Abstract
Background
Persistent postsurgical pain is a major source of dissatisfaction after knee arthroplasty. Postoperative pain trajectories allow a dynamic view of pain resolution after surgery and might help to identify patients at risk for persistent pain.
Questions/purposes
In this prospective observational study, we examined the relationship between postoperative pain trajectories and persistent pain, specifically neuropathic pain, at 3 months after knee arthroplasty.
Methods
Over a 1-year period, all patients undergoing elective unilateral knee arthroplasty for osteoarthritis by one surgeon were invited to participate in the study, provided they had not had prior knee surgery and their American Society of Anesthesiologists grade was 3 or lower; 128 patients fulfilled these criteria. Patients filled in a diary questioning postoperative pain at rest and during mobilization and maximal pain from Day 1 until Day 8 after surgery. At 3 months, the patients were questioned concerning the presence of persistent pain and its nature and intensity using the Douleur Neuropathique 4 [Neuropathic Pain 4] and Brief Pain Inventory questionnaires. At 3 months, 112 of the 128 patients (87%) were successfully contacted.
Results
At 3 months, 47 of the 112 (42%) patients were totally pain free and 65 (58%) reported persistent pain at the surgical site. Among the latter, 12 patients (11%) presented with a neuropathic component and more severe persistent pain. Pain trajectories highlighted higher acute pain scores for maximal pain (from Day 1 until Day 8) and for pain at mobilization (from Day 3 until Day 8) in patients with neuropathic persistent pain (p < 0.05 at all time points compared with the no persistent pain group).
Conclusions
Postoperative pain trajectories constructed from patient’s pain diary suggest that a subgroup of patients who will present with higher pain at 3 months after knee arthroplasty might be identified early in the postoperative period and might benefit from preventative treatment.
Level of Evidence
Level III, diagnostic study. See Instructions for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Persistent postsurgical pain is a well-known clinical entity that has become a major focus of interest in the last decade. Although the exact nature of this type of pain remains unclear, strong associations between sensory abnormalities and persistent pain seem to point to a neuropathic etiology in many patients with persistent pain [10, 11]. Persistent pain has been identified as a particular problem after TKA; two recent reports, a systematic review of prospective studies [1] and a large cross-sectional survey [13], mention an incidence of persistent pain of 10% to 34% and up to 53%, respectively, after TKA.
Persistent pain after TKA can result in patient dissatisfaction [1, 13], and its prevention now represents a challenge, as it is an indicator of healthcare quality. To develop effective preventative strategies, it will be important to better understand the mechanisms underlying the persistence of pain and to determine the risk factors with the aim to target high-risk patients [12]. Among the risk factors for developing persistent pain, including after TKA, the most striking one is the severity of acute postoperative pain [11, 13, 17]. TKA, a major joint surgery, is a very painful procedure that carries a prevalence of 60% or more of severe and poorly relieved postoperative pain [23]. However, some patients presenting with severe acute pain will never develop persistent pain, and so the question arises: How should physicians best target those who will be at risk? The current assessment of postoperative pain is usually limited to the first 24 to 48 hours and very often does not include movement-evoked pain; a more dynamic view of the postoperative pain evolution of patients [12] seems necessary. The development of pain trajectories (from Day 1 until Days 5–6) may allow us to characterize individual postoperative pain and thereby identify abnormal acute pain resolution [5]. According to Chapman et al. [5], a substantial proportion of patients (37% in their series) experience unresolved postoperative pain at Day 6 after surgery.
Our working hypothesis is that patients with more severe early pain trajectories may be at risk for more severe pain several months into recovery and that these patients’ pain may be more neuropathic in origin. In this observational prospective study, we therefore examined the relationship between postoperative pain trajectories and the persistence of pain at 3 months after knee arthroplasty. We also questioned the nature of persistent pain, specifically focusing on the neuropathic component of the pain, as others have shown that neuropathic pain is usually more severe and more difficult to alleviate than nociceptive pain [21].
Patients and Methods
After institutional ethical committee approval and written consent, all eligible adult patients scheduled for an elective unilateral knee arthroplasty for osteoarthritis by a single surgeon (ET) were invited to participate in a prospective observational study from January 2012 until December 2012. The exclusion criteria were patient’s refusal to participate, language barrier, inability to fill in pain score questionnaires, history of previous open knee surgery, and infection. Patients with an American Society of Anesthesiologists grade of greater than 3 (ie, very sick patients) were not asked to participate. One hundred twenty-eight patients (106 TKAs, 22 unicompartmental knee arthroplasties [UKAs]) were included in the study. There were 83 women and 45 men, with a mean ± SD age of 68 ± 10 years.
After general or spinal anesthesia, patients were prepared and the tourniquet was inflated at 100 mm Hg above systolic blood pressure. A medial skin incision of 12 cm was made for all patients. A minimally invasive far-medial subvastus approach was used for both UKA and TKA [20]. For UKA, an extramedullary tibial guide and tibia-first technique were used. A gap-balancing technique was used for the distal femoral cut. A cemented fixed bearing implant (Zimmer® Unicompartmental Knee; Zimmer, Inc, Warsaw, IN, USA) was used for all patients. For TKA, an intramedullary guide was used for the distal femoral cut and an extramedullary guide for the tibial cut. A cemented fixed bearing implant (Vanguard®; Biomet, Inc, Warsaw, IN, USA) with resurfacing of the patella was used in all cases. Local infiltration analgesia with a cocktail of ropivacaine 0.2%, epinephrine 1:200,000, and clonidine 150 μg, 220-mL total volume, was used for both UKA and TKA. According to the progression of the surgical procedure, different zones of the knee were injected with the ropivacaine cocktail: first, the suprapatellar pouch and gutters, followed by the femoral and tibial periost; then, the posterior capsule after femoral and tibial bone cuts; and finally, the subfascial layers both medially and laterally. No drains were used. Mobilization was done by active and passive ROM exercises twice a day with a trained physiotherapist. Patients performed straight leg raising immediately after surgery, and when sufficient recovery was attained, patients were allowed to walk without aid. When independent ambulation, stair ascent, and 90° of flexion were attained, discharge criteria were reached. Patients received deep vein thrombosis prophylaxis with 10 days of enoxaparin injections.
All patients were managed with intra- and postoperative multimodal analgesia. On the day of surgery, 1 hour before the procedure, patients were premedicated with pregabalin 300 mg, celecoxib 200 mg, and paracetamol 1 g. The technique of anesthesia, either general anesthesia or spinal anesthesia (15 mg hyperbaric bupivacaine with 2.5 μg sufentanil), was left at the discretion of the anesthesiologist in charge of the patient. All patients received an intraoperative dose of dexamethasone 4 mg and a local infiltration of the operative field by the surgeon as previously described. In the ward, postoperative pain control was provided by oral analgesics, including the systematic administration of paracetamol and celecoxib. An opioid analgesic, tramadol or oxycodone, was available according to the degree of pain. All patients underwent early mobilization and were included in an active rehabilitation program. The same analgesics were prescribed to the patients when they left the hospital and continued for 3 weeks postoperatively.
During their hospital stay, the patients were taught how to fill in a pain diary from Day 1 until Day 8 after surgery. At the end of every postoperative day, they were instructed to use a numerical rating scale (NRS; 0 = no pain to 10 = worst pain) to rate their average pain at rest and during mobilization, as well as the maximal pain felt during the day. The diary also included analgesics consumption and medication name and dose. All patients left the hospital with their diary and were told to send it back after completion by regular mail. Three months after surgery, 128 patients who had completed and mailed their postoperative pain diary were contacted by telephone by a research nurse (MNF). We were able to contact 112 patients (87%), and the remaining 16 patients were excluded from the data analysis. During the telephone interview, the patients were questioned concerning the persistence of pain related to the arthroplasty. Patients who reported pain at rest and/or at mobilization were invited to answer the French versions of the Brief Pain Inventory (BPI) and Douleur Neuropathique 4 [Neuropathic Pain 4] (DN4) questionnaires. The BPI is a short but concise questionnaire that has been largely used to assess the intensity and impact of pain, specifically neuropathic pain, on the quality of life [6, 7]. The DN4 is a simple four-question questionnaire that attempts to distinguish neuropathic pain from nonneuropathic pain [3]. A cutoff score of 4 has a predictive value of 86%, with a sensitivity of 83% and a specificity of 90%.
Other patient data included preoperative pain intensity (NRS, 0–10), presence of comorbidities such as diabetes, and preoperative medications. Before surgery, all patients were also asked to fill in a questionnaire assessing their mental state of pain catastrophization (Pain Catastrophizing Scale; score from 0 to 52) [19] and their state of anxiety (State-Trait Anxiety Inventory for Adults; score from 0 to 60) [18].
At 3 months after knee arthroplasty, 47 of the 112 (42%) patients were completely pain free and 65 (58%) reported persistent pain at the surgery site (maximal pain intensity 4.3 ± 2.0 on the NRS). For the subsequent analysis of the data, the patients were separated into three groups: patients without persistent postsurgical pain (n = 47), patients with persistent pain not characterized as neuropathic pain (n = 53), and patients with persistent pain involving a neuropathic component (n = 12).
We performed statistical analysis using SigmaStat® 3.5 (Systat Software GmbH, Erkrath, Germany). Results are expressed as mean ± SD or median (interquartile range). The normal distribution of the data was assessed by the Kolmogorov-Smirnov test. Several of the data were not normally distributed and were analyzed using Kruskal-Wallis ANOVA on ranks with Dunn’s method for pairwise multiple comparisons or Mann-Whitney test for unpaired data. Comparisons of the observed proportions were performed using chi-square analysis and the Fisher exact test if appropriate. A p value of less than 0.05 was considered significant.
Results
Among the 65 patients with persistent pain, 12 (18%) presented with a neuropathic component of the pain, ie, they had a positive DN4 questionnaire (≥ 4 of 10 points). The incidence of neuropathic pain at 3 months after knee arthroplasty was 11% (12 of 112 patients).
We were unable to identify any preoperative demographic or clinical predictors of persistent pain at 3 months after surgery (Table 1). The group with neuropathic pain reported more pain than the group with pain without a neuropathic component (Table 2), but the differences in clinical pain scores between groups were small and of questionable clinical importance (ie, 2 points for the average pain). The negative impact of persistent pain on the daily quality of life was globally higher in the group with neuropathic pain than in the the group with pain without a neuropathic component (Table 2). Mood and common working tasks seemed particularly affected in patients with neuropathic pain, but here also the clinical relevance may be questioned as indicated by the small differences between the scores reported (Table 2). With the numbers available, the pain-relieving effects of the analgesics used were similar in the two groups (40% versus 60%, p = 0.085) (Table 2).
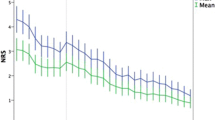
Postoperative pain trajectories were constructed for the three groups of patients, based on the pain diaries. Maximal postoperative pain was higher in the group with neuropathic pain than in the group without pain from Day 1 until Day 8 (Fig. 1). Postoperative pain associated with mobilization was also more severe in the group with neuropathic pain than in the group without pain from Day 3 until Day 8 (Fig. 2). No differences were found among pain trajectories for pain at rest (data not shown).
A graph shows the pain trajectories for maximal pain reported by the patients in their diaries from Day 1 to Day 8 after surgery for the three groups: the group with no persistent postsurgical pain (No PPSP), the group with pain but without a neuropathic component (PPSP), and the group pain but with a neuropathic component (NeuP PPSP). Pain scores are expressed as median values. p values are shown for comparisons between the group without pain and the group with neuropathic pain.
A graph shows the pain trajectories for pain during mobilization reported by the patients in their diaries from Day 1 to Day 8 after surgery for the three groups: the group with no persistent postsurgical pain (No PPSP), the group with pain but without a neuropathic component (PPSP), and the group pain but with a neuropathic component (NeuP PPSP). Pain scores are expressed as median values. p values are shown for comparisons between the group without pain and the group with neuropathic pain.
Discussion
The persistence of pain after joint arthroplasty is an important problem because most patients undergo the surgery to relieve pain; hence, not surprisingly, persistent pain after a procedure such as knee arthroplasty is the primary predictor of dissatisfaction [1, 13]. Our results suggest that a subgroup of patients who report pain 3 months after knee arthroplasty, specifically those with the highest persistent pain scores and for whom a neuropathic component of the pain is found, might be identified early after the surgical procedure. The use of pain trajectories constructed from patient’s pain diary from Day 1 until Day 8 after surgery demonstrated that patients at risk displayed a delayed postoperative pain resolution with higher pain scores, particularly during mobilization, than other patients.
There are several limitations related to this observational study. First, in accordance with the International Association for the Study of Pain’s current definition of persistent postsurgical pain [14], ie, pain related to a surgical procedure and lasting more than 2 months, our study included only followup at 3 months. Nonetheless, some patients presenting with persistent pain may yet recover as the duration of healing processes remains unknown and depends on surgery and extent of tissue trauma. Second, although reaching statistical significance, the clinical relevance of some of the results reported in Table 2, including pain scores and impact of neuropathic pain on the patient’s quality of life, must be interpreted cautiously, as the effect sizes of the differences between the scores of the patients with and without a neuropathic component of their pain are low. Another limitation concerns the absence of physical examination of the patients with persistent pain and the use of a single questionnaire, ie, the DN4, to confirm neuropathic pain. The choice of DN4 was based on its simplicity and ease of application, particularly during a telephone interview, but other validated tools also distinguish neuropathic from nonneuropathic pain, eg, the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) questionnaire, which has also been used during telephone interviews after TKA [14]. Finally, the inclusion of both UKAs and TKAs in the study, as well as the use of either spinal or general anesthesia during the procedure, may deserve some discussion, although there were no demographic differences among the groups of patients (Table 1). It is worth noting that all the patients benefited from a standardized surgical procedure (minimally invasive technique, single surgeon) and a standardized postoperative analgesic regimen (multimodal analgesia including local infiltration analgesia). To date, there are no relevant data showing the preventative effect of any intraoperative anesthetic technique on the development of persistent pain after knee arthroplasty. Further, whether UKA is associated with faster recovery and a lower incidence of persistent pain remains unknown.
We found that patients with more severe early pain as highlighted by postoperative pain trajectories in the first week were more likely to continue to report pain and limitations 3 months after surgery. Others have suggested that risk factors for the development of persistent pain after surgery include preoperative factors (ie, anxiety, catastrophization, patient’s expectations), intraoperative factors (ie, degree of tissue injury including nerve lesion), and postoperative factors [11]. Several of these risk factors have differed across studies [13, 16]; likewise, in our study, we did not identify any demographic predictors of persistent pain. A majority of studies, including several after knee arthroplasty, point to the severity of acute postoperative pain as a predictor of persistent pain [13, 17]. However, knee arthroplasty is a highly painful procedure, and as many as 70% of patients experience severe acute pain during the first days [23] while only 20% will develop persistent pain [1] and only 6% will present with neuropathic pain [8, 22].
Postoperative pain is a dynamic process. The recent development of acute pain trajectories may afford a more precise approach to measurement of postoperative pain than conventional evaluation and allow identification of abnormal pain resolution [5]. Using this method, Morze et al. [16] shaped the evolution of pain from week to week until 12 weeks after TKA and identified patients with a low preoperative pain score and those with a small reduction in their worst pain score by Week 4 as a group at risk for poor pain outcome. Another study, using similar pain trajectories, found that either higher postoperative pain score at Day 1 or worsening of pain scores during the first week predicted patients at risk for persistent pain after living donors for liver transplantation [2]. Our results support the utility of these pain trajectories as they may point out very early patients with a delayed pain resolution.
The nature of persistent pain is as important as its prevalence. One reason for this is that certain types of pain, such as neuropathic pain, will require specific treatment. Another is the possibility that a better understanding of the pathophysiologic mechanisms underlying persistent pain will contribute to designing preventative strategies. Chronic pain of neuropathic origin is associated with higher pain intensity and poorer quality of life than chronic nonneuropathic pain. Its prevalence among patients with persistent pain differs in various types of surgery and seems low; one study found that approximately 6% of patients will develop it after knee arthroplasty, often involving the infrapatellar branch of the saphenous nerve [8]. Our results, 11% at 3 months (DN4 questionnaire), are in agreement with others, with a frequency of 8.7% at 3 months and approximately 6% at 6 months (LANSS questionnaire) [4] and later (PainDETECT questionnaire) [22]. Although persistent neuropathic pain only concerns a small percentage of patients undergoing arthroplasty, it can cause severe pain that is often difficult to alleviate. We here noted that 67% of our patients with neuropathic pain were taking analgesics 3 months after surgery while their pain was poorly relieved (40% analgesic effectiveness).
Although data remain scarce, some studies have shown that neuropathic pain can be detected very early after surgery [9, 15, 18]. In one of those studies, Martinez et al. [15] demonstrated the predictive value of a positive DN4 score at 48 hours for the development of neuropathic pain after iliac crest bone harvest. Identification of patients at risk soon after surgery would permit more aggressive and earlier secondary prevention. To date, few clinical studies have been able to show a preventative effect on the development of persistent pain after knee arthroplasty, except for the administration of pregabalin 14 days after TKA [4]. One could hypothesize that pregabalin, which is used as first-line treatment in neuropathic pain, has targeted some high-risk patients.
In conclusion, we found that neuropathic pain after knee arthroplasty only concerns a small percentage of patients, but those patients report more pain that badly interferes with their daily quality of life. Our results suggest that patients who will experience persistent pain with a neuropathic component may be identifiable early in the postoperative period using pain trajectories and adequate questionnaires. These findings need to be confirmed at longer term to ascertain the validity of these tools. Future directions of research should focus on such early identification of high-risk patients to develop preventative strategies.
References
Beswick A, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2:e000435.
Bonnet A, Lavand’homme P, France MN, Reding R, De Kock M. [Postoperative pain trajectories to identify risk of chronic postsurgical pain in living donors for liver transplantation] [in French]. Ann Fr Anesth Reanim. 2012;31:945–949.
Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lantéri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29–36.
Buvanendran A, Kroin J, Della Valle C, Kari M, Moric M, Tuman K. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110:199–207.
Chapman C, Donaldson G, Davis J, Bradshaw D. Improving individual measurement of postoperative pain: the pain trajectory. J Pain. 2011;12:257–262.
Cleeland C, Ryan K. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138.
Erdemoglu AK, Koc R. Brief Pain Inventory score identifying and discriminating neuropathic and nociceptive pain. Acta Neurol Scand. 2013 April 18 [Epub ahead of print].
Haroutiunian S, Nikolajsen L, Finnerup N, Jensen T. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154:95–102.
Hayes C, Browne S, Lantry G, Burstal R. Neuropathic pain in the acute pain service: a prospective study. Acute Pain. 2002;4:45–48.
Johansen A, Romundstad L, Nielsen C, Schirmer H, Stubhaug A. Persistent postsurgical pain in a general population: prevalence and predictors in the Tromso study. Pain. 2012;153:1390–1396.
Kehlet H, Jensen T, Woolf C. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625.
Lavand’homme P. The progression from acute to chronic pain. Curr Opin Anaesthesiol. 2011;24:545–550.
Liu S, Buvanendran A, Rathmell J, Sawhney M, Bae J, Moric M, Perros S, Pope A, Poultsides L, Della Valle C, Shin N, McCartney C, Ma Y, Shah M, Wood M, Manion S, Sculco TP. A cross-sectional survey on prevalence and risk factors for persistent postsurgical pain 1 year after total hip and knee replacement. Reg Anesth Pain Med. 2012;37:415–422.
Macrae WA, Davies HT. Chronic postsurgical pain. In: Crombie IK, Linton S, Croft P, Von Korff M, LeResche L, eds. Epidemiology of Pain. Seattle, WA: IASP Press; 1999:125–142.
Martinez V, Ben Ammar S, Judet T, Bouhassira D, Chauvin M, Fletcher D. Risk factors predictive of chronic postsurgical neuropathic pain: the value of the iliac crest bone harvest model. Pain. 2012;153:1478–1483.
Morze C, Johnson N, Williams G, Moroney M, Lamberton T, McAuliffe M. Knee pain during the first three months after unilateral total knee arthroplasty: a multi-centre prospective cohort study. J Arthroplasty. 2013;28:1565–1570.
Puolakka P, Rorarius M, Roviola M, Puolakka T, Nordhausen K, Lindgren L. Persistent pain following knee arthroplasty. Eur J Anaesthesiol. 2010;27:455–460.
Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
Sullivan M, Lynch M, Clark A. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain. 2005;113:310–315.
Thienpont E. Faster quadriceps recovery with the far medial subvastus approach in minimally invasive total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:2370–2374.
Torrance N, Smith B, Bennett M, Lee A. The epidemiology of chronic pain of predominantly neuropathic origin: results from a general population survey. J Pain. 2006;7:281–289.
Wylde V, Hewlett S, Learmonth I, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152:566–572.
Wylde V, Rooker J, Halliday L, Blom A. Acute postoperative pain at rest after hip and knee arthroplasty: severity, sensory qualities and impact on sleep. Orthop Traumatol Surg Res. 2011;97:139–144.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Lavand’homme, P.M., Grosu, I., France, MN. et al. Pain Trajectories Identify Patients at Risk of Persistent Pain After Knee Arthroplasty: An Observational Study. Clin Orthop Relat Res 472, 1409–1415 (2014). https://doi.org/10.1007/s11999-013-3389-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-013-3389-5