Abstract
Background
Tranexamic acid (TXA) has shown safety and efficacy in reducing blood loss associated with various surgical procedures. However, to our knowledge there are no studies evaluating the effect of TXA on blood loss and transfusion requirements associated with periacetabular osteotomy (PAO).
Questions/purposes
The main purpose of this study is to determine whether TXA reduces blood loss and transfusion use in patients undergoing PAO for symptomatic acetabular dysplasia. Our secondary purpose was to compare the frequency of symptomatic thromboembolic events between patients undergoing surgery with and without TXA.
Methods
A consecutive series of 100 periacetabular osteotomies performed by one surgeon was reviewed to compare the groups immediately before and after implementation of routine use of tranexamic acid (two retrospective cohorts). TXA dosing followed an established protocol with a standard dose of 1 g infused intravenously during 10 minutes before skin incision and an additional 1 g intravenously at wound closure. Outcome measures include total estimated blood loss perioperatively and transfusion requirements. Total estimated blood loss was calculated using a formula built from the National Surgical Quality Improvement Program data regarding surgical blood loss.
Results
The mean perioperative total estimated blood loss was less in the patients receiving TXA compared with blood loss in patients who did not receive TXA (706 mL versus 1021 mL; p < 0.001; 95% CI, −495 to −134). Twenty-six (52%) of the 50 patients who did not receive TXA had postoperative blood transfusions compared with 15 (30%) of 50 who received TXA (odds ratio, 0.395; 95% CI, 0.174–0.899; p = 0.0414). No symptomatic deep vein thromboses or symptomatic pulmonary emboli were identified in either group.
Conclusions
TXA reduces estimated blood loss and the frequency of transfusions in patients undergoing PAO for treatment of symptomatic acetabular dysplasia. Future prospective studies should confirm our findings to determine whether patients undergoing PAO should receive routine perioperative TXA.
Level of Evidence
Level III, therapeutic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antifibrinolytic agents such as tranexamic acid (TXA) have shown safety and efficacy in reducing blood loss associated with various surgical procedures [3, 4, 6, 9, 13, 16]. TXA inhibits fibrinolysis by reversibly binding to plasminogen and thus blocking the binding of plasminogen to fibrin. Its effectiveness in reducing postoperative blood loss and minimizing transfusions has been documented in various surgical subspecialties including cardiac, gastrointestinal, urologic, transplant, and orthopaedic surgery [3, 4]. The effectiveness of TXA in total joint arthroplasty was shown in two recent meta-analyses [13, 16].
Compared with total joint arthroplasty, patients undergoing periacetabular osteotomy (PAO) lose more blood intraoperatively and receive transfusions more frequently [12]. Minimizing blood loss in young active patients undergoing pelvic osteotomy facilitates recovery and avoids the risk of complications associated with transfusions. However, to our knowledge, there are no studies evaluating the effect of TXA on blood loss and transfusion requirements associated with PAO. Multiple approaches including autologous blood donation and blood salvage have been used for PAO, but each has associated risks and costs. Therefore, there is a need for more effective blood management strategies for patients undergoing PAO. Pharmacologic agents, including antifibrinolytic drugs, are a possible solution to minimize blood loss and decrease transfusion rates and associated risks in this patient population. A concern associated with antifibrinolytic agents such as TXA is the possible increased risk of thrombosis, but reviews and multiple studies on this topic have identified no increased risk [1, 2, 4, 11]. After PAO, the incidence of symptomatic venous thromboembolism, including symptomatic deep vein thrombosis (DVT) and symptomatic pulmonary embolism (PE), is low [17].
The primary purposes of this study were to determine whether TXA reduces blood loss and transfusions in patients undergoing PAO for symptomatic acetabular dysplasia. Our secondary purpose was to compare the frequency of symptomatic thromboembolic events between patients undergoing surgery with and without TXA.
Patients and Methods
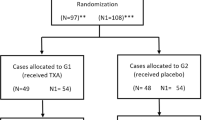
An initial query was run for all PAOs performed by one surgeon (JCC) from March 2011 to January 2013 to identify a consecutive series of patients immediately before and after institution of routine use of TXA. The study design was submitted to the institutional review board and received approval. Inclusion criteria were all PAOs performed at one hospital as an individual procedure without additional open procedures. Patients undergoing associated arthroscopy in the same setting were included. Patients undergoing revision or combination open procedures (including surgical dislocation, proximal femoral osteotomy) were excluded. A total of 104 procedures were identified and four were excluded (one revision, three combination procedures) to compile a final study group of 100 procedures in patients meeting inclusion criteria with two prospective longitudinal cohorts of 50 procedures (No TXA and TXA). In 34 cases, hip arthroscopy was performed in the same surgical setting as the PAO with 11 in the No TXA group and 23 in the TXA group. Separate analysis was performed to identify any variations based on the inclusion of an arthroscopic procedure before the PAO and no differences were seen for the included outcome measures. Therefore, all results are reported for two groups based only on administration of TXA.
All patients underwent PAO for symptomatic acetabular dysplasia using the Bernese surgical approach with slight modifications [5]. Briefly, the PAO allows for repositioning of the acetabulum for improved femoral head coverage and loading of the acetabular rim [10]. Surgical technique did not change during the course of this study. All patients received general anesthesia with a goal mean arterial pressure of 60 to 70 mmHG and epidural catheters for postoperative pain management. No changes were made to anesthetic and pain management protocols during the study period. Autologous blood recovery was used intraoperatively for all patients and was reinfused if greater than 125 mL was able to be prepared.
Outcome measures include perioperative total estimated blood loss (EBL), and transfusion use (frequency and total number of units transfused). Preoperative and postoperative laboratory values including hemoglobin and hematocrit were evaluated for both groups. At baseline there were no differences between groups for hemoglobin and hematocrit. Perioperative EBL included intraoperative EBL, visual estimation and documentation by the anesthesiologist, and postoperative drain output. The formula by Wu et al. [14, 15] built from the National Surgical Quality Improvement Program data regarding surgical blood loss (EBL in milliliters = [31.265 * preoperative hematocrit] – [29.83 * postoperative hematocrit] + [269.67 * units of red blood cells transfused]) was used to confirm the accuracy of EBL (p = 0.503; 95% CI, −128.6 to 63.40), Preoperative autologous blood donation was offered to all patients and varied from zero to two units depending on patient preferences. Clinical records from routine followups were available for all patients and checked for incidence of DVT or PE. Ultrasound evaluation was used for diagnosis of thromboembolic events if warranted symptomatically. Routine screening for DVT was not performed in these patients.
Demographics
Evaluation of demographic data identified no differences between groups (Table 1). The average age of the patients for the entire study was 27 years (range, 14–49 years) with no significant difference between groups (28 versus 27 years, p = 0.572). The average BMI for the study was 23 kg/m2 (range, 17–33 kg/m2) with no significant difference between groups (24 versus 23 kg/m2, p = 0.118). The patient population was predominantly female (85%) with only 18% male (nine of 50) in the No TXA group and 12% (six of 50) in the TXA group and no significant difference between groups (p = 0.406). Among the included patients, 34 underwent arthroscopy during the same surgical setting (11 in the No TXA group, 23 in the TXA group). No differences were seen for demographics between patients who did and did not undergo arthroscopy.
Tranexamic Acid Protocol
One gram of TXA was infused intravenously (IV) during the 10 minutes before skin incision and an additional 1 g IV was administered at wound closure. All patients during the study period met criteria for the low-risk dosing protocol. Only one patient did not receive any TXA after implementation of its use and was included in the No TXA group for data analysis purposes.
Anticoagulation Protocol
All patients before and after the use of TXA were treated with the same venous thromboembolism prophylaxis protocol. Patients younger than 18 years do not receive prophylaxis. All patients older than 18 years receive a contralateral mobile pneumatic compression device intraoperatively and bilateral mobile pneumatic compression devices for 10 days postoperatively. Chemical prophylaxis includes 325 mg enteric-coated aspirin twice daily for 6 weeks.
Indications for Transfusion
No standardized transfusion protocol was in place during the study period, but a consistent clinical approach for one surgeon was used throughout the study. Transfusion was considered for patients with a hematocrit less than 26% and symptoms related to anemia. A total of eight (three without TXA and five with TXA) transfusions were given for symptomatic patients with a hematocrit of 26% or greater. Transfusions were given one unit at a time and symptoms were monitored. Furthermore, evaluation of baseline and postoperative laboratory values for anemia (hemoglobin and hamatocrit) allow for direct comparison between groups and aid in eliminating possible bias related to transfusion triggers.
Data Analysis
Descriptive statistics were analyzed for demographic data including average age, BMI, and sex for the study groups. Student’s t-test was used to compare continuous variable outcome measures between the two groups. Categorical data for transfusion frequencies were assessed with Fisher’s exact test. Results were considered statistically significant with a p value less than 0.05.
Results
Perioperative blood loss was lower among patients who received TXA. The mean intraoperative EBL was lower in the TXA group (mean, 489 mL vs 639 mL respectively; p < 0.007; 95% CI, −260 to −40.6) (Table 2). Mean intraoperative autologous blood recovery reinfusion amounts were greater in the No TXA group than in the TXA group (192 mL and 114 mL, p = 0.005). The mean total recorded perioperative blood loss was lower in the TXA group (706 mL versus 1021 mL; 95% CI, −495 to −134; p < 0.001). The overall transfusion requirements for the study period was 41% with a significant difference between groups (OR, 0.395; 95% CI, 0.174–0.899; p = 0.0414). Transfusion use was lower among patients who received TXA. In cases without administration of TXA, patients had a 52% overall transfusion rate (26 of 50) with 1.02 units transfused per patient (range, 0–3 units). With the use of TXA, patients had a 30% overall transfusion rate (15 of 50) at 0.28 units transfused per patient (range, 0–2 units). After exclusion of autologous transfusion units, a statistically significant difference remained for allogenic blood transfusion between groups (48% no TXA group versus 24% THA group; p = 0.021)
No patients had symptomatic postoperative DVT or symptomatic PE in either group.
Discussion
TXA has been shown to reduce blood loss and transfusion use in many surgical fields and seems safe to use [3, 4, 6, 9, 13, 16]. To our knowledge, no previous studies have evaluated the use of TXA in conjunction with PAO. In our study, patients undergoing PAO appeared to lose less blood and receive fewer transfusions when treated with TXA during surgery.
The study is weakened by the inherent limitations associated with nonrandomized, retrospective studies. Patients were not randomized to treatment groups for TXA, but a consecutive series was evaluated and strengthened by a single-surgeon cohort. No changes were made in clinical practice or surgical technique during this period and all patients before and after institution of TXA met low-risk criteria for TXA dosing based on an established protocol. Second, calculating blood loss retrospectively has inherent difficulties and is dependent on anesthesia provider estimation and drain output measurements. While the methods were not standardized prospectively the data collection process was similar for both groups. Third, preoperative autologous blood donation was not analyzed as a contributing factor to perioperative blood loss. Nevertheless, given the findings in this study we no longer use preoperative blood donation for patients undergoing PAO. Larger future studies are needed to fully clarify noninferiority of outcomes after TXA use with the PAO. Future, prospective, randomized controlled trials could further clarify the effectiveness of TXA in patients undergoing PAO, but our study results provide initial data in a consecutive, single-surgeon series. No standardized transfusion protocol was in place during the study period, but a consistent clinical approach for a single surgeon was used throughout the study. Furthermore, evaluation of baseline and postoperative laboratory values for anemia (hemoglobin and hematocrit) allow for direct comparison between groups and aid in eliminating possible bias related to transfusion triggers. Hemoglobin and hematocrit levels were higher in the TXA group even with a lower transfusion frequency. A higher percentage of patients without TXA received blood transfusions and the associated increase in hemoglobin and hematocrit values, yet the patients receiving TXA maintained higher laboratory values. Finally, larger clinical studies focused on the safety of TXA use related to symptomatic and subclinical DVT and PE are warranted to confirm the findings of this study.
Various blood management protocols have been evaluated and instituted in hopes of decreasing transfusion rates and the associated risks and costs [8]. Meta-analyses from the transfusion and intensive care literature [2, 7] showed reduced blood loss and units transfused after IV administration of TXA in patients undergoing THA or TKA. A meta-analysis of 15 randomized controlled trials of TXA use in patients undergoing TKAs identified less blood loss and fewer transfusions in patients receiving TXA [16]. The trials included varied dosing regimens, study quality, and venous thromboembolism prophylaxis, but there was no identified increase in symptomatic DVT or PE [16]. Although different protocols were followed regarding timing and dosing of TXA, the combined data show safe and effective use of TXA in patients undergoing TKA. PAO is a longer procedure with more potential blood loss than primary joint arthroplasty and similar effectiveness was seen in this study.
In a series of 108 PAOs, Pulido et al. [12] identified a 20% allogeneic transfusion rate and an overall rate of 94% when including autologous transfusions and intraoperative autologous blood recovery. Each patient received an average of 2.14 units [12]. The allogeneic transfusion rate in our study was higher before institution of TXA use (48% versus 20%), but this variation may result from various factors including preoperative autologous blood donation, clinical transfusion triggers, and availability of autologous blood. Thus, comparison of these data with our data is difficult owing to these potential differences in perioperative blood management. Our current allogeneic transfusion rate is comparable to that of Pulido et al. (24% versus 20%).
The risk for symptomatic DVT or PE after PAO has been shown to be low. Specifically, results from a multicenter database showed four cases of PE and seven cases of DVT for a crude incidence of 9.4 per 1000 procedures [17]. Across the six centers, various prophylaxis strategies were used. The data from Zaltz et al. [17] suggest that the risk of symptomatic DVT is low but proper studies with effective screening methods are needed to fully evaluate this risk.
Our data indicate the use of IV TXA before skin incision and at wound closure reduces blood loss and transfusion use in patients undergoing PAO for treatment of symptomatic acetabular dysplasia. Future randomized controlled studies should confirm our findings to determine whether patients undergoing PAO should receive routine perioperative TXA.
References
Alvarez JC, Santiveri FX, Ramos I, Vela E, Puig L, Escolano F. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion. 2008;48:519–525.
Cid J, Lozano M. Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty: results of a meta-analysis of randomized controlled trials. Transfusion. 2005;45:1302–1307.
Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–1032.
Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18:132–138.
Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias: technique and preliminary results. Clin Orthop Relat Res. 1988;232:26–36.
Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):78–82.
Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31:529–537.
Keating EM, Meding JB. Perioperative blood management practices in elective orthopaedic surgery. J Am Acad Orthop Surg. 2002;10:393–400.
Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21:2611–2617.
Leunig M, Siebenrock KA, Ganz R. Rationale of periacetabular osteotomy and background work. Instr Course Lect. 2001;50:229–238.
Lozano M, Basora M, Peidro L, Merino I, Segur JM, Pereira A, Salazar F, Cid J, Lozano L, Mazzara R, Macule F. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95:39–44.
Pulido LF, Babis GC, Trousdale RT. Rate and risk factors for blood transfusion in patients undergoing periacetabular osteotomy. J Surg Orthop Adv. 2008;17:185–187.
Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93:39–46.
Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM, Uttley G, Mor V, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252:11–17.
Wu WC, Trivedi A, Friedmann PD, Henderson WG, Smith TS, Poses RM, Uttley G, Vezeridis M, Eaton CB, Mor V. Association between hospital intraoperative blood transfusion practices for surgical blood loss and hospital surgical mortality rates. Ann Surg. 2012;255:708–714.
Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94:1153–1159.
Zaltz I, Beaule P, Clohisy J, Schoenecker P, Sucato D, Podeszwa D, Sierra R, Trousdale R, Kim YJ, Millis MB. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93(suppl 2):62–65.
Acknowledgments
We thank Debbie Long, Department of Orthopaedic Surgery, Washington University School of Medicine (St Louis, MO) for assistance with manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by the Curing Hip Disease Fund (JCC) (Washington University School of Medicine, St Louis, MO, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Wingerter, S.A., Keith, A.D., Schoenecker, P.L. et al. Does Tranexamic Acid Reduce Blood Loss and Transfusion Requirements Associated With the Periacetabular Osteotomy?. Clin Orthop Relat Res 473, 2639–2643 (2015). https://doi.org/10.1007/s11999-015-4334-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-015-4334-6