Abstract
Purpose
While the effects of tranexamic acid (TXA) use on transfusion rates after acetabular fracture surgery are unclear, previous evidence suggests that holding deep vein thrombosis (DVT) chemoprophylaxis may improve TXA efficacy. This study examines whether holding DVT chemoprophylaxis in patients receiving TXA affects intraoperative and postoperative transfusion rates in acetabular fracture surgery.
Methods
We reviewed electronic medical records (EMR) of 305 patients who underwent open reduction and internal fixation of acetabular fractures (AO/OTA 62) and stratified patients per the following perioperative treatment: (1) no intraoperative TXA (noTXA), (2) intraoperative TXA and no preoperative DVT prophylaxis (opTXA/noDVTP), or (3) intraoperative TXA and preoperative DVT prophylaxis (opTXA/opDVTP). The primary outcomes were need for intraoperative or postoperative transfusion. Risk factors for each primary outcome were assessed using multivariable regression.
Results
Intraoperative or postoperative transfusion rates did not significantly differ between opTXA/opDVTP and opTXA/noDVTP groups (46.2% vs. 36%, p = 0.463; 15.4% vs. 28%, p = 0.181). Median units transfused did not differ between groups (2 ± 1 vs. 2 ± 1, p = 0.515; 2 ± 1 vs. 2 ± 0, p = 0.099). There was no association between preoperative DVT chemoprophylaxis and TXA with intraoperative or postoperative transfusions. EBL, preoperative hematocrit, and IV fluids were associated with intraoperative transfusions; age and Charlson Comorbidity Index (CCI) were associated with postoperative transfusions.
Conclusion
Our findings suggest holding DVT prophylaxis did not alter the effect of TXA on blood loss or need for transfusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of deep venous thrombosis (DVT) and pulmonary embolism (PE) in pelvic and acetabular fractures has been shown to be as high as 61% and 10%, respectively [1,2,3]. Venous thromboembolism (VTE) chemoprophylaxis has been shown to reduce rates of DVTs and PEs [4]. However, bleeding complications and transfusions lead to worse outcomes, putting patients at risk for anemia, reduced wound healing, increased length of stay, infection, and VTE [5,6,7].
Tranexamic acid (TXA) is synthetic lysine derivative that inhibits fibrin degradation and has been shown to effectively reduce perioperative bleeding in total joint arthroplasty [8,9,10,11]. Previous literature in total hip arthroplasty has shown that TXA efficacy is not affected by anticoagulants and may balance the risk of increased bleeding [12]. The effect of TXA on transfusion during and after acetabular fracture surgery is less clear [1, 8, 13]. One previous study demonstrated that TXA efficacy was reduced by concomitant DVT chemoprophylaxis; however, TXA dosing was not standardized throughout the study [1]. This study aims to compare the efficacy of a standard TXA dosing regimen with and without preoperative DVT prophylaxis in acetabular fracture surgery. We hypothesized that holding DVT prophylaxis would improve TXA efficacy.
Methods
This protocol was approved by the Institutional Review Board (protocol #56,011) and performed in accordance with the ethical standards and national laws. Patients with acute acetabular fractures who underwent open reduction and internal fixation (ORIF) between 2004 and 2020 were retrospectively reviewed using Current Procedural Terminology (CPT) codes 27,226, 27,227, and 27,228. Patients with pathologic fractures, concomitant injuries and procedures, fractures treated with percutaneous fixation, and patients < 18 years old were excluded from the study. Any fractures treated with a combined dual anterior and posterior approach or extended iliofemoral approach were excluded. The procedures were performed at a single institution’s Level 1 trauma center by one of three fellowship trained orthopedic traumatologists.
Patients were stratified into three cohorts: (1) patients who received no intraoperative TXA (noTXA), (2) patients who received intraoperative TXA and no preoperative DVT prophylaxis (opTXA/noDVTP), and (3) patients who received intraoperative TXA and preoperative DVT prophylaxis (opTXA/opDVTP). TXA was administered intravenously (1 g) prior to surgical incision and prior to wound closure (1 g). Patients given TXA were selected at the discretion of the surgeon, and no patients had contraindications for receiving TXA. Intraoperative blood transfusions were performed in accordance with surgeon discretion. In patients without concomitant diseases, postoperative blood transfusions were performed for patients with a hemoglobin < 7 g/dL. In patients with concurrent cardiac disease or other risks to acute anemia, postoperative blood transfusions were performed for patients with a hemoglobin of less than 7–8 g/dL.
Patient demographics, body mass index, Charlson Comorbidity Index (CCI), American Society of Anesthesiologists (ASA) score, Injury Severity Score (ISS), tobacco use, diabetes mellitus, fracture type, and surgical approach were collected. Fracture type was classified using the AO-OTA classification [14]. Surgical approach was grouped into the posterior approach (including Kocher-Langenbeck and Gibson approach), full ilioinguinal approach, iliofemoral/ilioinguinal lateral window, and anterior intrapelvic. The primary outcomes assessed were intraoperative and postoperative transfusions. Secondary outcomes included length of stay (LOS), operative time, IV fluids, estimated blood loss (EBL), perioperative complications (infection, venous thromboembolism (VTE), postoperative delirium, postoperative ileus, urinary retention), cell saver use, and preoperative and postoperative cell count and coagulation factors (hemoglobin, hematocrit, international normalized ratio (INR), platelets). EBL was determined subjectively based on volume of fluid in the suction container, subtracting amount administered for irrigation. VTE included any DVT or PE recorded in the patient chart during admission. Infection included any documented accounts of sepsis, pneumonia, or urinary tract infection during admission.
Continuous variables were compared using analysis of variance (ANOVA) and Kruskal–Wallis with a two-sided significance level of p < 0.05. Categorical variables were compared using chi-squared and Fisher’s exact tests. Multivariate logistic regression was used to assess risk factors for intraoperative or postoperative transfusion. Each variable was assessed for significance using backward variable elimination until only significant (p < 0.05) or trending (p < 0.10) predictors remained. Interaction between variables, specifically preoperative VTE chemoprophylaxis and TXA use, was analyzed. Intraoperative blood transfusion was additionally assessed via logistic regression as a predictor for postoperative blood transfusion. Statistical analysis was performed using SAS Enterprise Guide (Cary, NC).
To determine sample size, a power analysis was conducted, which determined that 305 patients were necessary to provide a power of 80%. ANOVA with a two-sided significance of α = 0.05 was used to detect a 20% difference in transfusion rates.
Results
Three hundred and five patients were included in the study with an average age of 52 years. Two hundred and twenty-five patients were male. Two hundred and sixteen patients received no treatment (noTXA), 50 patients received TXA without DVT prophylaxis (opTXA/noDVTP), and 39 patients received TXA treatment with preoperative DVT prophylaxis (opTXA/opDVTP). Baseline demographics did not differ between groups, aside from ISS score, which was lower in the opTXA/opDVTP group (p < 0.01). Demographic data are summarized in Table 1. All patients receiving TXA received perioperative IV infusion and did not receive intraoperative topical TXA.
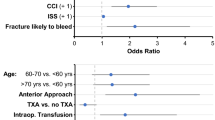
After controlling for other variables, TXA use and preoperative DVT chemoprophylaxis were not associated with intraoperative transfusions (p = 0.931, p = 0.854) (Fig. 1A). EBL, preoperative hematocrit, and IV fluids were shown to be associated with intraoperative transfusion (p < 0.001, p = 0.004, p = 0.048). Age, diabetes, CCI, ISS, fracture pattern, surgical approach, operative time, and preoperative hemoglobin were not associated with intraoperative transfusion. Results of multivariable logistic regression for intraoperative transfusions are shown in Fig. 1A.
TXA use and preoperative DVT chemoprophylaxis were not associated with postoperative transfusion (p = 0.074, p = 0.513) (Fig. 1B). Age and CCI were associated with postoperative transfusions (p = 0.016, p = 0.0081). Operative time and IV fluids were associated with EBL (p = 0.0025, p < 0.001). The remaining variables were not associated with postoperative transfusions or EBL. Results of multivariable logistic regression for operative transfusion are shown in Fig. 1B.
There was no difference in EBL (p = 0.09), intraoperative (p = 0.46), and postoperative transfusions (p = 0.18) between opTXA/noDVTP and opTXA/opDVTP (Table 2). The opTXA/noDVTP group had a smaller postoperative drop in platelets relative to the opTXA/opDVTP group (2.04 ± 59.6 vs. 28.9 ± 38.5; p = 0.007). Patients in the noTXA group had a longer mean operative time (251.5 min; p < 0.01), but there was no difference in mean operative time between patients in the opTXA/noDVTP group and opTXA/opDVTP group (218.7 min, 202.5 min). While patients in the noTXA control group had a higher mean volume of IV fluids (2992.9 mL; p = 0.01), opTXA/noDVTP and opTXA/opDVTP groups showed similar results (2451.0 mL, 2560.3 mL). Length of stay and perioperative complications were similar between groups. Table 2 summarizes outcomes data from this cohort.
Discussion
Patients undergoing acetabular fracture surgery are at an increased risk of VTE postoperatively which must be balanced against the risk of perioperative bleeding. The purpose of this study was to determine if preoperative DVT chemoprophylaxis modifies TXA efficacy in acetabular fracture surgery. Our present study found that holding DVT chemoprophylaxis in patients who received TXA did not affect number of intraoperative or postoperative transfusion rates. Patients did experience a significantly lower reduction in platelets compared to TXA with DVT prophylaxis.
Transfusion rates as high as 57% have been reported in pelvic and acetabular fractures [15], and allogenic blood transfusions put patients at risk for transfusion-associated complications, infection, and increased mortality [16, 17]. Evidence regarding TXA use for this indication is mixed. While one RCT found that TXA decreases transfusion [1, 18], more recent evidence finds no such difference [8, 13, 19]. Notably Cohen-Levy et al. [1] found that holding DVT prophylaxis and administering TXA prior to surgery and 3 h following resulted in a 20.7% reduction in blood product transfusion. However, our study found that holding DVT prophylaxis did not improve TXA efficacy in reducing blood transfusions intraoperatively or postoperatively. Interestingly, it did improve postoperative platelet levels. The difference observed between these studies may be attributable to variation in TXA administration as the current study administered 1 g of TXA prior to surgery and 1 g following wound closure, while the other administered 1–3 g initially and 1 g after 3 h.
TXA is safe and effective for reducing blood loss and transfusions in patients undergoing total joint arthroplasty while receiving DVT prophylaxis [20,21,22]. Additionally, choice of DVT chemoprophylaxis has been shown to not significantly effect TXA efficacy [12, 23]; however, these studies compared patients receiving anticoagulants with or without TXA use and did not compare TXA without chemoprophylaxis. Interestingly, a study by Sharfman et al. compared TXA efficacy in patients receiving VTE chemoprophylaxis versus TXA and intermittent pneumatic compression device (IPCD) without chemoprophylaxis. The study found TXA with IPCD and discontinuation of enoxaparin completely eliminated blood transfusions and did not increase the rate of thromboembolic events in patients undergoing total joint arthroplasty [24]. Not administering enoxaparin reduced transfusion rates by 19.1%, and addition of TXA further reduced transfusion rates by 5.6%., but the study did not assess interaction of TXA and enoxaparin.
Yakkanti et al. attempted to develop recommendations for bleeding and VTE prophylactic protocols for acetabular and pelvic fractures. They advised routine use of intraoperative TXA and DVT chemoprophylaxis postoperatively [25]. These recommendations were made in part due to the literature showing no increase in DVT, PE, and bleeding rates, while some lower quality evidence shows a direct benefit [25]. A meta-analysis by Shu et al. supported these findings with TXA reducing blood transfusions without increase in risk of VTE, DVT, or PE. Our present study found no increase in DVT, PE, or infection rates postoperatively (p = 0.938). While no clear benefit in transfusion and bleeding rates was shown, we observed that patients receiving TXA experienced shorter operative times (p < 0.01), which has been reported elsewhere in the literature [1, 26]. In our cohort, patients prescribed TXA with DVT chemoprophylaxis had lower ISS severity scores than patients given TXA without DVT chemoprophylaxis (p < 0.0001). This study attempted to control for this difference in demographics using multivariable regression, but ultimately these cohorts may be fundamentally different.
There are several limitations in this study. This study was retrospective in nature which inherently introduces bias. For example, patients were not prospectively randomized into each cohort and there was no standardized protocol for which patients were selected to receive TXA; patients may have been more likely to receive TXA if they were perceived to have a higher risk of bleeding. Additionally, topical TXA was not evaluated in the present study. Future prospective studies comparing standardized approaches to TXA administration with and without preoperative anticoagulant use could better assess the efficacy of TXA in acetabular and pelvic fractures.
Conclusion
Holding DVT chemoprophylaxis does not affect IV TXA efficacy in reducing blood loss and transfusion rates in acetabular fracture surgery. There was no association between TXA administration and intraoperative or postoperative transfusion rates.
References
Cohen-Levy WB, Rush AJ, Goldstein JP et al (2020) Tranexamic acid with a pre-operative suspension of anticoagulation decreases operative time and blood transfusion in the treatment of pelvic and acetabulum fractures. Int Orthop 44:1815–1822. https://doi.org/10.1007/s00264-020-04595-w
Wang P, Kandemir U, Zhang B et al (2019) Incidence and risk factors of deep vein thrombosis in patients with pelvic and acetabular fractures. Clin Appl Thromb 25:1076029619845066. https://doi.org/10.1177/1076029619845066
Dwyer EP, Moed BR (2019) Venous thromboembolism after hospital discharge in pelvic and acetabular fracture patients treated operatively. J Orthop Surg Hong Kong 27:2309499019832815. https://doi.org/10.1177/2309499019832815
Pirkle S, Cook DJ, Kaskovich S et al (2021) Comparing bleeding and thrombotic rates in spine surgery: an analysis of 119 888 patients. Glob Spine J 11:161–166. https://doi.org/10.1177/2192568219896295
Carson JL, Duff A, Berlin JA et al (1998) Perioperative blood transfusion and postoperative mortality. JAMA 279:199–205. https://doi.org/10.1001/jama.279.3.199
Chaudhry YP, MacMahon A, Rao SS et al (2022) Predictors and outcomes of postoperative hemoglobin of <8 g/dl in total joint arthroplasty. JBJS 104:166. https://doi.org/10.2106/JBJS.20.01766
Shohat N, Ludwick L, Goh GS et al (2021) Blood transfusions increase the risk for venous thromboembolism events following total joint arthroplasty. Sci Rep 11:21240. https://doi.org/10.1038/s41598-021-00263-0
Lack WD, Crist BD, Seymour RB et al (2017) Effect of tranexamic acid on transfusion: a randomized clinical trial in acetabular fracture surgery. J Orthop Trauma 31:526–530. https://doi.org/10.1097/BOT.0000000000000968
Cao G, Huang Q, Huang Z et al (2019) The efficacy and safety of multiple-dose oral tranexamic acid on blood loss following total hip arthroplasty: a randomized controlled trial. Int Orthop 43:299–305. https://doi.org/10.1007/s00264-018-3925-8
Hines JT, Hernandez NM, Amundson AW et al (2019) Intravenous tranexamic acid safely and effectively reduces transfusion rates in revision total hip arthroplasty. Bone Jt J 101(6):104–109. https://doi.org/10.1302/0301-620X.101B6.BJJ-2018-1376.R1
Wang C, Kang P, Ma J et al (2016) Single-dose tranexamic acid for reducing bleeding and transfusions in total hip arthroplasty: a double-blind, randomized controlled trial of different doses. Thromb Res 141:119–123. https://doi.org/10.1016/j.thromres.2016.02.027
Deng Z, Zhang Z, Sheng P et al (2020) Effect of 3 different anticoagulants on hidden blood loss during total hip arthroplasty after tranexamic acid. Medicine 99:e22028. https://doi.org/10.1097/MD.0000000000022028
Wadhwa H, Tigchelaar SS, Chen MJ et al (2022) Tranexamic acid does not affect intraoperative blood loss or in-hospital outcomes after acetabular fracture surgery. Eur J Orthop Surg Traumatol 32:363–369. https://doi.org/10.1007/s00590-021-02985-3
Marsh JL, Slongo TF, Agel J et al (2007) Fracture and dislocation classification compendium - 2007: orthopaedic trauma association classification, database and outcomes committee. J Orthop Trauma 21:S1-133. https://doi.org/10.1097/00005131-200711101-00001
Magnussen RA, Tressler MA, Obremskey WT, Kregor PJ (2007) Predicting blood loss in isolated pelvic and acetabular high-energy trauma. J Orthop Trauma 21:603–607. https://doi.org/10.1097/BOT.0b013e3181599c27
Viberg B, Gundtoft PH, Schønnemann J et al (2018) Introduction of national guidelines for restrictive blood transfusion threshold for hip fracture patients–a consecutive cohort study based on complete follow-up in national databases. J Orthop Surg 13:116. https://doi.org/10.1186/s13018-018-0828-8
Bochicchio GV, Napolitano L, Joshi M et al (2008) Outcome analysis of blood product transfusion in trauma patients: a prospective, risk-adjusted study. World J Surg 32:2185–2189. https://doi.org/10.1007/s00268-008-9655-0
Sharaby MMF, El-Deeb YM (2022) Is intravenous tranexamic acid effective in reduction of blood loss during pelvic and acetabular surgery? Int Orthop 46:1721–1729. https://doi.org/10.1007/s00264-022-05416-y
Sen RK, Attar MU, Saini G, Tripathy SK (2022) Safety and efficacy of perioperative tranexamic acid infusion in acetabular fracture fixation: a randomized placebo-controlled double-blind prospective study. Injury 53:3361–3364. https://doi.org/10.1016/j.injury.2022.08.036
Clavé A, Gérard R, Lacroix J et al (2019) A randomized, double-blind, placebo-controlled trial on the efficacy of tranexamic acid combined with rivaroxaban thromboprophylaxis in reducing blood loss after primary cementless total hip arthroplasty. Bone Jt J 101(2):207–212. https://doi.org/10.1302/0301-620X.101B2.BJJ-2018-0898.R1
Karampinas PK, Megaloikonomos PD, Lampropoulou-Adamidou K et al (2019) Similar thromboprophylaxis with rivaroxaban and low molecular weight heparin but fewer hemorrhagic complications with combined intra-articular and intravenous tranexamic acid in total knee arthroplasty. Eur J Orthop Surg Traumatol Orthop Traumatol 29:455–460. https://doi.org/10.1007/s00590-018-2307-7
Hourlier H, Fennema P (2018) Tranexamic acid use and risk of thrombosis in regular users ofantithrombotics undergoing primary total knee arthroplasty: a prospectivecohort study. Blood Transfus 16:44–52. https://doi.org/10.2450/2016.0160-16
Fraval A, Duncan S, Murray T et al (2019) OBTAIN E: outcome benefits of tranexamic acid in hip arthroplasty with enoxaparin: a randomised double-blinded controlled trial. HIP Int 29:239–244. https://doi.org/10.1177/1120700018780125
Sharfman ZT, Campbell JC, Mirocha JM, Spitzer AI (2016) Balancing thromboprophylaxis and bleeding in total joint arthroplasty: impact of eliminating enoxaparin and predonation and implementing pneumatic compression and tranexamic acid. J Arthroplasty 31:1307–1312. https://doi.org/10.1016/j.arth.2015.11.046
Yakkanti RR, Mohile NV, Cohen-Levy WB et al (2023) Perioperative management of acetabular and pelvic fractures: evidence-based recommendations. Arch Orthop Trauma Surg 143:1311–1321. https://doi.org/10.1007/s00402-021-04278-0
Kenmegne GR, Zou C, Lin Y et al (2023) A prophylactic TXA administration effectively reduces the risk of intraoperative bleeding during open management of pelvic and acetabular fractures. Sci Rep 13:12570. https://doi.org/10.1038/s41598-023-39873-1
Funding
No funding was received to assist with the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wadhwa, H., Rohde, M., Oquendo, Y. et al. Interaction of preoperative chemoprophylaxis and tranexamic acid use does not affect transfusion in acetabular fracture surgery. Eur J Orthop Surg Traumatol 34, 1025–1029 (2024). https://doi.org/10.1007/s00590-023-03763-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-023-03763-z