Abstract
Castor (Ricinus communis L.) is an important oil seed crop having its main cultivated area in India, China, and Brazil in dry land farming. Castor husk is generated as waste in castor oil production. Use of castor husk waste as substrate is studied for alkaline protease production by Bacillus altitudinis GVC11 in solid-state fermentation. Various parameters like moisture content, incubation period, particle size, effect of carbon and nitrogen sources are studied and optimized for enzyme production. Highest enzyme production of 419,293 units per gram husk is obtained. Cost of enzyme production can be reduced by using castor husk as substrate.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proteases constitute one of the most important groups of industrial enzymes and have applications in different industries like detergent, food, feed, pharmaceutical, leather, silk, and for recovery of silver from used X-ray films [1–3]. Among all proteases, alkaline proteases are robust in nature and are primarily used as detergent additives [4]. They account for 60% of the total worldwide enzyme sales and this trend is expected to increase in near future [5].
Large number of microorganisms produce proteases, but Bacillus spp. are recognized as important sources of commercial alkaline proteases because of their ability to secrete large quantities of enzyme with high activity [6, 7].
Most of the microbial products at industrial scale are generally produced using submerged fermentation [8–10]. However, solid-state fermentation has gained renewed interest and attention from researchers because of its edge in biomass energy conservation, solid waste treatment, and its application to produce secondary metabolites over submerged fermentation [11–14]. Production of enzymes using agro-industrial wastes as substrates in solid-state fermentation provide several advantages in productivity, cost-effectiveness in labor, time, medium components, and less effluent production, thus being environment friendly [11–15].
There are several reports describing use of agro-industrial residues for production of alkaline protease, e.g., nug meal and Bacillus sp. AR009 [16], pigeon pea, and Bacillus sp. JB-99 [17], wheat bran, and Rhizopus oryzae [18]. Mitra et al. [19] reviewed production of proteolytic enzymes in SSF systems and emphasized greater economic feasibility. Studies were carried out to compare alkaline protease production in SmF systems and SSF systems [20]. The total protease activity present in one-gram bran (SSF) was equivalent to 100 mL broth (SmF).
Given the potential uses of proteases and the need for development of economical methods for enzyme production with the aim of reducing the overall cost of the industrial process, SSF using cheaper agro-byproducts is an excellent alternative in achieving higher enzyme yields. Till now, there are no reports available on production of alkaline protease using castor husk as substrate. Castor husk is generated in large quantities during processing for production of castor oil where global castor seed production is around 1 million tons per year. Castor husk contains 18.6 g nitrogen, 2.6 g phosphorous, 45 g potassium, 6.7 g calcium, 3.7 g magnesium in addition to lignocellulosic biomass per kg of husk. Leading castor-producing countries are India, China, and Brazil. India is the world's largest producer of castor and its derivatives contributing to almost 65% share. Hence, the present study was aimed to exploit the locally available, inexpensive agro-industrial waste, castor husk, as substrate for alkaline protease production using B. altitudinis GVC11 in solid-state fermentation and optimization of various parameters for improved enzyme production.
Materials and Methods
Microorganism and Culture Conditions
Bacillus altitudinis GVC11 used in this study was isolated and maintained in our lab [21]. It was maintained on nutrient agar medium in refrigerator and subcultured every 30 days. Inoculum of about 108 cells per milliliter was obtained by growing the culture in nutrient broth at 37°C for 18 h at 200 rpm.
Substrates
Castor husk is collected from the local market, washed, and dried. It is grinded and processed using different sieve to obtain mean particle size of <1 mm, 1–2 mm, 2–3 mm, >3 mm and stored till further use.
Moisture Content Estimation in Castor Husk
The moisture content present in castor husk was determined by drying to constant weight [22]. The husk (20 g) was weighed in a weighing bottle and placed in the oven and sample was dried at 105°C to 110°C to constant weight and calculated as follows.
Solid-State Fermentation
Five grams of substrate was taken in 250 ml Erlenmeyer flasks, and to this, a predetermined quantity of mineral salts medium [composition (grams per liter): (NH4)2 SO4 0.1, K2HPO4 6, KH2PO4 3, MgSO4·7H2O 0.01, CaCl2·2H2O 0.5, MnSO4·2H2O 0.01, FeSO4·7H2O 0.001, ZnSO4·7H2O 0.001, tri-sodium citrate 10, Dextrose 1] was added to achieve the desired moisture content, mixed thoroughly, and autoclaved at 121°C for 15 min. After cooling to room temperature, the flasks were inoculated with 2% inoculum (0.8 OD at 660 nm) under sterile conditions. The contents of the flasks were well mixed and incubated at 37°C for predetermined time period. One flask contents at every 24 h were used for enzyme extraction and estimation of protease activity. For studying the effect of particle size, different particle size substrate was used, while for moisture content, the solid material was provided with calculated amount of water with respect to solid material and mixed well before autoclaving. pH adjustment of solid medium was achieved by adjusting the pH of moisturizing medium before addition to the solid material. Moisture content of the solid medium was maintained by increasing the quantity of moisturizing medium.
Enzyme Extraction
The enzyme was extracted according to the method described by Nagamine et al. [23]. Five grams of fermented medium was mixed thoroughly with about 20–30 ml of 0.1 M glycine–NaOH buffer (pH 10) and homogenized on a rotary shaker at 200 rpm for 1 h. The extract was obtained by squeezing through a cheese cloth. This process was repeated three times and extracts were pooled and made up to 100 ml, centrifuged at 17,600×g, at 4°C for 15 min, and the supernatant was used as enzyme source for protease assay.
Measurement of Alkaline Protease Activity
Alkaline protease activity was determined with alkali soluble casein as substrate and 0.5 mL enzyme was mixed with 2 mL of 1% (w/v) casein in 0.1 M glycine–NaOH buffer (pH 10) and incubated for 30 min at 40°C [16]. The reaction was terminated by adding 2.5 mL of 10% (w/v) tri-chloro acetic acid and centrifuged. To 1 mL supernatant, 5 mL of 0.5 M sodium carbonate and 0.5 mL 1 M Folin-Ciocalteu's phenol reagent were added. The reaction mixture was allowed to stand for 20 min at room temperature, and absorbance was read at 660 nm against tyrosine standard in UV–Vis spectrophotometer.
One unit of alkaline protease is defined as amount of enzyme which released 1 μg tyrosine per minute under standard assay conditions and reported in terms of protease activity per gram fermented substrate.
Results and Discussion
Optimization of Incubation Period
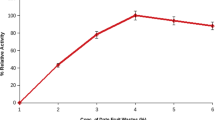
The incubation time for achieving maximum enzyme production is governed by the characteristics of the culture and is based on growth rate and enzyme production. Result of this study showed that protease production increased with incubation period (Fig. 1). Maximum enzyme production of 128,357 U/gram dry substrate (gds) was observed at 96 h of incubation. Further incubation has resulted in decreased enzyme production. Penicillium godlewskii SBSS 25 with wheat bran, Aspergillus oryzae with coffee byproducts, and Bacillus subtilis MTCC 441 with green gram husk are reported to give maximum yield of protease on fourth day of incubation [24–26].
Influence of Particle Size
In solid-state fermentation, the availability of surface area plays a vital role for microbial attachment, mass transfer of various nutrients, substrates, and subsequent microbial growth and product formation. The availability of surface area in turn depends on particle size of the substrate/support matrix. To study the influence of particle size, four different-sized particles of <1, 1–2, 2–3, >3 mm were taken. Maximum enzyme production (128,357 U/g dry substrate) was noticed with <1 mm particle size castor husk (Fig. 1). Maximum enzyme production of 83,825, 10,873, and 23,610 U/gds was observed with particle sizes of 1–2, 2–3, and >3 mm at 120, 48, and 168 h, respectively (Figs. 2, 3, and 4). The substrate particle size above this resulted in reduction of alkaline protease production. The observed reduction in protease production with altered particle size could be attributed to intra-particulate associated aeration, available surface area for microbial attachment, substrate mass transfer, and subsequent growth and enzyme production. Pushpa and Naidu [25] found optimum protease production with the particle size of 1 mm coffee by products. Whereas Murat and Antonio [27] found optimum alkaline protease production with the soybean particle size of 2 mm. According to Prakasham et al. [13], maximum protease production was observed with 1.0–1.4 mm particle size of green gram husk.
Influence of Moisture Content
Among the several factors that are important for microbial growth and enzyme production under solid-state fermentation using a particular substrate, moisture content/water activity is one of the most critical factors [28–30]. Because, solid-state fermentation processes are different from submerged fermentation, microbial growth and product formation occur at or near the surface of the solid substrate particle having low moisture contents [29]. Thus, it is crucial to provide optimized moisture level that controls the water activity (a W) of fermenting substrate for achieving maximum enzyme production. Reports on enzyme production by microbial species in solid-state fermentation indicated that the availability of water in lower or higher concentrations affected microbial activity adversely [31]. Moreover, water is known to have profound impact on the physico-chemical properties of solids and this, in turn, affects the overall process productivity [15]. To study the influence of moisture on protease production during SSF, castor husk was moistened with constant amount of mineral salt medium and different amounts of distilled water to get 40–80% moisture content prior to fermentation.
The data indicated that characteristic nature of enzyme production along with studied moisture level and moisture content played a critical role in alkaline protease production by B. altitudinis GVC11. It is generally observed that organisms grow well at higher moisture levels hence kept on the higher side during growth phase [32]. Maximum enzyme production was observed with 80% moisture content, which could give 128,357 U/gds biomass. Linearity between moisture content and enzyme production was observed up to 80% (Fig. 1) and thereafter further increase in moisture level in the fermentation medium resulted in separation of liquid medium from solid substrate. The observed reduction in enzyme production at reduced moisture level might be associated with reduced availability of water for microbial growth. However, optimum requirement of moisture content during solid-state fermentation process varied with the type of organism and agro-industrial waste material [31].
Moisture content of 80% was found to be optimum in alkaline protease production by Thermoactinomycetes thalpophilus PEE 14 [33], and 70% moisture content was found to be optimum in case of exoglucanase production by B. subtilis using banana stalk [34], lipase production by B. subtilis OCR-4 using ground nut oil cake [35], lipase production by B. subtilis using coconut oil cake [36] and 90% was found to be optimum in case of alpha-amylase production using agricultural byproducts by Humicola lanugilosa [37] and 73.38% moisture content was found to be optimum for subtilisin production by B. subtilis MTCC 441 by using green gram husk [26].
Effect of Different Carbon Sources on Protease Production
The selection of an ideal carbon source for enzyme production in solid-state fermentation process is one of the critical factors to be considered due to some of the nutrients may be available in sub-optimal concentrations, or even absent in the substrate, therefore addition of carbon sources externally may result in optimal yields. Carbon sources such as lactose, maltose, starch, glucose, xylose were selected and supplemented to the solid medium at 1% (w/w).
The data suggested that supplementation of external carbon source influenced alkaline protease production in this bacterial strain, and all the selected carbon sources showed positive effect on enzyme production. Improvement of cell growth and subsequent metabolite synthesis, in several microorganisms, was noticed upon supplementation of external carbon sources [38, 39]. However, enhancement in enzyme production levels varied with the type of carbon source. Of all the carbon sources tested, lactose showed maximum increase in enzyme production followed by xylose, starch, maltose, and glucose.
Glucose supplemented conditions slightly increased the enzyme production when compared to control (no external carbon source supplementation) (Fig. 5). This data suggested that glucose was not a repressor of protease enzyme in the bacterial strain under SSF, unlike the catabolite repression by glucose in B. subtilis and Bacillus licheniformis [40, 41].
Effect of Different Nitrogen Sources on Protease Production
Nitrogen source is one of the essential requirements for microbial growth and is required to produce several cellular organic compounds such as amino acids, nucleic acids, proteins, and cell wall components. Though most of the microorganisms metabolize inorganic and organic nitrogen sources, the preference varies with the genetic nature of microbe and type of product produced [17]. Therefore, influence of different nitrogen sources such as ammonium nitrate, ammonium sulphate, urea, yeast extract, and soya peptone on alkaline protease yield was investigated by supplementing 1% (w/w) selected nitrogen compound to solid medium under optimal fermentation conditions. Out of all nitrogen sources, ammonium nitrate, ammonium sulphate, and yeast extract have increased the enzyme production, whereas urea and soya peptone have resulted in decreased enzyme production (Fig. 6). Yeast extract as nitrogen source showed maximum influence by enhancing the enzyme production to that of other organic and inorganic nitrogen sources. Similar observations were noticed in the case of protease production by different microbial species [13, 25, 29].
Conclusion
In the present study, we have found castor husk as a good substrate for the production of alkaline protease in solid-state fermentation. Results presented in Figs. 1, 2, 3, 4, 5, and 6 indicate that various parameters influenced enzyme production by bacteria. It appears that the effect of initial moisture content, incubation period, and particle size on enzyme yield is very high. The physical nature and water holding capacity are important criteria for a solid substrate for its use in SSF process. The moisture content of the medium is a critical factor that determines the microbial growth and product yield in SSF. Addition of suitable carbon and nitrogen sources such as lactogen and yeast extract also enhanced the enzyme production three- to fourfold resulting in 419,293 U/gds with in 96 h by B. altitudinis GVC11 at moisture content of 80% with particle size of <1 mm. Thus, this study has proved that the optimization of growth parameters in a suitable solid-state medium has significant effect on improved production.
References
Anisworth, S. J. (1994). Soap and detergents. Chemical and Engineering News, 72, 34–59.
Fujiwara, N. (1993). Journal of Biotechnology, 30, 245–256.
Outtrup, H., Dambmann, C., Christiansen, M., Aaslying, D. A. (1995). US Patent Number 5466594.
Gupta, R., Beg, Q. K., & Lorenz, P. (2002). Applied Microbiology and Biotechnology, 59(1), 15–32.
Ellaiah, P., Adinarayana, K., Rajyalaxmi, P., & Srinivasulu, B. (2003). Asian Journal of Microbiology Biotechnology & Environmental Sciences, 5, 49–54.
Beg, Q. K., & Gupta, R. (2003). Enyme and Microbial Technology, 32, 294–304.
Joo, H. S., Kumar, C. G., Park, G. C., Palik, S. R., & Chang, C. S. (2004). Process Biochemistry, 39, 1441–1447.
Mahalaxmi, Y., Sathish, T., & Prakasham, R. S. (2009). Letters in Applied Microbiology, 49, 533–538.
Sathish, T., & Prakasham, R. S. (2010). Journal of Chemical Technology and Biotechnology, 85, 50–58.
Subba Rao, Ch, Sathish, T., Ravichandra, P., & Prakasham, R. S. (2009). Process Biochemistry, 44, 262–268.
Hymavathi, M., Sathish, T., Subba Rao, Ch, & Prakasham, R. S. (2009). Applied Biochemistry and Biotechnology, 159, 191–198.
Mahalakshmi, Y., Sathish, T., Subba Rao, Ch, & Prakasham, R. S. (2010). Process Biochemistry, 45, 47–53.
Prakasham, R. S., Subba Rao, Ch, & Sarma, P. N. (2006). Bioresource Technology, 97, 1449–1454.
Sathish, T., Lakshmi, G. S., Subba Rao, Ch, Brahmaiah, P., & Prakasham, R. S. (2008). Letters in Applied Microbiology, 47, 256–262.
Pandey, A., Soccol, C. R., Nigam, P., Brand, D., Mohan, R., & Roussos, S. (2000). Biochemical Engineering Journal, 6, 153–162.
Gessesse, A. (1997). Bioresource Technology, 62, 59–61.
Johnvesly, B., Manjunath, B. R., & Naik, G. R. (2002). Bioresource Technology, 82, 61–64.
Aikat, K., & Bhattacharyya, B. C. (2000). Process Biochemistry, 35, 907–914.
Mitra, P., Chakraverty, R., & Chandra, A. L. (1994). Journal of Scientific and Industrial Research, 55, 439–442.
Ortiz-Vazquez, E., Granados-Baeza, M., & Rivera-Munoz, G. (1993). Biotechnology Advances, 11, 409–416.
Vijay Kumar, E., Srijana, M., Kiran Kumar, K., Hari Krishna, N., & Gopal Reddy. (2010). Bioprocess and Biosystems Engineering, 34, 403–409.
Robert, J. S. (1967). Industrial Aspects. In: R. L. Whistler, & E. F. Paschall (Eds.), Starch: Chemistry and Technology (Vol. 2, Chapter 25, pp. 571–571). London: Academic Press.
Nagamine, K., Murashima, K., Kato, T., Shimoi, H., & Ito, K. (2003). Bioscience, Biotechnologyand Biochemistry, 67, 2194–2202.
Sindhu, R., Suprabha, G. N., & Shashidhar, S. (2009). African Journal of Microbiology, 3(9), 515–522.
Murthy, P. S., & Madhava Naidu, M. (2010). World Applied Sciences Journal, 8(2), 199–205.
Varun Bhaskar, Jones Raj, T. R., Kandasamy, S. K. J., Vijay Kumar, P., & Anant Achary. (2008). African Journal of Biotechnology, 7(13), 2286–2291.
Elibol, M., & Moreira, A. R. (2005). Process Biochemistry, 40(5), 1951–1956.
Pandey, A. (1994). In: A. Pandey (Ed.), Solid state fermentation (pp. 3–10). New Delhi: Wiley Eastern Publishers.
Pandey, A., Soccol, C. R., & Mitchell, D. (2000). Process Biochemidtry, 35, 1153–1169.
Nigam, P., & Singh, D. (1994). Journal of Basic Microbiology, 34, 405–422.
Ramesh, M. V., & Lonsane, B. K. (1990). Applied Microbiology and Biotechnology, 33, 501–505.
Perez-Guerra, N., Torrado-Agrasar, A., Lopez-Macias, C., & Pastrana, L. (2003). Electronic Jounal of Environmental, Agricultural and Food Chemistry, 2, 343.
Divakar, G., Sunitha, M., Vasu, P., Udaya Shanker, P., & Ellaiah, P. (2006). Indian Journal of Biotechnology, 5, 80–83.
Shafique, S., Asgher, M., Sheikh, M. A., & Asad, M. J. (2004). International Journal of Agriculture and Biology, 06(3), 488–491.
Singh, M., Saurav, K., Srivastava, N., & Kannabiran, K. (2010). Current Research Journal of Biological Sciences, 2(4), 241–245.
Chaturvedi, M., Singh, M., Chugh, M. R., & Rahul, K. (2010). International Journal of Biotechnology and Biochemistry, 6(4), 585–594.
Singh, R. K., Kumar, S., Kumar, S. (2009). Current Trends in Biotechnology and pharmacy, 3(2), 172–180.
Malathi, S., & Chakraborty, R. (1991). Applied and Environmental Microbiology, 57, 712–716.
Sen, S., & Satyanarayana, T. (1993). Indian Juornal Microbiology, 33, 43–47.
Frankena, J., Koningstein, G. M., Van Verseveld, H. W., & Stouthamer, A. H. (1986). Applied Microbiology, 24, 106–112.
Kole, M. M., Draper, I., & Gerson, D. F. (1998). Journal of Chemical Technology and Biotechnology, 41, 197–206.
Acknowledgment
The authors thank the Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi, for financial assistance to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhuri, A., Nagaraju, B., Harikrishna, N. et al. Production of Alkaline Protease by Bacillus altitudinis GVC11 using Castor Husk in Solid-State Fermentation. Appl Biochem Biotechnol 167, 1199–1207 (2012). https://doi.org/10.1007/s12010-012-9570-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9570-6