Abstract
L-asparaginase production was optimized using isolated Bacillus circulans (MTCC 8574) under solid-state fermentation (SSF) using locally available agricultural waste materials. Among different agricultural materials (red gram husk, bengal gram husk, coconut, and groundnut cake), red gram husk gave the maximum enzyme production. A wide range of SSF parameters were optimized for maximize the production of L-asparaginase. Preliminary studies revealed that incubation temperature, moisture content, inoculum level, glucose, and L-asparagine play a vital role in enzyme yield. The interactive behavior of each of these parameters along with their significance on enzyme yield was analyzed using fractional factorial central composite design (FFCCD). The observed correlation coefficient (R 2) was 0.9714. Only L-asparagine and incubation temperature, were significant in linear and quadratic terms. L-asparaginase yield improved from 780 to 2,322 U/gds which is more than 300% using FFCCD as a means of optimizing conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
L-Asparaginase (EC.3.5.1.1; asparagine amidohydrolase) has been widely used for the chemotherapy of acute lymphoblastic leukemia and is gaining commercial importance in the pharmaceutical sector. This enzyme is produced by a wide range of organisms including animals, microbes, plants, and in the serum of certain rodents but not in human beings [1]. L-asparaginase production pattern was studied in Escherichia coli, Erwinia cartovora, Corynebacterium glutamicum, Cylindrocarpon obtusisporum, Pseudomonas stutzeri, Rhodosporodium toruloids, Tetrahymena pyriformis, Pseudomonas aeuginosa 50071, Aspergillus tamari and Aspergillus terreus [2–11].
Most of the microbial enzymes are produced by submerged fermentation. In the recent years, solid-state fermentation (SSF) is increasingly used as an alternative economic process due to enhanced yield at low capital and energy cost using agricultural waste as medium [12, 13]. However, only a few publications are available on L-asparaginase production under SSF [1, 2, 14]. Literature reports suggested that each agricultural material is specific as source of energy and nutrients and influences the microbial metabolism and metabolite/enzyme production [1, 2, 14, 15]. Hence, selection of the most appropriate substrate and optimum fermentation conditions is crucial to develop the selected microbial strain at the commercial level. Therefore, an attempt was made to understand the significance of different agricultural waste materials and other medium components for L-asparaginase production using isolated Bacillus circulans (MTCC 8574) by response surface methodology and report the interaction of different medium components and their role on overall improvement of L-asparaginase yield.
Materials and Methods
Microorganism and Growth Conditions
Isolated B. circulans (MTCC 8574) was used for this study. The culture was maintained on M9 medium consisting of (g/L): Na2HPO4, 6.0; KH2PO4, 3; NaCl, 0.5; CaCl2, 0.011; MgSO4.7H2O, 0.12, L-asparagine, 5.0; and pH 7.0). The culture slants prepared with the above agar-based medium was sub-cultured at every 7-day interval and stored at 4 °C.
Preparation of Solid Substrates
Five grams of the selected substrate was taken separately for each 250-ml Erlenmeyer flasks and moistened with 2.5 ml of distilled water, unless otherwise specified. The flasks were sterilized and inoculated with 2 ml of 24-h-old cell suspension (having absorbance of 0.8 at 600 nm) of B. circulans. The flasks were mixed thoroughly and incubated at 37 °C in an incubator for 24 h. All experiments were performed in triplicate and average values were reported.
Enzyme Extraction
The crude enzyme was extracted by using 50 ml of 0.1 M phosphate buffer (pH 8.3) with simple contact method for 30 min at 150 rpm. The enzyme solution was filtered through the muslen cloth. This extraction process was carried out for two times and the filtrates were pooled together and centrifuged at 6,000 rpm for 15 min. The resultant supernatant was collected and used for enzyme assay.
Estimation of L-asparaginase Activity
L-asparaginase enzyme assay was performed by a colorimetric method, according to Wriston and Yellin [16] at 37 °C, using UV-visible spectrophotometer (CECIL instruments, UK), by estimating the ammonia produced during L-asparagine catalysis using Nessler’s reagent. Reaction mixture consisting of 0.5 ml of 0.08 mM L-asparagine, 1.0 ml of 0.05 mM borate buffer (pH 7.5) and 0.5 ml of enzyme solution. The reaction was terminated by the addition of 0.5 ml of 15% trichloroacetic acid solution after 30 min of incubation. The liberated ammonia was coupled with Nessler’s reagent and was quantitatively determined using a calibration curve from standard solutions of ammonia. One unit of the L-asparaginase (IU) is defined as that amount of enzyme capable of producing 1 μmol of ammonia per minute at assay conditions.
Experimental Design and Optimization
Five variables (moisture content, incubation temperature, glucose concentration, inoculum level, and asparagines concentration) were selected for RSM study. These factors were coded at five levels starting from −2, −1, 0, 1, and 2 defined by Eq. 1.
Where x i is the dimensionless coded value of the variable X i, X 0 the value of the X i at the center point and ΔX i the step change.
For statistical calculations, the variables X i were coded as x i according to the following transformation according to Eq. 1. The range and levels of the variables in coded units for RSM studies were reported in Table 1.The behavior of the system was explained by the following quadratic model Eq. 2.
Where Y is the predicted response, β 0 is the intercept term, β i is the linear effect, β ii is the squared effect, and β ij is the interaction effect.
In the present investigation, FFCCD design was employed to fit the second order polynomial model, which indicated 30 experimental tests (Table 1). For analysis of the data MATLAB 7.0 (Mathworks USA) software was used.
Results and Discussion
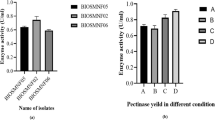
Preliminary studies on L-asparaginase production with B. circulans (MTCC 8574) in SSF was performed using various locally available agricultural materials. Among selected materials, red gram husk supported the maximum asparaginase production (780 U/gds) followed by bengal gram husk (600 U/gds). Ground nut (360 U/gds) and coconut oil cake (380 U/gds) indicating the importance of nature of agro-material in enzyme production by this isolate. This data suggested that the isolated bacterial strain of B. circulans (MTCC 8574) has higher potential in L-asparaginase production compared to the literature reported microbes such as 40 U/gds by Aspergillus niger [2] and 142.8 IU by P. aeruginosa [14] under solid-state fermentation and 24.5 IU/10 ml by Serratia marcescens [17] under submerged fermentation. Therefore, further optimization studies were performed using red gram husk as solid support/substrate medium for L-asparaginase production.
Conventional one-at-a-time experimental procedure revealed that five independent variables such as moisture content, incubation temperature, inoculum concentration, glucose (as carbon source) and asparagine (as nitrogen and as inducer of asparaginase enzyme) were the most influential factors for this enzyme production with red gram husk as solid medium (data not shown). These factors concentrations were optimized using a 26 − 2 factorial design (Table 1). The L-asparaginase production varied from 1,330 to 2,260 U/gds indicating the influence of selected parameters levels role on B. circulans metabolism and subsequent enzyme production as reported for P. aeruginosa [14]. Only ±6% variation in enzyme yields between experimental and software predicted amounts was noticed (Table 1) indicating the accuracy of experimentation. The calculated coefficient of determination (R 2) was 0.9714 (Fig. 1). The high value of R 2 (0.9081) suggested a higher significance of the model [18, 19]. The observed low difference (0.063) of the adjusted R 2 value (0.9081) and the R 2 value (0.9714) further confirm the data accuracy. At the same time, a relatively lower value of the coefficient of variation (CV = 3.03%) indicated a better precision and reliability of the performed experiments.
To understand an empirical relationship between the L-asparginase yield (Y) and test variables (in coded units) as well as to perform the analysis of variance (ANOVA), the data was analyzed using second order surface model using following Eq. 3.
Where Y, is response asparaginase production in U/gds.
Table 2 denoted the significance of each coefficient in terms of Student’s t test and p-values. Critical analysis of the data denoted that all other factors were significant in linear terms, except for asparagine and temperature which remained in quadratic terms. Similar results were reported by Abdel-Fattah and Olama [14] working on L-asparaginase production using P. aeruginosa under SSF using soya bean meal. The observed highest effect of inoculum, glucose, and asparagine concentrations in quadratic terms than their linear terms denoted that nutritional (carbon and nitrogen sources) and biological (initial biomass concentration) parameters are the most important factors and needs fine regulation for effective L-asparaginase production by B. circulans (MTCC 8574) (Table 2). However, at intercalative terms, the effect of temperature vs. moisture content interaction (−142.2) was higher than other factors interaction effects. Results identify that these two factors are critical in the production L-asparaginase by B. circulans (MTCC 8574) under SSF with red gram husk. The data also identified that the highest interaction was between glucose and asparagine (69.225) with their individual linear (37.09, −9.67) and quadratic terms (−48.14, −47.87) indicating their interactive importance in enzyme production (Table 2). Similar interaction influence was reported in other fermentation optimization studies [15, 20–23].
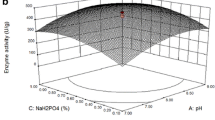
The regression model data presented in the form of contours revealed that L-asparaginase production was highly and interactively influenced by all selected fermentation parameters. L-asparaginase production predicted to be increased with increase in moisture content when incubation temperature is maintained in the selected lower level (Fig. 2a). However, higher selected level of moisture content is detrimental for enzyme production at lower glucose concentrations. Optimum L-asparaginase yield could be possible with 80–90% moisture content and in presence of 1–1.5 g of glucose supplementation (Fig. 2b). Similar trend could be predicted with respect to the interaction of moisture content and inoculum concentration (Fig. 2c) where supplementation of selected higher level of inoculum was expected to yield the maximum enzyme if moisture content is maintained in the range of 80–90% during fermentation. However, the interaction between moisture content and asparagine concentration denoted that optimum L-asparaginase was expected with moisture content in the range of 85–110% if asparagine is supplemented in the range of 0.8–1.5 g (Fig. 2d). Similar trend was noticed with studied parameters. Such data further revealed that each parameter interact differently with others and regulate the L-asparaginase production by B. circulans (MTCC 8574).
The predicted maximum enzyme production was 2,356 U/gds when fermentation was performed at 36.3 °C with the supplementation of 99.5% of moisture content, 1.17 gm of glucose and 1.24 gm of asparagine for 5 gm of solid material using 2.8 ml of inoculum. Experimental validation indicated 2,322 U/gds of L-asparaginase production revealing more than a 300% improvement. Such modelling defined improved enzyme productions were reported in literature for B. circulans (MTCC 6811) under solid-state fermentation [15] in Staphylococcus sp. [23] under submerged fermentation.
Overall, our results indicate that the isolated B. circulans (MTCC 8574) revealed higher enzyme production compared to those reported in the literature. Optimization studies showed more than a threefold improvement in L-asparaginase production using SSF. Each selected fermentation factor showed its influence at individual or interactive level either in linear or quadratic terms. The study emphasizes the selection of different process parameter levels that would influence economic production of L-asparaginase production.
References
El-Bessoumy, A. A., Sarhan, M., & Mansour, J. (2004). Journal of Biochemistry and Molecular Biology, 37, 387–393.
Mishra, A. (2006). Applied Biochemistry and Biotechnology, 135, 33–42. doi:10.1385/ABAB:135:1:33.
Mukherjee, J., Majumdar, S., & Scheper, T. (2000). Applied Microbiology and Biotechnology, 53, 180–184. doi:10.1007/s002530050006.
Mesas, J. M., Gil, J. A., & Martin, J. F. (1990). Journal of General Microbiology, 36, 515–519.
Raha, S. K., Roy, S. K., Dey, S. K., & Chakraborty, S. L. (1990). Biochemistry International, 21, 987–1000.
Manna, S., Sadhukhan, R., & Chakraborty, S. L. (1995). Current Microbiology, 30, 291–298. doi:10.1007/BF00295504.
Ramkrishnan, M. S., & Joseph, R. (1996). Canadian Journal of Microbiology, 42, 316–324.
Triantafillou, D. J., Georgatos, J. G., & Kyriakidis, D. A. (1998). Molecular and Cellular Biochemistry, 81, 43–51.
de Moura Sarquis, M. I., Oliveira, E. M. M., Santos, A. S., & da Costa, G. L. (2004). Memorias do Instituto Oswaldo Cruz, 99, 489–492.
Boeck, L. D., Squires, R. W., Wilson, M. W., & Ho, P. P. K. (1970). Applied Microbiology, 20, 964–969.
Narayana, K. J. P., Kumar, K. G., & Vijayalakshmi, M. (2008). Indian Journal of Microbiology, 48, 331–336. doi:10.1007/s12088-008-0018-1.
Mukherjee, P. S., Pandey, A., Selvakumar, P., Ashakumary, L., & Gurusamy, P. (1998). Journal of Scientific and Industrial Research, 57, 583–586.
Mishra, A., & Das, M. D. (2002). Applied Biochemistry and Biotechnology, 102, 193–199. doi:10.1385/ABAB:102-103:1-6:193.
Abdel-Fattah, Y. R., & Olama, Z. A. (2002). Process Biochemistry, 38, 115–122. doi:10.1016/S0032-9592(02)00067-5.
Prakasham, R. S., Subba Rao, Ch., & Sarma, P. N. (2006). Bioresource Technology, 97, 1449–1454. doi:10.1016/j.biortech.2005.07.015.
Wriston, J. C., & Yellin, T. O. (1973). Advances in Enzymology, 39, 185–248.
Sukumaran, C. P., Singh, D. V., & Mahadevan, P. R. (1979). Journal of Biosciences, 1, 263–269. doi:10.1007/BF02716875.
Box, G. E. P., Hunter, W. G., & Hunter, J. S. (1978). In Statistics for experimenters (pp. 291–334). New York: Wiley.
Cochran, W. G., & Cox, G. M. (1957). In Experimental design, 2nd ed (pp. 346–354). New York: Wiley.
Sreenivas Rao, R., Prakasham, R. S., Prasad, K. K., Rajesham, S., Sarma, P. N., & Rao, L. V. (2004). Process Biochemistry, 39, 951–956. doi:10.1016/S0032-9592(03)00207-3.
Subba Rao, Ch., Sathish, T., Laxmi, M. M., Laxmi, G. S., Rao, R. S., & Prakasham, R. S. (2008). Journal of Applied Microbiology, 104, 889–898. doi:10.1111/j.1365-2672.2007.03605.x.
Prakasham, R. S., Subba Rao, Ch., Rao, S., & Sarma, P. N. (2007). Journal of Applied Microbiology, 102, 204–211. doi:10.1111/j.1365-2672.2006.03058.x.
Prakasham, R. S., Subba Rao, Ch., Rao, R. S., & Sarma, P. N. (2007). Journal of Applied Microbiology, 102, 1382–1391. doi:10.1111/j.1365-2672.2006.03173.x.
Acknowledgements
M. Hymavathi is thankful for University Grants Commission and T. Sathish and Ch. Subba Rao are thankful for Council of Scientific and Industrial Research, New Delhi for financial support in the form of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hymavathi, M., Sathish, T., Rao, C.S. et al. Enhancement of L-Asparaginase Production by Isolated Bacillus circulans (MTCC 8574) Using Response Surface Methodology. Appl Biochem Biotechnol 159, 191–198 (2009). https://doi.org/10.1007/s12010-008-8438-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8438-2