Abstract
Given the well-known environmental drawbacks of using fossil fuels, advances in the field of alternative energy have become a worldwide technological priority. Special interest has been focused on the production of biodiesel obtained from oleaginous microorganisms. In the present research, lipid production by two species, microalgae Chlorella pyrenoidosa and yeast Rhodotorula mucilaginosa was assessed, independently and in mixed culture to evaluate a possible synergy. Fatty acid analysis was performed by gas chromatography. Among pure and mixed cultures of both strains and several culturing conditions, the highest biomass and lipid productivity was obtained by C. pyrenoidosa (8.05 and 1.62 g/L, respectively). The results of this study showed that both strains used are in fact oleaginous strains as they were found to reach up to 20 % of lipids, in addition, lipids in both pure and mixed cultures were mainly of triglycerides (>90 %), composed of fatty acid chains between 16 and 18 carbons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While energy demand soars as world population and the global economy continue to grow, recent research has warned that non-renewable energy sources such as oil are projected to be mostly depleted in less than 50 years. In addition to this, petroleum fuels generate harmful gases, primarily sulfur oxides (SOX) and carbon monoxide (CO) at higher rates than other fuels. For this reasons, alternative sources of fuels that are renewable, economical, and less harmful to the environment needs to be widely implemented. One such alternative is the use of biodiesel, whose production is based on the transesterification from long chain triglycerides from renewable sources using methanol [1].
Previous research has focused on both, finding new sources of raw materials for the production of biodiesel and the assessment of microorganisms such as microalgae and yeasts capable of accumulating lipids, mostly triglycerides, which could be later transesterified. Microalgae and yeasts are considered to have great potential due to their short life cycle, high lipid accumulation, and less nutritional and environmental requirements compared to plants [2, 3].
In the present study, we assessed the growth, lipid production, and fatty acid composition by Chlorella pyrenoidosa and Rhodotorula mucilaginosa, cultured alone or in combination, in order to evaluate their potential for biofuel production.
Material and Methods
Strains
The strains used in this study were obtained from Facultad de Ciencias Químicas at Universidad Autónoma de Nuevo León, and originally isolated in 2005 from the Río Pesquería, located in Escobedo, State of Nuevo León, México.
Enrichment Media
The yeast culture medium contained 25 g/L glucose, 12.5 g/L KH2PO4, 10 g/L yeast extract, and 5 g/L MgSO4 · 7H2O. The microalgae culture medium contained 10 mM KNO3, 2 mM KH2PO4, 4 mM MgSO4°7H2O, 13 mM Ca(NO3)2°4H2O, 92 μM H3BO3, 18.3 μM MnCl2 · 4H2O, 1.53 μM ZnSO4 · 7H2O, 640nM CuSO4 · 5H2O, 30nM (NH4)6Mo7O24 · 4H2O; 20 μM FeSO4 · 7H2O, and 20 μM Na2EDTA at pH = 4.9 [4].
Culture Media for Lipid Production
The nitrogen-limited culture medium (NLM) for yeasts contained 100 g/L glucose, 8 g/L yeast extract, and 3 g/L peptone at pH = 7.0 [5]. The modified BG11 culture medium for microalgae contained 50 g/L glucose, 1.5 g/L NaNO3, 0.4 g/L K2HPO4 · 3H2O, 0.075 g/L MgSO4 · 7H2O, 0.036 g/L CaCl2 · 2H2O, 0.02 g/L Na2CO3, 0.006 g/L citric acid, 0.006 g/L C6H8O7 · xFe3 + · yNH3, and 0.001 Na2EDTA at pH = 7.0 [6]. The medium for mixed algae and yeast culture contained 50 g/L glucose, 1.6 g/L yeast extract, 1.2 g/L NaNO3, 0.6 g/L peptone, 0.38 g/L K2HPO4 · 3H2O, 0.075 g/L MgSO4 · 7H2O, 0.036 g/L CaCl2 · 2H2O, 0.02 g/L Na2CO3, 0.006 g/L citric acid, 0.006 g/L C6H8O7 · xFe3 + · yNH3, and 0.001 g/L Na2EDTA, 0.001 at pH = 7. This culture medium was designed using the VisualMINTEQ® software for the combined growth of microalgae and yeast, and based on the medium for lipid production, as described above.
Culture Conditions
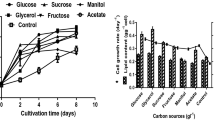
All strains were kept at 4 °C in semisolid media; R. mucilaginosa was maintained in potato dextrose agar (Difco, NJ, USA) and C. pyrenoidosa strain was preserved in the abovementioned microalgae enrichment medium, solidified with 1.5 % bacteriological agar. An isolated colony of semisolid culture of R. mucilaginosa was inoculated into 100 mL of yeast enrichment medium and incubated for 24 h at 30 °C and at 150 rpm; once the exponential growth phase was reached, 5 mL of this culture were transferred into 100 mL of nitrogen-limited medium and mixed-culture medium for lipid production and then incubated for 96 h and 120 h, respectively, to determine the highest lipid productivity of the strain in suitable conditions for yeasts and compare it with the productivity in the designed mixed-culture medium. Similarly, an isolated colony of C. pyrenoidosa was inoculated into 100 mL of microalgae enrichment medium and incubated for 72 h at 30 °C and 150 rpm, using a 100-W white bulb as a continuous light source; once the exponential growth phase was reached, 5 mL of the culture were transferred to 100 mL of modified BG11 and mixed-culture media for lipid production and incubated under the same conditions with the exception of the light source. Furthermore, the flasks were protected from any light source to promote the strain heterotrophic metabolism and incubated for 144 and 120 h, respectively. Similarly, a mixed culture with both strains was prepared by taking 4 mL of C. pyrenoidosa and 1 mL of R. mucilaginosa cultures at exponential phase, and inoculated to 100 mL of mixed-culture medium, incubated at 30 °C and 150 rpm for 144 h, in the absence of light. Incubation times were selected according to the growth curves (data not shown).
Biomass and Lipid Production
Once the incubation period was completed, the total biomass was collected by centrifugation, dissolved in 20 mL of distilled water and divided into two equivalent parts, one part was lyophilized to calculate biomass in g/L, whereas the other one was used for extraction of lipids by macerating biomass in a Bead Beater (Biospec 1107900–101) with two pulses of 2 min, following the Folch methodology [7], in which the organic phase was separated from the biomass and beads by centrifugation 5000 rpm, 5 min, and then washed with 0.85 % saline solution. The organic phase was transferred into a 100-mL previously weighted round-bottom flask and the solvents were removed using a rotary evaporator (Yamato RE200) until dryness, in this way, the lipid production in the samples (g/L of culture media and % of lipid content in strains dry biomass) was estimated.
Analysis of Fatty Acid Composition by gas Chromatography
The lipids extracted from the samples were transesterified by adding 6 mL of a methanolic solution of NaOH 0.5 N and 7 mL of a methanolic solution of BF3 at 14 % (Sigma-Aldrich, Toluca, México) and heating under reflux. Subsequently, 5 mL of petroleum ether and 15 mL of a NaCl-saturated solution were added; the resulting mixture was vigorously stirred for a few minutes and left aside until separation of the organic and aqueous phases. The organic phase containing fatty acid methyl esters (FAMEs) was analyzed by gas chromatography (Varian 3700, NY, USA) using a capillary-column 30 cm and 53 mm (Omegawax, Supelco, USA) with flame ionization detector 250 °C, and using nitrogen as a carrier gas (10 mL/min).
Results and Discussion
Biomass and Lipid Production of Strains Grown Separately
Both strains showed to have a proper growth in their respective media; however, a slight decrement in biomass and lipid production was observed when strains were grown in a mixed-culture media. The highest productivity was obtained growing C. pyrenoidosa in the modified BG11 media (8.05 g/L of dry biomass and 1.62 g/L lipids). In addition, it was observed that the strains tested in the present study could be considered as oleaginous species, due to the high percent of lipids obtained from the dry biomass, 20.1 and 20.8 %, in C. pyrenoidosa and R. mucilaginosa, respectively [8]. However, these results are lower compared with other strains of the same genus, such as R. mucilaginosa, reported by Karatay with a lipid production in dry biomass of 69.5 % [9], or Chlorella protothecoides used by Xu et al. which produced 55.2 % lipids from dried biomass samples [10]. It should be emphasized that both strains used in the present study achieved the production of more than 1 g/L of lipids, whose results are comparable to many other strains reported on the literature [11].
Biomass and Lipid Production in a Mixed Culture
Both strains showed good growth in a mixed culture. In fact when incubated together an enhanced productivity was observed, as compared with productivity in separate cultures, using the mixed-culture media. This could suggest a symbiotic relationship between C. pyrenoidosa and R. mucilaginosa. The observed synergistic relationship between some microalgae and yeast species is believed to be principally due to yeast providing CO2 to microalgae and in return microalgae providing O2 to yeast, which stimulates the growth of both species [12]. Although results showed higher productivity when growing both species together as compared with separate cultures in the mixed-culture media, and R. mucilaginosa in NLM medium, it was observed that the highest productivity resulted when growing C. pyrenoidosa using modified BG11 medium. The productivity of both strains growing together was 9.51 and 1.37 g/L of dry biomass and lipids, respectively. These results were higher than the productivity obtained by Xue et al. using mixed cultures of Rhodotorula glutinis and Spirulina platensis [13]; in contrast, our study resulted in a lower productivity compared with that reported by Cheirsilp et al. using a mixed culture of R. glutinis and Chlorella vulgaris [14]. The dry biomass and lipid productivity results from the present study are summarized in Table 1.
Analysis of Fatty Acids Produced by Pure and Mixed Cultures of R. mucilaginosa and C. pyrenoidosa
Lipids were analyzed to determine the viability of producing a biofuel from the fatty acids extracted from both species. In Table 2, it can be observed that a high percentage of triglycerides were obtained (>90 %), relative to the total lipids in the samples from two individual cultures and one mixed culture. These triglycerides were mostly composed of fatty acids from 16 to 18 carbons (Table 3). Comparing these results with similar studies, it can be suggested that lipids produced by the strains used in the present study are suitable for biodiesel production, in accordance to the American Society for Testing and Materials (ASTM) standards [10, 15, 16]. Moreover, a high percentage of oleic and linoleic fatty acids was observed, which in addition to using them for biofuel production, could also have important nutritional and therapeutic properties [17].
Conclusions
Based on the preceding results, we conclude that our experimental strains of R. mucilaginosa and C. pyrenoidosa provided evidence of suitability for lipids production with appropriate characteristics for becoming biodiesel or even useful as nutritional or therapeutic agents; however, our strain lipid productivity (at least at experimental scale) resulted lower than other strains reported in the literature, which could lead to disadvantages for large-scale production [18]; nevertheless, both strains can be considered oleaginous due to their lipid percentage production. It was also proved that culture media suitable for culturing of microbial consortia microalgae-yeast can be designed with both strains, showing promising results growing in separate cultures, even conducting to a synergistic relationship, increasing biomass and lipid production when both strains were grown in a mixed culture.
References
Campbell, M. N. (2008). Guelph Engineering Journal, 1, 2–7.
Li-Xia, P., Deng-Fen, Y., Li, S., Wei, L., Gui-Guang, C., & Zhi-Qun, L. (2009). Food Technology and Biotechnology, 47, 215–220.
Li, P., Miao, X., Li, R., & Zhong, J. (2011). Journal of Biomedicine and Biotechnology. doi:10.1155/2011/141207.
Lopez-Chuken, U., Young, S., & Guzman-Mar, L. (2010). Enviromental Technology, 31, 307–318.
Cuan-chao, D., Jie, T., Feng, X., Yi-jun, D., & Mo, Z. (2007). African Journal of Biotechnology, 6, 2130–2134.
Leesing, R., Kookkhunthod, S., & Nontaso, N. (2011). World academy of science. Engineering and Technology, 76, 499–502.
Folch, J., Lees, M., & Sloane-Stanley, G. (1956). Journal of Biological Chemistry, 226, 497–509.
Ratledge, C., & Wynn, J. (2002). Advances in Applied Microbiology, 51, 1–51.
Karatay, S. E., & Dönmez, G. (2010). Bioresource Technology, 101, 7988–7990.
Xu, H., Miao, X., & Wu, Q. (2006). Journal of Biotechnology, 126, 499–507.
Ajeitos, J., Vallejo, J., Veiga-Crespo, P., & Villa, T. (2011). Applied Microbiology and Biotechnology, 90, 1219–1227.
Cheirsilp, B., Suwannarat, W., & Niyomdecha, R. (2011). New Biotechnology, 4, 362–368.
Xue, F., Miao, J., Zhang, X., & Tan, T. (2010). Applied Biochemistry and Biotechnology, 160, 498–503.
Cheirsilp, B., Suwannarat, W., & Niyomdecha, R. (2011). New Biotechnology, 28, 362–368.
Danielo, O. (2005). Biofutur, 255, 1–4.
Chisti, Y. (2007). Biotechnology Advances, 25, 294–306.
Pereira, H., Barreira, L., Figueiredo, F., Custodio, L., Vizetto-Duarte, C., Polo, C., Resek, E., Engelen, A., & Varela, J. (2012). Marine Drugs, 10, 1920–1935.
Mata, M., Martins, A., & Caetano, S. (2010). Renewable and Sustainable Energy Reviews, 14, 217–232.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reyna-Martínez, R., Gomez-Flores, R., López-Chuken, U.J. et al. Lipid Production by Pure and Mixed Cultures of Chlorella pyrenoidosa and Rhodotorula mucilaginosa Isolated in Nuevo Leon, Mexico. Appl Biochem Biotechnol 175, 354–359 (2015). https://doi.org/10.1007/s12010-014-1275-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1275-6