Abstract
Vegetative insecticidal protein (Vip) is a class of insecticidal proteins produced by many Bacillus thuringiensis strains during their vegetative growth stage. The vip3LB gene of B. thuringiensis strain BUPM95, which encodes a protein active against the Lepidoptera olive tree pathogenic insect Prays oleae, was cloned into pET-14b vector and overexpressed in Escherichia coli. The expressed Vip3LB protein, found in the E. coli cytoplasmic fraction, was purified and used to produce anti-Vip3LB antibodies. Using the midgut extract of P. oleae, the purified Vip3LB bound to a 65-kDa protein, whereas Cry1Ac toxin bound to a 210-kDa midgut putative receptor. This result justifies the importance of the biological pest control agent Vip3LB that could be used as another alternative particularly in case of resistance to Cry toxins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of Bacillus thuringiensis to produce parasporal crystals composed of insecticidal δ-endotoxins has been the subject of numerous studies [1–3]. On the other hand, a number of secreted insecticidal proteins called vegetative insecticidal proteins (Vip) have been identified during the vegetative growth phase of B. thuringiensis strains [4–7]. This class of insecticidal proteins includes the toxins Vip1-Vip2 active on Coleoptera and Vip3 active on Lepidoptera [4, 5, 7–9].
Bacillus thuringiensis δ-endotoxins have been successfully used to control many crop pests by either traditional spray applications or transgenic plant approaches. However, a number of cases of insect resistance to the B. thuringiensis δ-endotoxins have been reported as a result of laboratory and, more rarely, field selections [10].
Binding of B. thuringiensis toxins to the receptors located on the epithelial brush border membrane has been identified as a critical step in their action and also the modification of this binding has been identified as a likely resistance mechanism [10, 11]. Therefore, searching for a new family of insecticidal toxins, with a mode of action or midgut receptors different from those of δ-endotoxins, is one facet of current strategies designed to delay resistance development.
One of the interesting features of the Vip3A protein is that it shares no sequence homology with the known δ-endotoxins [4]. Moreover, in ligand blotting experiments with brush border membrane vesicles (BBMV) from the tobacco hornworm, Manduca sexta, activated Cry1Ab and Vip3A bound to different molecules receptors [12]. These differences between Cry and Vip3 toxins of B. thuringiensis are very important to resolve the pest resistance problems.
Prays oleae is a pest that causes a lot of damages to olive crops. In this report, we demonstrate that the insecticidal proteins Vip3LB and Cry1Ac bind to different putative receptor proteins of P. oleae larvae midgut, predicting complementary Vip3LB and Cry1Ac insecticidal activities that could be exploited for pest biological control in the Mediterranean basin.
Materials and Methods
Heterologous Expression of vip3LB in Escherichia coli
Overexpression of vip3LB (GenBank Accession No. AY739665) was achieved by cloning its ORF in the pET-14b vector (Novagen). NdeI and BamHI restriction enzyme sites were created by PCR, respectively, upstream the initiation codon (ATG) and downstream the stop codon of the vip3LB gene of B. thuringiensis strain BUPM95, using the following primers: VipM1 (5′-AAGATGCA*TATGAACAAGAATAATA-3′) and VipM2 (5′-GATG*GAT CCCGATCTTACTTAATAG-3′). The resulting 2.37 kb fragment was cloned into pMOSBlue vector (Amersham) and recovered by digesting it with NdeI and BamHI restriction enzymes. This fragment was cloned in frame in its 5′ end with the His-tag sequence of the E. coli expression vector pET-14b (Novagen). The resulting plasmid, pET-vip3LB, was transformed into E. coli cells BL21(DE3) (ompT hsdS (rB− mB−) dcm + Tetr gal (DE3) endA Hte).
Recombinant E. coli clones carrying the vip3LB gene were grown at 37°C overnight in Luria–Bertani medium supplemented with 100 μg/ml ampicillin. These cells were used as seed culture to inoculate fresh cultures (50 ml) with an initial absorbance at 600 nm of 0.05. When the optical density reached 0.6, vip3LB gene expression was induced by isopropyl thiogalactoside (IPTG) 0.4 mM, and cells were grown for further 3 h at 37°C. After centrifugation at 2,400g for 10 min, the cell pellet was resuspended in sonication buffer [PBS 1× (pH 7.5); 4 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride] and sonicated with three pulses of medium intensity for 30 s each. The suspension was centrifuged at 2,400g for 10 min at 4°C, and the supernatant was collected.
Bioassays
Bioassays were carried out using third instar larvae of P. oleae. Sixty μl of E. coli(pET-vip3LB) protein extract was poured on the surface of olive leaves and left in Petri dishes for 4 h, then 10 larvae of P. oleae were added and incubated at room temperature to expose larvae to the diet containing Vip3LB toxin. As negative control, 10 larvae were fed with olive leaves treated with E. coli(pET-14b) protein extracts. The mortality was recorded each day and results are the average of three trials.
Purification and Proteolysis of the Vip3LB Protein
The supernatant collected, after sonication of the induced E. coli(pET-vip3LB) culture, was loaded onto His-Trap column (1 ml matrix volume) (Amersham) preequilibrated with a binding buffer (PBS 1×, imidazole 40 mM). After washing the column with 10 ml of the same binding buffer, the bound proteins were eluted using elution buffers containing increasing concentrations of imidazole in PBS 1× (5 ml for each concentration of imidazole). Proteolysis reactions were performed at room temperature in 0.1 M glycine–NaOH buffer (pH 9.0) using Vip3LB toxin (8 μg) and trypsin (20 μg/ml).
Antibody Preparation
Trypsin-activated soluble pure Vip3LB protein was mixed with Freund’s adjuvant and injected subcutaneously to immunize two rabbits, followed by two booster intradermally injections given within a 15-day gap. The obtained antiserum was treated with E. coli cell lysate for background reduction.
Cry1Ac Toxin Preparation and Proteolysis
Cry1Ac toxin preparation was carried out from the recombinant strain BNS3Cry−(pHTcry1Ac), expressing the Cry1Ac δ-endotoxin, previously constructed and investigated in our laboratory [2, 13]. The solubilization of parasporal insecticidal crystals and the proteolysis of Cry1Ac toxin were performed as reported by Rouis et al. [14].
Midgut Extract Preparation
Third instar larvae of P. oleae, kindly provided by the Sfax Institute of Olive Tree (Tunisia), were chilled on ice during 30 min. Midgut extract was prepared from the whole of the P. oleae larvae as described by Rouis et al. [14]. The supernatants were recovered and the protein contents were determined as described below.
Protein Quantification
The concentrations of midgut extract proteins, Vip3LB and Cry1Ac, were measured with the Bradford assay (Bio-Rad), using bovine serum albumin as a standard.
Toxin–Receptor Interaction
Prays oleae midgut proteins prepared in MET buffer (mannitol, 300 mM; EDTA, 5 mM; Tris, 20 mM; pH 7.2) containing 0.1 mM phenylmethylsulfonyfluoride (PMSF) as described by Rouis et al. [14] were separated by electrophoresis in 7.5% polyacrylamide gel and blotted onto a nitrocellulose membrane by electrotransfer (Bio-Rad, France). Proteins were visualized by Ponceau S staining. The membranes were blocked with 5% milk for 1 h, and then reacted with Vip3LB or Cry1Ac toxins for 2 h at room temperature. After three 5-min washing cycles using a buffer containing 1× PBS and 0.1% Tween 20 (pH 7.0), membranes were developed using primary antiserum (anti-Vip3LB or anti-Cry1Ac), secondary monoclonal antirabbit IgG-peroxidase conjugate, and ECL+ Kit (Amersham Pharmacia Biotech., France).
Results
Overexpression of vip3LB in E. coli: Study of the Insecticidal Activity and Purification of the Corresponding Toxin
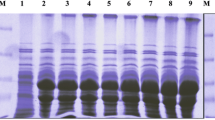
The vip3LB gene of B. thuringiensis strain BUPM95 [7] was cloned in the pET-14b vector as described in Materials and Methods section. After sonication of IPTG-induced E. coli (pET-vip3LB) and E. coli (pET-14b) used as negative control, supernatant proteins were analyzed by SDS-PAGE on which the two strains showed apparently similar protein patterns with the major exception of a 90-kDa Vip3LB-sized protein, which was produced by E. coli containing pET-vip3LB and not by E. coli containing pET-14b (Fig. 1).
Heterologous expression of vip3LB in E. coli: SDS-PAGE analysis of E. coli supernatants. Lanes: 1, Supernatant proteins of sonicated culture of E. coli strains containing pET-14b; 2, Molecular weight markers (170, 116, 76, and 53 kDa); and 3, Supernatant proteins of sonicated culture of E. coli strains containing pET-vip3LB
The recombinant E. coli(pET-vip3LB) protein extract was used for insect bioassays on P. oleae larvae. Mortality of 96% of larvae was reached after 5 days, whereas exposure of larvae to E. coli(pET-14b) protein extract, used as negative control, did not affect larval growth increase, weight gain, or morphology. These findings demonstrate clearly that the cloned vip3LB expressed in E. coli is active against P. oleae.
The six-histidine tail-Vip3LB fusion was purified using the His-trap column and applying increasing gradient of imidazole. The toxin was mainly eluted at the second and the third milliliters of the 5-ml fraction corresponding to 300 mM of imidazole (Fig. 2). The protein with the highest purity was obtained with 500 mM of imidazole (Fig. 2). Such purified Vip3LB was used for producing anti-Vip3LB antibodies and for searching the possible receptor proteins of Vip3LB in susceptible midgut larvae.
Purification of the Vip3LB toxin: SDS-PAGE (10% gel) stained with Coomassie stain. Lanes: 1, Supernatant proteins of sonicated culture of E. coli strain containing pET-vip3LB; 2, Molecular weight markers (170, 116, 76, and 53 kDa); and 3–10, Second and third milliliters collected using 5-ml elution buffers containing increasing concentration of imidazole: 100, 200, 300, and 500 mM, respectively
Antibody Preparation
Antibody preparation was performed as described in Materials and Methods section, using pure Vip3LB protein (Fig. 2, lane 10). The produced polyclonal anti-Vip3LB antibodies were tested to check the serum quality by Western blot. Using the sonicated culture of E. coli (pET-14b) (Fig. 3, lane 1) as negative control, we demonstrated that the anti-Vip3LB antibodies specifically recognized the Vip3 protein having a molecular weight of 90 kDa (Fig. 3, lane 2). The presence of two other spots corresponding to proteins with molecular weights of 62 and 45 kDa (Fig. 3, lane 2) was due to Vip3LB proteolysis by E. coli proteases as described by Yu et al. [15].
Verification of the anti-Vip3LB antibodies quality: Western blot analysis of proteins transferred onto a nitrocellulose membrane and blotted with anti-Vip3LB antibodies. Lanes: 1, Supernatant proteins of sonicated culture of E. coli strains containing pET-14b (negative control); 2, Supernatant proteins of sonicated culture of E. coli strains containing pET-vip3LB; and 3, Purified Vip3LB protein
Midgut Extracts Ligand Blotting
The active form of Vip3LB toxin (Fig. 4), produced by trypsin activation of the purified 90-kDa protein, was applied to the interaction study with total extract larvae of P. oleae by ligand blot. As shown in Fig. 5, the activated Vip3LB toxin of B. thuringiensis bound to a protein of 65 kDa (Fig. 5, lane 2). Using the same midgut extract, interaction of activated Cry1Ac with a protein of 210 kDa was shown (Fig. 5, lane 4). This result clearly demonstrates that Vip3LB and Cry1Ac toxins recognize different proteins in the midgut of the susceptible P. oleae larvae, suggesting that they bind to different receptors in vivo.
Proteolytic processing of the Vip3LB protein by trypsin. SDS-PAGE (10% gel) stained with Coomassie stain. Lanes: 1, Unprocessed Vip3LB protein; 2, Molecular weight markers (170, 116, 76, and 53 kDa); and 3, Vip3LB proteolytic pattern; arrowheads indicate the most prominent proteolytic bands derived from the Vip3LB protein
Discussion
The B. thuringiensis toxin mode of action depends largely on their binding to specific receptor(s) on the surface of the midgut membrane of susceptible larvae. Binding affinity, binding site concentrations, and irreversible binding phenomena have been demonstrated as critical factors in determining insecticidal activity and specificity. In vitro BBMV binding studies with susceptible and resistant Heliothis virescens strains, alterations in the receptor binding properties have been observed in the resistant strains [16–19], indicating that receptor binding is a critical factor in resistance development.
To prevent or delay resistance development to B. thuringiensis toxins, a multiple toxin approach has been suggested [20, 21]. The Vip3A protein is a novel insecticidal protein with activity against a broad spectrum of Lepidopteran insects [4]. Lee et al. [12] have shown that the mode of action of Vip3A differs in several steps compared with that of Cry1Ab δ-endotoxin of B. thuringiensis. Therefore, the incorporation of Vip3A into insect control program might serve to address δ-endotoxin resistance concerns in addition to its own value as a biological control agent.
Vip3LB protein is an insecticidal protein, secreted by B. thuringiensis strain BUPM95, having interesting insecticidal activities against Lepidopteran pests such as Ephestia kuehniella [7, 9] and P. oleae. In this study, the corresponding gene, vip3LB fused to His-tag, was heterologously overexpressed in E. coli. The high synthesis level of this 90-kDa protein allowed its purification and use to produce anti-Vip3LB antibodies. The purified Vip3LB protein bound to a 65-kDa midgut putative receptor of the susceptible Lepidopteran pest P. oleae after trypsin activation, whereas activated Cry1Ac toxin bound to another putative receptor having a molecular size of about 210 kDa.
This difference between Vip3 and Cry toxin putative receptors on the midgut membrane of susceptible larvae correlates with the result shown by Lee et al. [12] demonstrating that in Manduca sexta, Vip3A toxin does not bind to the well-known Cry1A toxin receptors of 120- and 250-kDa protein corresponding, respectively, to aminopeptidase-like and cadherin-like molecule, but Vip3A does bind to distinct 80- and 100-kDa BBMV proteins.
In our case of P. oleae, another putative receptor size of 65 kDa would support the use of Vip3LB as a biological control agent, especially when a resistance to Cry toxins would be detected in this redoubtable Lepidopteran pest that attacks olive crops.
Abbreviations
- B :
-
Bacillus
- P :
-
Prays
- IgG:
-
Immunoglobulin G
References
Höfte, H., & Whiteley, H. R. (1989). Insecticidal crystal proteins of Bacillus thuringiensis. Microbiological Reviews, 53, 242–255.
Tounsi, S., J’Mal, A., Zouari, N., & Jaoua, S. (1999). Cloning and nucleotide sequence of a novel cry1Aa-type gene from Bacillus thuringiensis subsp. kurstaki. Biotechnology Letters, 21, 771–775.
Tounsi, S., & Jaoua, S. (2003). Characterization of a novel cry2Aa-type gene from Bacillus thuringiensis subsp. kurstaki. Biotechnological Letters, 25, 1219–1223.
Estruch, J. J., Warren, G. W., Mullins, M. A., Nye, G. J., Craig, J. A., & Koziel, M. G. (1996). VIP3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proceedings of the National Academy of Sciences of the United States of America, 93, 5389–5394.
Warren, G. W. (1997). Vegetative insecticidal proteins: Novel proteins for control of corn pests. In N. B. Carozzi & M. Koziel (Eds.), Advances in insect control: The role of transgenic plants (pp. 109–121). London: Taylors & Francis.
Estruch, J. J., & Yu, C. G. (2001) Plant pest control. U.S. Patent 6,291,156 B1.
Mesrati, A. L., Tounsi, S., & Jaoua, S. (2005). Characterization of a novel vip3-type gene from Bacillus thuringiensis and evidence of its presence on a large plasmid. FEMS Microbiology Letters, 244, 353–358.
Chen, J. J., Yu, J. X., Tang, L. X., Tang, M. J., Shi, Y. X., & Pang, Y. (2003). Comparison of the expression of Bacillus thuringiensis full-length and N-terminally truncated vip3A gene in Escherichia coli. Journal of Applied Microbiology, 95, 310–316.
Mesrati, A. L., Karray, D. M., Tounsi, S., & Jaoua, S. (2005). Construction of a new high copy-number shuttle vector of Bacillus thuringiensis. Letters in Applied Microbiology, 41, 361–366.
Ferre, J., & Van Rie, J. (2002). Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annual Review of Entomology, 47, 501–533.
Schnepf, H. E., Crickmore, N., Van Rie, J., Lereclus, D., Baum, J., Feitelson, J., et al. (1998). Bacillus thuringiensis and its pesticidal crystal proteins. Microbiology and Molecular Biology Reviews, 62, 775–806.
Lee, M. K., Walters, F. S., Hart, H., Palekar, N., & Chen, J. S. (2003). The mode of action of the Bacillus thuringiensis Vegetative Insecticidal Protein VIP3A differs from that of Cry1Ab δ-endotoxin. Applied and Environmental Microbiology, 69, 4648–4657.
Tounsi, S., Dammak, M., Rebai, A., & Jaoua, S. (2005). Response of larval Ephestia kuehniella (Lepidoptera/Pyralidae) to individual Bacillus thuringiensis kurstaki toxins and toxin mixtures. Biological Control, 35, 27–31.
Rouis, S., Chakroun, M., Saadaoui, I., & Jaoua, S. (2007). Proteolysis, histopathological effects and immunohistopathological localization of delta-endotoxins of Bacillus thuringiensis subsp. kurstaki in the midgut of lepidopteran olive tree pathogenic insect Prays oleae. Molecular Biotechnology, 35, 141–148.
Yu, C. G., Mullins, M. A., Warren, G. W., Koziel, M. G., & Estruch, J. J. (1997). The Bacillus thuringiensis vegetative insecticidal protein VIP3A lyses midgut epithelial cells of susceptible insects. Applied and Environmental Microbiology, 63, 532–536.
Lee, M. K., Rajamohan, F., Gould, F., & Dean, D. H. (1995). Resistance to Bacillus thuringiensis Cry1A δ-endotoxins in a laboratory-selected Heliothis virescens strain is related to receptor alteration. Applied and Environmental Microbiology, 61, 3836–3842.
Jurat-Fuentes, J. L., Gould, F. L., & Adang, M. J. (2002). Altered glycosylation of 63- and 68-kilodalton microvillar proteins in Heliothis virescens correlates with reduced Cry1 toxin binding, decreased pore formation, and increased resistance to Bacillus thuringiensis Cry1 toxins. Applied and Environmental Microbiology, 68, 5711–5717.
Jurat-Fuentes, J. L., Gould, F. L., & Adang, M. J. (2003). Dual resistance to Bacillus thuringiensis Cry1Ac and Cry2Aa toxins in Heliothis virescens suggests multiple mechanisms of resistance. Applied and Environmental Microbiology, 69, 5898–5906.
Jurat-Fuentes, J. L., Gahan, L. J., Gould, F. L., Heckel, D. G., & Adang, M. J. (2004). The HevCaLP protein mediates binding specificity of the cry1A class of Bacillus thuringiensis toxins in Heliothis virescens. Biochemistry, 43, 14299–14305.
Gould, F. (1998). Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annual Review of Entomology, 43, 701–726.
Roush, R. T. (1998). Two-toxin strategies for management of insecticidal transgenic crops; can pyramiding succeed where pesticide mixtures have not? Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences, 353, 1777–1786.
Acknowledgments
This work was supported by grants from the Ministry of Higher Education, Scientific Research and Technology. We thank the Institut de l’olivier de Sfax, Tunisia for providing us with P. oleae larvae. We wish to thank Pr. Jamil Jaoua, Head of the English Unit at the Sfax Faculty of Science for having proofread this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdelkefi-Mesrati, L., Rouis, S., Sellami, S. et al. Prays oleae Midgut Putative Receptor of Bacillus thuringiensis Vegetative Insecticidal Protein Vip3LB Differs from that of Cry1Ac Toxin. Mol Biotechnol 43, 15–19 (2009). https://doi.org/10.1007/s12033-009-9178-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-009-9178-4