Abstract
Tuta absoluta is a destructive moth of Solanaceae plants and especially tomatoes. Here, we considered the entomopathogenic activity of the Bacillus thuringiensis Vip3Aa16 protein heterologously produced by Escherichia coli against T. absoluta. Purified Vip3Aa16 showed lower lethal concentration 50 % against third instar larvae (Toxin/tomato leaf) (335 ± 17 ng/cm2) compared to that of B. thuringiensis kurstaki HD1 δ-endotoxins (955 ± 4 ng/cm2) (P < 0.05). Action mode examination showed that Vip3Aa16 (88 kDa) was more sensitive to proteolysis activation by the chymotrypsin than the trypsin or the larvae gut soluble proteases, yielding derivative proteins essentially of about 62 and 33 kDa. The gut-soluble proteases could contain trypsin-like enzymes implicated in Vip3Aa16 activation since the proteolysis was inhibited using specific protease inhibitors. Additionally, we showed that the histopathological effect of Vip3Aa16 on T. absoluta larva midguts consisted on a microvillus damage and an epithelial cell rupture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuta absoluta (Lepidoptera: Gelechiidae) is a small lepidopteron moth with high reproductive potential. It feeds principally on tomatoes and less importantly on eggplant, pepper, and potato leaves as well as various other plants [1]. The larva development needs four instars to reach the chrysalides and then the moth. There are about 10–12 generations per year when development conditions are favorable. Its damage occurred throughout the entire growing cycle reaching up to 100 % in untreated cultivations. T. absoluta devastated tomato production in South America, but it was introduced accidentally to Spain in 2006 and then it spread rapidly in Afro-Eurasia [2, 3].
Actually, we lack ecologically acceptable methods for its control. Hence, despite the large use of numerous chemical insecticides, T. absoluta larvae evolved strains with reduced susceptibility to some of them [4–7]. However, the necessity to limit the use of chemical insecticides implies the development of potential bio-control agents such predatory bugs and egg parasitoids [8], despite that the pheromone trap was used for early detection and for control of T. absoluta. Interestingly, it is largely reported that the bacterium Bacillus thuringiensis was successfully used as biopesticide against several pests. It produced the Cry insecticidal proteins (δ-endotoxins) forming crystalline inclusion bodies during sporulation which are toxic to a variety of insect orders such as Lepidoptera, Diptera, and Coleoptera [9, 10]. In addition to δ-endotoxins, B. thuringiensis could produce and secrete the vegetative insecticidal proteins (Vip) during vegetative growth stage. The Vip proteins have no sequence homology with the Cry proteins and have been classified into four groups according to their sequence homology: Vip1, Vip2, Vip3, and Vip4 [9]. The binary toxins Vip1 and Vip2 are toxic to some coleopterans [11] while the Vip3 proteins are toxic to a wide range of lepidopteron insects [12–14].
Upon ingestion by susceptible larvae, Vip3A protein was activated by proteolysis and then bound to specific receptors on the midgut brush border membrane causing pore formation and cell lyses. By using brush border membrane vesicles (BBMV) from the midgut tissues, the Vip3Aa16 interacted with a 62-kDa receptor from Ephestia kuehniella, Prays oleae, and Agrotis segetum, with about 55 and 100 kDa receptors from Spodoptera littoralis, while the activated Vip3A toxin bound to 80 and 110 kDa receptors from Manduca sexta [15–17]. The recognized Vip3A receptors could be different from those of Cry1 proteins. In fact, Cry1Ac toxin interacted with 210 and 120 kDa putative receptors in P. oleae and Agrotis ipsilon midguts, respectively, while the Cry1Ab interacted with a 120-kDa putative receptor in M. sexta [18, 19]. Receptors variability indicated a very low cross-resistance potential between Vip3A and Cry1Ab and supports the use of Vip3A toxins as a biological control agent, especially to resolve the problems of Cry-resistance emergence. Despite the previously described Cry proteins for biological control of T. absoluta [20], we evaluated the toxicity of B. thuringiensis Vip3Aa16 protein against this pest. We also examined the capability of gut-soluble proteases to activate Vip3Aa16 as well as the midgut histopathological aspect after being exposed to this protein.
Materials and Methods
Preparation of Gut-Soluble Proteases from T. absoluta Larvae
T. absoluta early-fourth-instar larvae growing at 26 ± 2 °C were collected from tomato leaves, cooled on ice for 20 min, and dissected to isolate the guts. Each ten guts were homogenized in water and conserved at −20 °C. Before using, they were thawed but not disrupted and then centrifuged at 15,000×g and 4 °C for 15 min. The recovered supernatant constituted the gut-soluble proteases. The protein content was determined according to the method of Bradford [21] by using Bio-Rad Protein Assay (München, Germany).
Production of Vip3Aa16 Protein and δ-Endotoxins
The recombinant Escherichia coli strain BL21 harboring the plasmid pET-vip3LB was used for the heterologous production of the B. thuringiensis Vip3Aa16 protein [22] N-terminally supplemented with a histidine tag. We note that vip3LB gene correspond to vip3Aa16 gene related to Crickmore nomenclature [9]. The strain was grown at 37 °C in Luria-Bertani (LB) medium supplemented with ampicillin (60 μg/ml), and the gene expression was induced with 100 mM IPTG for 3 h. The Vip3Aa16 was purified from the bacterial lysate soluble fraction by HisTrap resin (Amersham Biosciences) and collected in 100 mM sodium phosphate buffer pH 7.2. A fraction of the purified protein was dialyzed against 50 mM Na2CO3 buffer, pH 9.6 at 4 °C. The protein concentration was determined by using the Bio-Rad Protein Assay (München, Germany) according to the method of Bradford [21]. The δ-endotoxins (Cry proteins) were prepared from B. thuringiensis kurstaki reference strain HD1 as described by Jamoussi et al. [20].
Vip3Aa16 Proteolytic Activation by T. absoluta Gut-Soluble Proteases and Effect of Protease Inhibitors
To inspect the activation kinetic of Vip3Aa16, the purified protein was mixed with the T. absoluta larvae gut-soluble proteases, the commercial trypsin, or the commercial chymotrypsin in 100 mM sodium phosphate buffer (pH 7.2) or in 50 mM Na2CO3 buffer (pH 9.6). Standard trypsin (2.739 USP units/mg), extracted from bovine pancreas was purchased from Amersham Life science. Alpha-chymotrypsin, extracted from bovine pancreas (1600.2 E/mg), was purchased from BioChemica. The Vip3Aa16/proteases (trypsin, chymotrypsin, or gut-soluble proteins) ratio was of 20/1 (μg/μg). The mixtures were incubated with constant agitation at 37 °C for diverse incubation times, and the proteolytic reaction was stopped by adding the protease inhibitor phenylmethanesulfonyl fluoride (PMSF) at 1 mM. Protein extracts suspended in Laemmli sample buffer (3×) were boiled for 5 min, analyzed by SDS-PAGE (9 %), and stained using Coomassie blue [23].
To examine the protease inhibitors effect, Vip3Aa16 was incubated with T. absoluta gut-soluble proteases in 50 mM Na2CO3 buffer (pH 9.6), using Vip3Aa16/gut-soluble proteins ratio of 5/1 (μg/μg), and with the PMSF (5 mM), the benzamidine (5 mM), or the soybean trypsin inhibitor (SBTI) (1 mg/ml) for 30 min at 30 °C. Then, the protein extracts were analyzed by SDS-PAGE and stained using Coomassie blue [23].
Zymogram Analysis
An aliquot of the gut-soluble proteins (100 μg) was mixed with Laemmli sample buffer and separated by SDS-PAGE 13 % Tris-glycine. The gel was washed in Triton X-100 (2.5 %) for 30 min and then washed three times in water (3× 15 min). It was incubated in 50 mM Na2CO3 buffer (pH 9.6) including 2 % casein or 10 mg/ml of the BL21 (pET-vip3LB) lysate containing Vip3Aa16 protein, at 37 °C for 3 h. Clear bands of protease activities were visible after Coomassie staining [24].
Insecticidal Bioassays
The Vip3Aa16 protein was displayed on tomato leaves surface at concentrations varying from 100 to 1500 (ng/cm2) (Vip3Aa16 protein/leaf surface) in order to establish the 50 % lethal concentration (LC50) after 3 days. One tomato leaf and ten T. absoluta third instar larvae were placed in Petri plate and then incubated in the insect culture room at 26 ± 2 °C and under a photoperiod of about 14 h light/10 h dark. The experiment condition was repeated three times, and the LC50 was calculated from pooled raw data by probit analysis using programs written in the R language [25].
Preparation and Sectioning of the Insect Tissues
T. absoluta larvae were alimented with tomato leaves containing the Vip3Aa16 protein. The larva guts were excised after 24 to 36 h and collected in 10 % formol. They were dehydrated via ethanol solutions with increasing concentrations, washed in 100 % toluene, and fixed in paraffin wax. The sections were placed in carriers loaded with a mix of 1.5 % egg albumin and 3 % glycerol in distilled water. They were deparaffinated in 100 % toluene and stained with hematoxylin-eosin (HE) [26].
Results and Discussion
Toxicity of the Vip3Aa16 Protein Against T. absoluta Larvae
T. absoluta attacks the Solanaceae major crops and principally the tomato crops in South America and Mediterranean countries. Hence, the efficacy of the vegetative insecticidal protein Vip3Aa16 was assayed against T. absoluta third instar larvae. By comparing to the normal development of the control larvae feeding tomato leaf, the presence of the Vip3Aa16 protein allowed the typical symptoms of the intoxication consisting on cessation of feeding, body retraction, overall paralysis, and then death of numerous larvae throughout the test. Quantitatively, the determined LC50 was about 335 ± 17 ng/cm2 (Vip3Aa16/tomato leaf), constituting the first demonstration of the Vip3A activity against a member of the family Gelechiidae. We also determined the LC50 of the δ-endotoxins produced by B. thuringiensis kurstaki reference strain HD1 against T. absoluta third instar larvae (955 ± 4 ng/cm2 (δ-endotoxins/tomato leaf)) and demonstrated that it was largely higher than that of Vip3Aa16. However, the estimated LC50 of Vip3A toward S. littoralis, A. segetum, Spodoptera frugiperda, and E. kuehniella were about 305, 86, 49.3, and 36 ng/cm2, respectively [16–18, 27].
Vip3Aa16 Activation by the T. absoluta Gut-Soluble Proteases
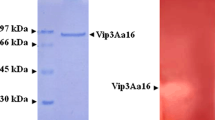
Like Cry proteins, the toxicity variation of Vip3A protein toward the lepidopteron species might be due to its activation by the gut proteases or the following step consisting on its affinity and interaction with the midgut-specific receptors. Hence, the Vip3Aa16 activation process by the midgut proteases was studied. Firstly, the zymogram analysis of the larvae gut-soluble proteases revealed similar patterns using the recombinant BL21 (Vip3Aa16) lysate or the casein as universal substrate and indicated that at least six discernible activities (A1–A6) could be implicated in Vip3Aa16 proteolysis (Fig. 1). Secondly, the Vip3Aa16 protein was incubated with trypsin, chymotrypsin, or larvae gut-soluble proteases for different incubation times at pH 7.2 and pH 9.6 since the pH of lepidopteran midgut larvae is alkaline [28]. The SDS-PAGE analysis revealed that the Vip3Aa16 (88 kDa) without any added proteases remained stable during the incubation times (Fig. 2). Furthermore, at pH 7.2 or pH 9.6, this protein was progressively proteolyzed by the gut-soluble proteases or the trypsin into the active form of about 62 kDa as well as the proteolysis products of about 33, 45 (at pH 7.2), and 22 kDa (at pH 7.2), but the full protein (88 kDa) still remained in slight amount after 120 min. Interestingly, Vip3Aa16 proteolysis occurred rapidly via the chymotrypsin as no detectable full protein was found after 1 min, showing the highest specificity of the chymotrypsin recognition site in Vip3Aa16. It produced essentially the 62 kDa active form which remains stable during incubation times, the 33 kDa, and the 22 kDa proteolysis products.
Time course of Vip3Aa16 proteolytic processing. Vip3Aa16/proteases (trypsin, chymotrypsin, or gut-soluble proteins) ratio was of 20/1 (μg/μg). The reactions were done at pH 7.2 (a, b) and pH 9.6 (c, d) for 1, 15, 45, and 120 min at 37 °C. Reaction products were separated by SDS-PAGE and the gels were stained with Coomassie blue. M: LMW (low molecular weight, Amersham); P: purified Vip3Aa16 without incubation, asterisk midgut-soluble proteases without Vip3Aa16 protein. White arrows indicated the full-length protein and black arrows indicated proteolysis products
Previous studies reported the Vip3A activation by susceptible larvae midgut proteases or trypsins but not with chymotrypsins. In fact, Yu et al. [29] and Abdelkefi-Mesrati et al. [16] demonstrated that Vip3A and Vip3Aa16 can be processed into four major proteolysis products of approximately 62, 45, 33, and 22 kDa by some lepidopteran gut fluids or S. littoralis gut fluid, respectively. Using the midgut proteases of E. kuehniella and A. segetum to activate the Vip3Aa16, the obtained bands were about 62 and 45 kDa and 62, 45, and 22 kDa, respectively [17, 18]. Likewise, Lee et al. [19] demonstrated that a major and stable 62 kDa protein was formed by the action of the lepidopteran M. sexta gut juice on the Vip3A-F (90 kDa). Differences in the Vip3A processing by different lepidopteran midgut proteases could play a crucial role in species susceptibility.
The Vip3Aa16 proteolysis showed similar kinetic activation patterns when the protein was incubated with the gut-soluble proteases or the trypsin, but some pattern differences were observed when it was incubated with chymotrypsin. To confirm the implication of trypsin-like activity, the Vip3Aa16 activation was done by T. absoluta gut-soluble proteases with either the PMSF as a common serine and cysteine proteases inhibitor, the SBTI as trypsin-like serine proteases inhibitor, or the benzamidine as competitive inhibitor of trypsin, trypsin-like enzymes, and serine proteases (Fig. 3). Hence, the SDS-PAGE analysis demonstrated the inhibition of the Vip3Aa16 activation by PMSF, SBTI, and benzamidine inhibitors, confirming the implication of T. absoluta trypsin-like proteases in the activation of the B. thuringiensis Vip3Aa16.
SDS-PAGE analysis of Vip3Aa16 proteolysis inhibition by protease inhibitors. M: LMW (low molecular weight, Amersham). Vip3Aa16 (5 μg): without protease (C1), without protease but incubated 30 min at 30 °C (C2), incubated with larvae gut-soluble proteases (1 μg) (C3), incubated with larvae gut-soluble proteases, and the SBTI (1 mg/ml), the PMSF (5 mM), or the benzamidine (5 mM)
Numerous studies on the Vip3A action mode described the protein proteolysis in the insect alkaline midgut environment to the 62 kDa active form. The activated Vip3A toxin could interact specifically with appropriate receptors on the surface of the midgut brush border membrane vesicles (BBMV) of the target insects. These interactions caused the insertion of Vip3A protein into the membrane to form ion channels, leading to colloid osmotic lyses [16–19].
Vip3Aa16 Histopathological Effects on T. absoluta Larvae
Histopathological observations of the Vip3Aa16 effects on T. absoluta were done on the early-fourth-instar larvae (Fig. 4a). For the unexposed larvae to Vip3Aa16, the gut cross sections showed a midgut wall typical morphology composed of an epithelium and a peritrophic membrane [30, 31]. The midgut epithelium was composed of columnar cells containing numerous microvilli which form the brush border membrane, and goblet cells that were intercalated with the columnar cells. In contrast, wide damages to the T. absoluta midgut epithelium were clearly induced by Vip3Aa16 (Fig. 4b). Mostly, histopathological changes included Brush border membrane alteration and degeneration of the epithelium columnar cells conducting to larvae death.
Histopathological effects of Vip3Aa16 on T. absoluta midgut. Sections through the midgut epithelium from larvae not exposed to toxins (a) and larvae fed diet containing Vip3Aa16 (b). In b, black arrows indicated lyses of columnar cells in the apical region of cells and arrowhead showed brush border membrane detachment. Me midgut epithelium, Gc goblet cell, Cc columnar cell, N nucleus, Am apical membrane (Bb brush border membrane), Bm basement membrane, L lumen. a, b Magnification ×100
In conclusion, the B. thuringiensis Vip3Aa16 could be a promising entomopathogenic protein for biological control of T. absoluta as it was more toxic than the δ-endotoxins of B. thuringiensis kurstaki reference strain HD1. This protein was activated by T. absoluta gut-soluble proteases, harboring trypsin-like proteases, producing principally a 62-kDa toxin which could cause the midgut epithelium damage and the larvae death.
Abbreviations
- B :
-
Bacillus
- T :
-
Tuta
References
Galarza, J. (1984). IDIA Nos 421/424, 30–32
Desneux, N., Wajnberg, E., Wyckhuys, K. A. G., Burgio, G., Arpaia, S., Narváez-Vasquez, C. A., González-Cabrera, J., Catalán Ruescas, D., Tabone, E., Frandon, J., Pizzol, J., Poncet, C., Cabello, T., & Urbaneja, A. (2010). Journal of Pest Science, 83, 197–215.
Desneux, N., Luna, M. G., Guillemaud, T., & Urbaneja, A. (2011). Journal of Pest Science, 84, 403–408.
Lietti, M. M. M., Botto, E., & Alzogaray, R. A. (2005). Neotropical Entomol, 34, 113–119.
Silva, G. A., Picanço, M. C., Bacci, L., Crespo, A. L., Rosado, J. F., & Guedes, R. N. (2011). Pest Management Science, 67, 913–920.
Siqueira, H. A. A., Guedes, R. N. C., & Picanco, M. C. (2000). Agricultural and Forest Entomology, 2, 147–153.
Siqueira, H. A. A., Guedes, R. N. C., Fragoso, D. B., & Magalha, L. C. (2001). Int J Pest Manag, 47, 247–251.
Miranda, M. M. M., Picanco, M., Leite, G. L. D., Zanuncio J. C., & De Clerq, P. (1998). Sampling anon-action levels for predators and parasitoid of virus vectors and leaf miners of tomato in Brazil. 50th International Symposium on Crop Protection, PTS I-IV 50, 519–526.
Crickmore, N., Zeigler, D. R., Schnepf, E., Van Rie, J., Lereclus, D., Baum, J., Bravo, A., & Dean, D. H. (2014). Available from: www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt/toxins2.html
Schnepf, E., Crickmore, N., Van Rie, J., Lereclus, D., Baum, J., Feitelson, J., Zeigler, D. R., & Dan, D. H. (1998). Microbiology and Molecular Biology Reviews, 62, 775–806.
Shi, Y., Xu, W., Yuan, M., Tang, M., Chen, J., & Pang, Y. (2004). Journal of Applied Microbiology, 97, 757–765.
Estruch, J., Warren, G. W., Mullins, M. A., Nye, G. J., Craig, J. A., & Koziel, H. G. (1996). Proceedings of the National Academy of Sciences of the United States of America, 93, 5389–5394.
Yu, X., Zheng, A., Zhu, J., Wang, S., Wang, L., Deng, Q., Li, S., Liu, H., & Li, P. (2010). Current Microbiology, 62, 752–757.
Sellami, S., Jamoussi, K., Dabbeche, E., & Jaoua, S. (2011). Current Microbiology, 63, 289–294.
Lee, M. K., Miles, P., & Chen, J. S. (2006). Biochemical and Biophysical Research Communications, 339, 1043–1047.
Abdelkefi-Mesrati, L., Boukedi, H., Chakroun, M., Kamoun, F., Azzouz, H., Tounsi, S., Rouis, S., & Jaoua, S. (2011). Journal of Invertebrate Pathology, 107, 198–201.
Abdelkefi-Mesrati, L., Boukedi, H., Dammak-Karray, M., Sellami-Boudawara, T., Jaoua, S., & Tounsi, S. (2011). Journal of Invertebrate Pathology, 106, 250–254.
Ben Hamadou-Charfi, D., Boukedi, H., Abdelkefi-Mesrati, L., Tounsi, S., & Jaoua, S. (2013). Journal of Invertebrate Pathology, 114, 139–143.
Lee, M. K., Walters, F. S., Hart, H., Palekar, N., & Chen, J. S. (2003). Applied and Environmental Microbiology, 69, 4648–4657.
Jamoussi, K., Sellami, S., Nasfi, Z., Krichen-Makni, S., & Tounsi, S. (2013). Journal of Microbiology and Biotechnology, 23, 1099–1106.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Abdelkefi-Mesrati, L., Rouis, S., Sellami, S., & Jaoua, S. (2009). Molecular Biotechnology, 43, 15–19.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Garcia-Carreño, F., Dimes, L., & Haard, N. (1993). Analytical Biochemistry, 214, 65–69.
Venables, W. N., Smith, D. M., et al. (2004). Available from: www.r-project.org/
Ruiz, L. M., Segura, C., Trujillo, J., & Orduz, S. (2004). Memórias do Instituto Oswaldo Cruz, 99, 73–79.
Sena, J. A., Hernández-Rodríguez, C. S., & Ferré, J. (2009). Applied and Environmental Microbiology, 75, 2236–2237.
Chougule, N. P., Doyle, E., Fitches, E., & Gatehouse, J. A. (2008). Journal of Insect Physiology, 54, 563–572.
Yu, C. G., Mullins, M. A., Warren, G. W., Koziel, M. G., & Estruch, J. J. (1997). Applied and Environmental Microbiology, 63, 532–536.
Lehane, M. J. (1997). Annual Review of Entomology, 42, 525–550.
Tellam, R. L., Wijffels, G., & Willadsen, P. (1999). Insect Biochemisry and Molecular Biology, 29, 87–101.
Acknowledgments
This study was supported by grant from the Tunisian “Ministère de l’Enseignement Supérieur et de la Recherche Scientifique (MESRS).”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sellami, S., Cherif, M., Abdelkefi-Mesrati, L. et al. Toxicity, Activation Process, and Histopathological Effect of Bacillus thuringiensis Vegetative Insecticidal Protein Vip3Aa16 on Tuta absoluta . Appl Biochem Biotechnol 175, 1992–1999 (2015). https://doi.org/10.1007/s12010-014-1393-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1393-1