation was continued for an additional 4 h.
Purification of recombinant proteins under native conditions was performed by using the Ni–NTA His · Bind Resins (Novagen, Germany). Briefly, the bacterial pellet was resuspended in ice-cold 1× Ni–NTA binding buffer, lysed in 3 consecutive freezing (−35°C) and thaw (room temperature) cycles and sonicated on ice (20 s intervals, 10 min). The bacterial lysate was then centrifuged at 16,000×g for 30 min, and the supernatant was added to the Ni–NTA HisBind slurry and mixed gently by shaking for 1 h. After washing the column, the protein was eluted with 250 mM imidazole elution buffer. Purity of the recombinant protein was assessed on 12% SDS-PAGE gel according to the method of Laemmli [19] and visualized with Coomassie brilliant blue R250.
Lysozyme antibacterial activity assay
The activity of recombinant banana shrimp lysozyme against three Vibrio sp. included Vibrio alginolyticus, Vibrio parahemolyticus and Vibrio vulnificus was studied by solid phase [12] and turbidimetric assays [31]. Bacterial cultures grown up to OD600 of 0.5 in TSB with 1% NaCl were used for both the assays. For solid phase assay bacterial lawn was prepared on TSB agar plates. Three Vibrio sp. cultures in 1% warm (50°C) melting agarose (50 mM phosphate buffer, pH 6.2) was poured onto three area of one plate which were separated by sheet metal, respectively. The recombinant banana prawn lysozyme (250 μg), hen egg-white lysozyme (250 μg, Sigma) (positive control) and elution buffer (negative control) were put in individual wells (3-mm) in the agarose plate and incubated at 30°C for 24 h. All experiments were carried out in triplicate and diameter of the zone of inhibition was measured.
For kinetic determination of antibacterial activity against Vibrio sp., the turbidimetric method of Shugar [31] was modified. A 5-ml sample of fresh Vibrio culture was centrifuged at 11,000×g for 5 min, the supernatant was discarded and the pellet was washed twice with 50 mM sodium phosphate, pH 8.0. The bacterial pellet was resuspended in the same buffer and the change of optical density was followed over time at 450 nm. Initial measurement was made as close as possible to OD 0.5, either by adding more bacteria or buffer. The assay was started by addition of 3 μg of recombinant shrimp lysozyme C and the kinetic absorbance was obtained by recording data every 30 s over 20 min in a Cary 50 spectrophotometer (Varian Inc.). Additionally, the antibacterial activity of lysozyme C was assayed on E. coli using a CFU assay [22]. Cells were incubated at 37°C for 2 h in LB medium, and the number of CFU was determined by plating the diluted cell suspension on to LB agar plates.
Time-kill studies
Mid-logarithmic-phase cultures of V. alginolyticus in poor broth (105–106 CFU/ml) were incubated at 37°C in presence of recombinant lysozyme C or 50 mM phosphate buffer, pH 12 (control). The final concentration of the molecule tested was 10 times over the MIC value. Ten microliter aliquots were carried out at 0, 1, 2, 3, 6, 12, and 24 h. The samples at each time point were diluted serially 10-fold, plated on to the LB agar and incubated for 24 h at 37°C. Viable counts obtained were calculated to give the CFU/ml and the time-kill curves were plotted with time against the logarithm of the viable count. Each experiment was performed twice. The limit of count detection was 200 CFU/ml. Bactericidal activity was defined as a 3-log decrease in CFU/ml (99.9% kill). Bacteriostatic activity was defined as 99.9% kill.
Results and discussion
Cloning and sequence analysis of banana prawn lysozyme C
The Banana prawn lysozyme C is 971 bp long including 5′ and 3′ untranslated regions and coding for 158 amino acid residues (Fig. 1). The initiation codon begins with ATG and extends to a stop codon TAG. The polyadenylation signal (AATAAA) is located upstream of the poly (A) tail. A continuous ORF was obtained and consisted of 158 amino acids (Fig. 1). Protein sequence analysis showed that the polypeptide contained an α-lactalbumin/lysozyme C signature motif [8, 27], and was highly similar to the tiger prawn (93% identity), chicken (44% identity), mouse (39% identity), and human (37% identity) lysozyme C (Fig. 2). A BLAST search in the GenBank database and a multiple alignment of selected mature lysozymes (Fig. 2) revealed that the Banana prawn lysozyme is of the c-type [1, 37]. It possesses all of the 8 conserved cysteine residues and two major residues responsible for the catalytic activity of c-type lysozymes: glutamate E51 and aspartate D68 (numbered according to the Banana prawn lysozyme). We designated this polypeptide as Banana prawn lysozyme C. The c-type lysozymes are classified into two different subfamilies, i.e., the calcium binding and non-calcium binding families. Since it lacks an aspartic acid residue at positions 101, 106, and 107 that have been shown to be necessary for calcium binding [27], the Banana prawn lysozyme C appears, when compared with mammalian and avain lysozyme C, to belong to the non-calcium binding family. The multiple alignment by Clustal W (Fig. 2) shows that three gaps and two insertions were noted in the sequence of banana prawn lysozyme C. The lysozymes of all shrimp also had the three gaps. A seven-residue insertion from amino acid 120–126 (TERFRGR) is novel and is seen only in shrimp lysozyme. A nine-residue extension at the C-terminus (GSNSVFPF) have been found in shrimps and other invertebrates [12] when compared with mammalian and avian lysozyme C. This variability of C terminal region has been cited earlier [30].
Complete cDNA sequence of the banana prawn lysozyme C and its deduced amino acid sequence. Nucleotides are numbered on the left from the first base of the cDNA. The deduced amino acid sequence is shown below the nucleotide sequence in single letter code. Codons for initiation, termination, polyadenylation and poly(A) tail are indicated as bold letters
Multiple sequence alignment of banana prawn lysozyme C with selected lysozymes from different species. The numbers given in parentheses show the percentage of residues identical to banana prawn lysozyme C. The star symbols (★) mark the conserved cysteine residues; the diamond symbols (◆) indicate two major residues (Glu 51 and Asp 68) responsible for the catalytic activity of c-type lysozymes; the triangle symbols (▲) indicate Asp residues involved in Ca2+ binding in α-lactalbumins. GenBank accession numbers of these genes are as follows: banana prawn lysozyme C, EU591712; tiger prawn lysozyme C, ABU75288; kuruma shrimp lysozyme C, BAC57467; chicken lysozyme C, P00698; human lysozyme C, P00695; mouse lysozyme C, CAA35922
Phylogenetic analysis
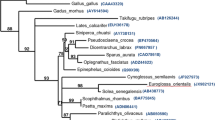
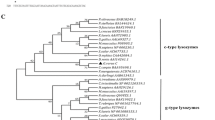
In the phylogenetic tree constructed, the c-type, g-type and i-type lysozymes are each clustered together correctly. Among the c-type lysozyme cluster, lysozyme C of banana prawn is between the insect and bird, based on the phylogenetic tree. This implies that banana prawn lysozyme C evolved at an early time point. The banana prawn lysozyme is closely related to that of shrimp homologues both in terms of identities and phylogenetic analyses. There are quite a few different hypotheses regarding the relationship between c-, i-, and g-type lysozymes [12, 26, 36]. Phylogenetic analysis (Fig. 3) in this study reveals that i-type, g-type lysozymes, phage lysozyme, and lysozyme M1 are strongly clustered, and are more closely related than either is to c-type, and that c-type lysozyme is basal (ancestral) to i- and g-type lysozymes. This apparently favors the notion that c-type lysozymes are structurally closest to the lysozyme ancestor [11, 12].
Molecular phylogenetic tree constructed from the amino acid sequences of lysozyme genes. The phylogeny was estimated by PAUP program. The amino acid sequence of α-lactalbumin was used as the outgroup. The accession numbers of the sequences of lysozyme G are perch, AAU86896; grouper, Q90X99; cassowary, Q7LZR3; swan, P00717. Lysozyme C genes in the following: mosquito, AAC47326; banana prawn, EU591712; kuruma shrimp, BAC57467; tiger prawn, ABU75288; pigeon, P00708; mouse, CAA35922; tamarin, AAB41210; chicken, AAL69327; bobwhite, P00700. Fruit fly: Lysozyme A NP_524869; Lysozyme B, NP_523882. Phage lysozyme: lysozyme R of prophage, P_287803; Bacteriophage, AAK28884; LYSOZYME M1 (Brucella melitensis) NP_541760
Lysozyme expression in tissue post V. alginolyticus injection
Semiquantitative RT-PCR was employed to determine the tissue specific expression of the banana prawn lysozyme C mRNA. The lysozyme C transcripts were mainly detected in the tissues of gill and gonad, and marginally detectable in hemolymph and lymphoid organ, while there seemed to be no signal in the kidney, hepatopancreas and tail muscle of healthy banana prawn. However, in banana prawn injected with heat-killed V. alginolyticus, the lysozyme C mRNA levels in all the detected tissues were higher than those in the unchallenged banana prawn, especially in the tissues of hemolymph, gill, gonad and lymphoid organ (Fig. 4).
The high numbers of lysozyme C mRNA transcripts in gill and gonad compared to other organs and hemolymphs suggest these two organs are the main sites of lysozyme C synthesis and are likely the major contributors of lysozyme C to plasma. Most noteworthy is our finding that lysozyme C was particularly abundant and evidently increased in gonad, gills, hemolymph and lymphoid organ of the challenged banana prawn, which is first defence to the external pathogens, but at the same time, can be used as a portal of entry by shrimp pathogens. After heat-killed V. alginolyticus challenge, remarkably higher expression levels could be observed in all detected tissues. These results suggested that lysozyme C was a constitutive and inducible acute-phase protein that could play a crucial role in host defense. Hikima et al. [12] reported that lysozyme from Marsupenaeus japonicus was strongly expressed by RT-PCR in samples from hemocytes, moderately expressed in the epidermis, and weakly expressed in the gills, midgut and muscle. Our results that are partly different to previous study of Hikima is possibly due to non-specific biodefense functions in innate immunity [17] or phyletic difference.
The temporal expression of banana prawn lysozyme C mRNA after bacterial challenge
The temporal expression of lysozyme C gene after M. luteus or V. alginolyticus challenge was shown in Fig. 5. In the M. luteus challenged shrimps, there was an up-regulation in the relative expression level of lysozyme C in hemocytes. At 2 and 6 h after bacterial challenge, there was an 11.3-fold and 18.3-fold increase in the relative abundance of the lysozyme C mRNA compared with control. At 8 h post injection, the lysozyme C gene expression reached the maximum level and was 25.4-fold higher than that observed in control shrimps. As time progresses, the expression of lysozyme C transcript dropped back to the original level at 16 h post injection. In the V. alginolyticus challenged group, significant difference was observed in the expression level of lysozyme C among the blank, control and challenged group. At 4, 6, and 8 h after bacterial challenge, there was a significant increase in the relative abundance of lysozyme C mRNA. At 16 h, the expression increased 15.7-fold and reached the highest level. As the time progresses, the expression of lysozyme C transcription dropped at 24 h. An unpaired, two-tailed t-test with the control and challenged groups showed significant difference in lysozyme gene expression at 2, 4, 6, and 8 h post injection in the M. luteus challenged group and from 4 to 32 h post injection in the V. alginolyticus challenged group (P < 0.05).
The temporal expression of banana prawn lysozyme C mRNA in hemocytes after M. luteus and V. alginolyticus stimulation. Data was expressed as the ratio of the banana prawn lysozyme C mRNA to the β-actin mRNA. The relative banana prawn lysozyme C expression level was determined for each group and values were shown as mean ± S.E., n = 3. The unstimulated and stimulated (injected with PBS) shrimps were used as the blank and control groups, respectively
A clear time-dependent pattern of lysozyme C gene expression was observed in hemocytes after bacterial challenge. In the M. luteus and V. alginolyticus challenged shrimps, the lysozyme mRNA levels in hemocytes varied at different infection time. In the V. alginolyticus challenged group, the expression of Lysozyme C gene was up-regulated, but the expression pattern was different from the M. luteus challenged group. As a whole, the expression level in V. alginolyticus was lower than that in M. luteus challenged group, and the highest level occurred at 16 h post injection rather than 8 h in M. luteus challenged group. The up-regulation of lysozyme gene expression observed after bacterial challenge suggested that banana prawn lysozyme gene is undoubtedly related to the non-specific immune defense and hemocytes may also play an important role in prawn immune mechanism. These results are consistent with the findings of Muñoz et al. [23], who examined penaeidin expression in L. vannamei injected with Vibrio alginolyticus. de Lorgeril et al. [6] reported very similar lysozyme expression from L. stylirostris infected with Vibrio and speculated that this response was due to hemocytes regulation of lysozyme transcription, or due to granular hemocytes leaving the circulation and infiltrating tissues.
Expression of the recombinant lysozyme C in bacteria and assay of its antibacterial activity
A high level expression was observed in DE3 cells transformed with pET-32a-lysozyme with IPTG induction when cultured between 20 and 37°C, and after gentle sonication on ice, the recombinant lysozyme was released into the supernatant. The recombinant protein could be eluted specifically from the Ni–NTA column with buffer containing 250 mM imidazole. The final preparation was subjected to SDS-PAGE analysis and a single band corresponding to a molecular mass of 15 kDa was detected after Coomassie blue staining (Fig. 6). Its molecular mass, computed from the amino acid sequence, is 14745.54 Da, and the theoretic isoelectric point is 9.1.
Expression and purification of banana prawn recombinant lysozyme C in E. coli cells. The collected cell lysates and the purified proteins were separated on 12% SDS-PAGE reducing gels. Lane 1: protein molecular standard; 2: pET-32a without insert; 3: pET-32a lysozyme without IPTG induction; 4: pET-32a-lysozyme with IPTG induction; 5: purified recombinant lysozyme
We found that the banana prawn lysozyme has bactericidal activity against Vibrio alginolyticus, Vibrio parahemolyticus and Vibrio vulnificus. The solid phase lytic assay against Vibrio sp. produced clear halos (Fig. 7). The recombinant banana prawn c-type lysozyme showed a relatively higher level of lytic ability than commercial hen egg-white lysozyme. These results are consistent with the findings of Hikima et al. [12], who examined c-type lysozyme lytic ability of kuruma shrimp. However, we did not determine a linear relationship between halo radius and lysozyme concentration, therefore it was not considered to be quantitative.
Lytic activity of recombinant banana prawn c-type lysozyme against Vibrio sp.. Small central circles are the wells containing the samples; larger circles represent lysed halos formed by the lysozyme on Vibrio sp. substrate. 1: control/V. alginolyticus; 2: control/V. parahemolyticus; 3: control/V. vulnificus; 4: purified recombinant c-type lysozyme/V. alginolyticus; 5: purified recombinant c-type lysozyme/V. parahemolyticus; 6: purified recombinant c-type lysozyme/V. vulnificus; 7: hen egg-white lysozyme/V. alginolyticus; 8: hen egg-white lysozyme/V. parahemolyticus; 9: hen egg-white lysozyme/V. vulnificus
The turbidimetric assay was developed to measure lysozyme activity against its substrate peptidoglycan using the Gram positive bacteria M. luteus [18]. In this assay, we found that banana prawn lysozyme reduced the optical density of a M. luteus suspension and cultured live Vibrio species (Fig. 8). To further confirm that decrease of OD reflects antibacterial activity against Gram negative bacteria, we quantified the CFU before and after interaction with lysozyme (Table 2). Using the classical Shugar assay with M. luteus as a standard, we estimated a relative bacteriolytic activity based on the negative slopes from Fig. 8. Our studies indicated that banana prawn lysozyme was more effective against V. alginolyticus by comparison of bacteriolytic activity.
To study the nature of the inhibition mechanism displayed by the recombinant lysozyme C in terms of bacteriostatic or bactericidal effects, the time-kill experiment was performed using the most sensitive bacteria V. alginolyticus to the recombinant lysozyme C. The recombinant lysozyme C was incubated with the bacteria at a concentration 10-fold higher than its MIC value to overcome a possible dose dependent effect. Bactericidal activity, as defined by a 3-log reduction viable count (99.9% kill), occurred after 6 h exposure to recombinant lysozyme C (Fig. 9). Therefore, recombinant lysozyme C possessed the bactericidal activity.
Results of banana prawn lysozyme antibacterial activity showed that it has potent bactericidal effect against both Gram positive and Gram negative bacteria. This observation were also indicated in scallop [25] and shrimp [5, 12]. Crustacean lysozyme has antibacterial activity against Gram negative bacteria due to two novel sequence features: an insertion and a hydrophobic extension at the C-terminus which are found only in Crustacean c-type lysozymes [5, 14, 15, 38]. Hikima et al. [12] hypothesize that the lysozymes of marine invertebrates evolve a wider range of activities than those of terrestrial invertebrates in order to cope with a greater range of bacterial strains and species in the marine environment.
In conclusion, a lysozyme has been isolated from banana prawn F. merguiensis. It is the first time that banana prawn lysozyme with complete primary structure was reported. Based on its potent bactericidal activity, lysozyme, in combination with other antimicrobial organic molecules [12], might play important roles in innate defense system in banana prawn.
References
Bachali S, Jager M, Hassanin A, Schoentgen F, Jollès P, Fiala-Medioni A, Deutsch JS (2002) Phylogenetic analysis of invertebrate lysozymes and the evolution of lysozyme function. J Mol Evol 54:652–664. doi:10.1007/s00239-001-0061-6
Bu XJ, Du XJ, Zhou WJ, Zhaoa XF, Wang JX (2008) Molecular cloning, recombinant expression, and characterization of lysozyme from Chinese Shrimp Fenneropenaeus chinensis. Chin J Biotechnol 24(5):723–732. doi:10.1016/S1872-2075(08)60037-0
Burge EJ, Madigan DJ, Burnett LE, Burnett KG (2007) Lysozyme gene expression by hemocytes of Pacific white shrimp, Litopenaeus vannamei, after injection with Vibrio. Fish Shellfish Immunol 22:327–339. doi:10.1016/j.fsi.2006.06.004
Canfield RE (1963) The amino acid sequence of egg white lysozyme. J Biol Chem 238:2698–2707
de-la-Re-Vega E, García-Galaz A, E. Díaz-Cinco M, Sotelo-Mundo RR (2006) White shrimp (Litopenaeus vannamei) recombinant lysozyme has antibacterial activity against Gram negative bacteria: Vibrio alginolyticus, Vibrio parahemolyticus and Vibrio cholerae. Fish Shellfish Immunol 20:405–408
de Lorgeril J, Saulnier D, Janech MG, Gueguen Y, Bachère E (2005) Identification of genes that are differentially expressed in hemocytes of the Pacific blue shrimp (Litopenaeus stylirostris) surviving an infection with Vibrio penaeicida. Physiol Genomics 21:174–183
FAO (2006) Fishstat Plus: Universal software for fishery statistical. Time Series 1950–2004. Version 2.30. FAO Fisheries Department, Fishery Information, Data and Statistics Unit. http://www.fao.org/fi/statist/fisoft/FISHPLUS.asp
Fujiki K, Shin DH, Nakao M, Yano T (2000) Molecular cloning of carp (Cyprinus carpio) leucocyte cell-derived chemotaxin 2, glia maturation factor b, CD45 and lysozyme C by use of suppression subtractive hybridization. Fish Shellfish Immunol 10:643–650
Gao FY, Ye X, Bai JJ, Lao HH, Wu RQ (2005) cDNA cloning and expression characterization of lysozyme gene in two freshwater prawn. Acta Hydrobiol Sin 29(6):615–620
Goarant C, Merien F, Berthe F, Mermoud I, Perolat P (1999) Arbitrarily primed PCR to type Vibrio spp. pathogenic for shrimp. Appl Environ Microbiol 65:1145–1151
Grütter MG, Weaver LH, Matthews BW, Grütter MG (1983) Goose lysozyme structure: an evolutionary link between hen and bacteriophage lysozymes? Nature 303:828–831
Hikima S, Hikima J, Rojtinnakorn J, Hirono I, Aoki T (2003) Characterization and function of kuruma shrimp lysozyme possessing lytic activity against Vibrio species. Gene 316:187–195
Hoang T (2001) The banana prawn—the right species for shrimp farming? World Aquac 32(4):40–44
Ibrahim HR, Yamada M, Kobayashi K, Kato A (1992) Bactericidal action of lysozyme against Gram-negative bacteria due to insertion of a hydrophobic pentapeptide into its C-terminus. Biosci Biotechnol Biochem 56:1361–1363
Ibrahim HR, Yamada M, Matsushita K, Kobayashi K, Kato A (1994) Enhanced bactericidal action of lysozyme to Escherichia coli by inserting a hydrophobic pentapeptide into its C terminus. J Biol Chem 269:5059–5063
Ito Y, Yoshikawa A, Hotani T, Fukuda S, Sugimura K, Imoto T (1999) Amino acid sequences of lysozymes newly purified from invertebrates imply wide distribution of a novel class in the lysozyme family. Eur J Biochem 259:456–461
Jollés P, Jollés J (1984) What’s new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem 63:165–189
Krieg NR, Gerhardt P (1994) Solid, liquid/solid, and semisolid culture. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology, vol 32. American Society of Microbiology, USA, pp 216–223
Laemmli UK (1970) Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 227:680–685
Lightner DV (1996) A handbook of shrimp pathology and diagnostic procedures for disease of cultured penaeid shrimp. World Aquaculture Society, Baton Rouge, LA, USA
Loongyai W, Avarre JC, Cerutti M, Lubzens E, Chotigeat W (2007) Isolation and functional characterization of a new shrimp ovarian peritrophin with antimicrobial activity from Fenneropenaeus merguiensis. Mar Biotechnol 9:624–637
Milochau A, Lassegues M, Valembois P (1997) Purification, characterization and activities of two hemolytic and antibacterial proteins from coelomic fluid of the annelid Eisenia fetida andrei. Biochem Biophys Acta 1337:123–132
Muñoz M, Vandenbulcke F, Saulnier D, Bachère E (2002) Expression and distribution of penaeidin antimicrobial peptides are regulated by haemocyte reactions in microbial challenged shrimp. Eur J Biochem 269:2678–2689
Nicholas KB, Nicholas HB (1997) GENEDOC: a tool for editing and annotating multiple sequence alignments. http://www.cris.com/~ketchup/genedoc.shtml
Nilsen IW, Rverbr K, Sandsdalen E, Sandaker E (1999) Protein purification and gene isolation of chlamysin, a cold-active lysozyme-like enzyme with antibacterial activity. FEBS Lett 464:153–58
Nilsen IW, Myrnes B, Edvardsen RB, Chourrout D (2003) Urochordates carry multiple genes for goose-type lysozyme and no genes for chicken- or invertebrate-type lysozymes. Cell Mol Life Sci 60:2210–2218
Nitta K, Sugai S (1989) The evolution of lysozyme and a-lactalbumin. Eur J Biochem 182:111–118
Prager EM, Jollès P (1996) Animal lysozymes c and g: an overview. In: Jollès P (ed) Lysozymes: model enzymes in biochemistry and biology. Birkhäuser Verlag, Basel
Rittidacha W, Paijita N, Utarabhand P (2007) Purification and characterization of a lectin from the banana shrimp Fenneropenaeus merguiensis hemolymph. Biochem Biophys Acta 1770(1):106–114
Sotelo-Mundoa RR, Islas-Osuna MA, de-la-Re-Vega E, Hernández-López J, Vargas-Albores F, Yepiz-Plascencia G (2003) cDNA cloning of the lysozyme of the white shrimp Penaeus vannamei. Fish Shellfish Immunol 15:325–331
Shugar D (1952) The measurement of lysozyme activity and the ultra-violet inactivation of interferon. Biochem Biophys Acta 8:302–309
Simpson RJ, Begg GS, Dorow DS, Morgan FJ (1980) Complete amino acid sequence of the goose-type lysozyme from the egg white of the black swan. Biochemistry 19:1814–1819
Sotelo-Mundo RR, Islas-Osuna MA, de-la-Re-Vega E, Hernandez-Lopez J, Vargas-Albores F, Yepiz-Plascencia G (2003) cDNA cloning of the lysozyme of the white shrimp Penaeus vannamei. Fish Shellfish Immunol 15:325–331
Swofford DL (2002) PAUP*, Phylogenetic Analysis Using Parsimony (*and other methods), Version 4. Sinauer Associates, Sunderland, MA
Thompson JD, Higgins DG, Gibson TJ (1997) Clustal W: improving the sensitivity of progressive multiple sequence weighting, position-specific gap penalties and weight metric choices. Nucleic Acids Res 22:4673–4680
Thunnissen AMWH, Isaacs NW, Dijkstra BW (1995) The catalytic domain of a bacterial lytic transglycosylase defines a novel class of lysozymes. Proteins 22:245–258
Ting KL, Jernigan RL (2002) Identifying a folding nucleus for the lysozyme/a-lactalbumin family from sequence conservation clusters. J Mol Evol 54:425–436
Touch V, Hayakawa S, Saitoh K (2004) Relationships between conformational changes and antimicrobial activity of lysozyme upon reduction of its disulfide bonds. Food Chem 84:421–428
Acknowledgement
We acknowledge the help of Youhou Xu, Lifeng Wu and Peng Luo in running this experiment. This work was supported by the National High Technology Development Project of China under contract no. 2006AA10A406.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mai, Wj., Hu, Cq. Molecular cloning, characterization, expression and antibacterial analysis of a lysozyme homologue from Fenneropenaeus merguiensis . Mol Biol Rep 36, 1587–1595 (2009). https://doi.org/10.1007/s11033-008-9355-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-008-9355-8