Abstract
In recent years, a lot of interest has been given to renewable resources for their environmental friendliness and potential biodegradability in the synthesis of urethane-derived polymers. In this work, UV-curable castor oil-based polyfunctional polyurethane acrylate (COPUA) was prepared by the reaction of isophorone diisocyanate (IPDI) with castor oil and pentaerythritol triacrylate (PETA). The structures and molecular weights of the targeted IPDI–PETA and COPUA were characterized by FTIR, 1H NMR, and GPC, respectively. In addition, the effect of reactive diluent content on damping properties, thermal stabilities, and mechanical properties of COPUA was characterized by dynamic mechanical analysis (DMA), thermogravimetric analysis (TGA), and universal test machine. DMA revealed the copolymers had a glass transition temperature (T g) from 31.81 to 48.09°C. TGA showed that thermal initial decomposition temperatures were above 344.5°C, indicating the copolymers had certain thermal stability. Finally, some physical properties of curing films were studied by the contact angle and water absorption, and the results showed that the coatings exhibited good hydrophobicity. The COPUA obtained from castor oil can be used as eco-friendly materials and other applications alternative to the use of other petrochemicals in coatings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ultraviolet (UV)-curable technology has attracted wide attention because of its advantages, such as efficiency and being economical, energy saving and environmentally friendly (5E).1,2 Vegetable oil-based polymers from renewable resources are an interesting alternative chemical feedstock in UV-curable materials, addressing concerns about the environment and sustainability,3 and they have found numerous applications in coatings, inks, adhesives and related materials.4,5
Various crosslinked polymer materials based on vegetable oil have been prepared in decorative and protective coatings.6 Castor oil (CO) has low toxicity, avoids volatile organic chemical, and is an abundantly available, renewable raw material that has attracted research effort in the chemical and polymer industries.7 CO is a three glycerol ester of fatty acid, which contains hydroxyl (average hydroxyl is 2.7), improving in water resistance, crosslinking degree of the polymer, and mechanical properties of polymer resin. A great deal of research has been carried out in application of vegetable oils such as photocured hybrid materials based on acrylated castor oil,6 UV-curable urethane acrylate oligomers based on castor oil reactive diluents,8 biocompatible waterborne polyurethane based on castor oil,9,10 and multifunctional acrylate polyurethane coatings derived from castor oil.11,12
As is well known, polyurethane acrylates (PUAs) have been widely used as oligomers for various UV coating industries because of their excellent physical and mechanical properties, such as toughness, flexibility, as well as greater impact strength.13,14 Some studies have reported synthesis and characterization of PUA based on polyol;15 moreover, there are some reports on UV-curable PUA using castor oil instead of polyol as original material.16 Pentaerythritol triacrylate (PETA) was utilized as one of the most promising materials for multiple acrylates in the UV-curing coating field, but there are few reports on UV-curable castor oil-based PUA with acrylate monomer PETA with 100% solids.
The aim of this paper is to develop a polyfunctional UV-curable castor oil-based oligomer with 100% solids via isophorone diisocyanate (IPDI), castor oil (CO), and pentaerythritol triacrylate (PETA) as a core moiety gives the branched structure. The structure of castor oil-based polyurethane acrylate (COPUA) was characterized by Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR). Furthermore, the physical and mechanical properties of the COPUA were studied with dynamic mechanical analysis (DMA), thermogravimetric analysis (TGA), contact angle, and universal test machine.
Experimental
Materials
The castor oil (CO, M n = 932, average hydroxyl functionality of ∼2.7) and acetone were purchased from Nanjing Chemical Reagent Co. (China). Isophorone diisocyanate (IPDI, mixture of isomers, 99%) was purchased from 9 Ding Chemistry (Shanghai) Co. Ltd. (China). Dibutyltin dilaurate (DBTDL) was bought from Shanghai Titan Scientific Co. Ltd. (China). Pentaerythritol triacrylate (PETA, TG), dibutylamine (DBA, 99%), 2-hydroxy-2-methylpropiophenone (photoinitiator 1173, 98%), and 4-methoxyphenol (polymerization inhibitor, 98%) were obtained from Sahn Chemical Technology Co., Ltd. 2-Hydroxyethyl methacylate (reactive diluent) was supplied by Adamas Reagent Co. Ltd. Acetone, IPDI, CO, and PETA were dehydrated by immersion in 4-Å molecular sieves for one week. All other materials were used without further purification.
Preparation
Synthesis of the COPUA prepolymer
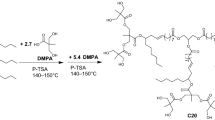
Polyurethane acrylate oligomer was synthesized by two steps, as shown in Fig. 1. Initially oligomer was synthesized by the reaction of IPDI and PETA. The IPDI dissolved in normal hexane, DBTDL used as a catalyst (0.1 wt% per mol of NCO), and 4-methoxyphenol used as polymerization inhibitor were added into the reactor, respectively, in a four-necked 500-mL glass flask equipped with mechanical stirrer, reflux condenser and nitrogen, which was used to avoid the ingress of atmospheric moisture and the urea linkages. When the temperature reached 45°C in the reactor, PETA was slowly dropped into the solution and mechanically stirred at the same time, which lasted for about 6 h at 60°C. The isocyanate content of the mixture was determined by dibutylamine–acetone method, and the reaction was stopped until the value of isocyanate reached the theoretical value of mono-isocyanate by titration. Next, the resultant mono-isocyanate was reacted with CO in the ratio 1:1 for hydroxyl equivalent to isocyanate equivalent at 60°C until the –NCO content was less than 0.5%. In the last step, the polyurethane acrylate oligomer was obtained after removal of the normal hexane by rotary vacuum evaporation.
Preparation of UV-curable coating formulation and curing
To form films for coating properties testing, photoinitiator 1173 (based on 3% of the amount of prepolymer), reactive diluent, and prepolymer COPUA were mixed fully in different proportions as summarized in Table 1; then, COPUA films were formed by casting the formulated mixture onto a polytetrafluoroethylene plate at room temperature and then radiating it under a medium pressure mercury lamp for several minutes. The UV irradiation conditions were as follows2: the main wavelength was 365 nm, the UV light intensity at the sample was 50 mW/cm2, and the irradiation distance was 8 cm.
Determination of NCO content24
The NCO content of the COPUA prepolymer was determined by the standard acetone–dibutylamine method back titration. 1.0000 g of the prepolymer was dissolved in acetone and mixed with 20 mL solution (0.1 molar dibutylamine–acetone mixture). Then the mixture was left to stand for 30 min. Bromocresol green indicator was used, and the solution was titrated by 0. 1 mol/L HCl until a color variation from blue to yellow was obtained. Parallel, a blank titration was carried out using the above procedure without any sample. The –NCO content of the COPUA prepolymer was calculated according to the following formula:
V b and V s represent the volume of HCl consumed in the blank test and titration of the sample, respectively; c is the molar concentration of the HCl; m s is the mass of the sample; and 42 was the molecular weight of NCO group.
Characterization
Fourier transform infrared spectroscopy (FTIR) analysis
FTIR was recorded with a Nicolet iS10 FTIR meter (Nicolet Instrument Crop., USA) by the attenuated total reflection (ATR) method, in the optical range of 4000–500 cm−1 and with 16 scans on average at a resolution of 4 cm−1.
1H Nuclear magnetic resonance (1H NMR) analysis
The 1H NMR spectra were conducted by an Avance-300 MHz spectrometer (Bruker Corporation, Germany) by using tetramethylsilane (TMS) as standard. The chemical shifts were expressed in parts per million (δ scale), and samples were dissolved in deuterated chloroform (CDCl3) as solvent.
Gel permeation chromatography (GPC)
Molecular weight and polydispersity (PDI) of the prepolymer were obtained by gel permeation chromatography (GPC) equipped with Styragel HR5E and HR2 (300 mm × 7.8 mm, Waters Corporation, USA) columns at the THF flow rate of 1 mL/min, and an Optilab rEX refractive index detector (Wyatt Technology Corporation, USA) was used.
Dynamic mechanical analysis (DMA)
Dynamic mechanical analysis was done by a DMA (Q800, TA Corporation, USA) in three-point bending geometry at a constant frequency of 1 Hz in the temperature range of −70 to 120°C. The viscoelastic response of COPUA systems was expressed in terms of dynamic moduli (E′) and damping ability (tan δ) by heating each film sample at a rate of 2°/min.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis (Netzsch, Germany) was used to measure the weight loss of the films and carried out on an STA 409 PC/PG analyzer. The sample (5–10 mg) was heated from 25 to 600°C at a heating rate of 10°C/min under a nitrogen atmosphere.
Tensile property testing of copolymers
The mechanical properties including tensile strength, elongation at break, and Young’s modulus of the copolymers matrices were carried out using a SANS7 CMT-4304 universal tester (Shenzhen Xinsansi Jiliang Instrument Corporation, China) according to the GB/T 2567-2008 Standard. Dumbbell specimens with a size of 50 mm × 10 mm × 4 mm at the narrow middle part were conducted for the tensile tests at a constant draw speed of 5.0 mm/min. An average value of five replicates of each sample group was taken. Testing temperature was 25°C.
Contact angle measurement
The contact angle of deionized water on the film measurements was performed at 50% relative humidity at 25°C and conducted using a DSA100 instrument from KRÜSS GmbH (Hamburg, Germany). Water drop volumes were 10 μL. The advancing and receding angles were determined using static method. The hydrophilic and hydrophobic nature of the films was studied by contact angle measurements. Contact angles were averaged from at least five different spots for each sample.
Water absorption
The water absorption of UV-curing films: the films were cut into the size of 1.5 cm × 1.5 cm and put into water for 24 h at 25°C. The water on the surface of the films was sucked by filter papers. The water absorption can be calculated by the following equation4:
m 1 is the weight of the original film, m 2 is the weight of the bibulous film.
Real-time Fourier transform infrared photopolymerization
The kinetics of the photopolymerization was recorded by real-time Fourier transform infrared (RT-FTIR) spectroscopy (Thermo-Nicolet 5700, Thermo Nicolet Instrument Corp., USA). The double bonds conversion was measured by monitoring the decrease in the intensity of absorbance associated with the carbon–carbon double bond peak at around 810 cm−1.
Results and discussion
FTIR analysis
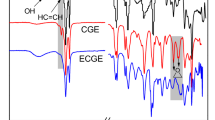
The chemical composition and structure of the samples were carried out using FTIR spectroscopy, as shown in Fig. 2. The FTIR spectrum of castor oil is shown in Fig. 2c; the obvious peak around 3452.57 cm−1 corresponds to the -OH stretching vibration, and the peaks around 2923.83 and 2853.45 cm−1 were ascribed to the stretching vibration of the –CH3 and –CH2 group, respectively. The strong peak of IPDI at 2265.81 cm−1 in Fig. 2e was attributed to the –NCO group, and there was the same characteristic peak in Fig. 2b of the IPDI–PETA. In contrast, when IPDI–PETA reacted with CO, the peak of 2265.81 cm−1 belonging to the –NCO group almost disappeared in Fig. 2a, implying the successful reaction between CO and IPDI–PETA to form urethane. The peaks observed at 3387.04 and 1725.15 cm−1 in the COPUA were the characteristic peaks of the N–H and C=O stretching from the urethane group, respectively.23 Furthermore, a newly formed characteristic peak at 1536.15 cm−1 was ascribed to the amide vibration of the –NHCOO– group of the synthesized COPUA. FTIR results revealed that the COPUA was successfully obtained through the chemical reaction of CO, IPDI, and PETA.
1H-NMR analysis
Figure 3 shows the 1H NMR spectra of the compounds including chemical formula and the chemical shift of corresponding different proton (∂/ppm). As for CO and PETA, the characteristic peaks are evidently shown in Figs. 3a and 3b, respectively. The peak at ∂ = 3.04 ppm was assigned to CH2 adjacent to the N atom of carbamate, and the peak at ∂ = 7.30 ppm in Figs. 3c and 3d can be assigned to both CDCl3 which was used as nuclear magnetic solvent and the proton adjacent to NH of the urethane group,17 indicating that carbamate was formed. Furthermore, in addition to the basic characteristic peak of PUA prepolymer Fig. 3c, the peaks of CO and PETA also appear in the spectra of PUA. At the same time, the weak peak at ∂ = 2.62 ppm appeared in the spectral patterns of both PETA and CO, which disappeared in both Figs. 3c and 3d. This may be because the hydroxyl groups on both PETA and CO have been completely reacted with PUA prepolymer. All the analysis mentioned above supports that both PETA and CO have been introduced into the molecule of COPUA. The obvious characteristic peaks indicate that the products have a high purity.
GPC analysis
GPC technique was employed to analyze molar masses of the obtained polymers, and the corresponding results of molar masses are listed in Table 2 and Fig. 4. The weight average molar mass (M w) calibrated with polystyrene standard was 1156, 361, and 9867 for CO, IPDI–PETA, and COPUA, respectively. It was observed that both the weight and number average molar masses (M w and M n) of COPUA polymers were larger than those of IPDI–PETA and indicated the occurrence between IPDI–PETA and CO.18 Nevertheless, the real reactions are so complicated that the final resins have broad polydispersity (PDI) of 1.78 for side reactions.19
DMA characterization
The DMA is an excellent tool to study the relaxation behavior of UV-cured urethane polymers, and their interaction has a predominant force on the physical, as well as the interaction between the hard and soft segments, which have a dominant influence on the physical and mechanical properties.20 Variation of storage modulus (E′) and loss tangent (tanδ) with temperature is shown in Figs. 5a and 5b.
As shown in Fig. 5a, all the storage modulus (E′) decreased rapidly from −40 to 40°C due to vibrations of the molecular segments; under −40°C, the composites exhibited a glassy state characteristic and the molecular segmental motions were frozen, and thus, E′ remained at a high level from 2600 to 3200 MPa. The decrease in storage modulus continued until its rubbery plateau, and further progress to the flowing region was restricted by the chemical crosslinks; and above 40°C, the frozen segmental structure began to relax gradually, and all E′ values were close to a constant level below 120 MPa. Generally, T g was determined from the temperature associated with the peak magnitude of the loss factor (tanδ),21 as shown in Fig. 5b. The T g of COPUA were 31.81, 40.48, 44.19, 45.40, 46.41, 48.09°C, respectively. The result suggested that the COPUA with higher content of reactive diluent had higher T g for the stronger hydrogen bonding and higher rigidity of the prepolymer backbone. All UV-cured samples showed a single T g, and the result showed the good miscibility between the hard and soft segments as well as the complete phase mixing of polyurethane.22
Thermal properties
TGA and derivative thermogravimetry (DTG) curves of COPUA are shown in Fig. 6. The initial decomposition temperature (IDT), which was assumed to the temperature of 5% weight loss, is shown in Table 3. As observed, thermal property presents a minor increasing trend as the reactive diluent content increases, which was due to the increase of crosslinking that could be introduced into the system. In addition, the residues at 800°C of COPUA are similar in Table 3. Degradation was found to be two stages. First, weight loss was in the range of 350–380°C, and this stage was mainly due to the decomposition of allophanate derived from the reaction between NCO and newly formed urethane group and formed volatile products such as carbon dioxide.23 The second in the range of 400–430°C was due to the decomposition crosslinking bonds and other chemical bonds or the oxidative decomposition of isocyanates compound.3
Contact angle characterization
The surface wettability properties of the COPUA coating materials were investigated by water contact angle measurements. Each contact angle value plotted in Fig. 7 represents an average of at least five readings, showing that the water contact angle increased with reactive diluent content added. The higher the contact angle, the more hydrophobic the surface was, which led to a self-cleaning effect. Dirt particles with an extremely reduced contact area were picked up by water droplets and thus easily cleaned off the surface. Coatings with increasing reactive diluent had a contact angle above 105.77° (COPUA-20), as compared to formulation COPUA-0 (87.41°). The contact angle increase may be due to reactive diluent which results in more crosslinking and leads to more dense and compact coatings. The phenomenon can be analyzed as follows: for one thing, CO contains hydrophobic long-chain nonpolar fatty acid chains, and when introduced into the COPUA molecule, hydrophobic property of the whole chain can be enhanced.24 For another, both CO and PETA contain multiple double bonds and can form such a pyknotic network structure during the curing process.25
Mechanical properties of the COPUA
Tensile strength, elongation at break, and Young’s modulus of the UV-cured films were determined; the results are represented in Table 4. The coatings showed an almost linear reduction of elongation at break (%) (from 46.58% to 13.54%) with increasing reactive diluent content. The result was attributed to the increasing condensation of the precursors and higher crosslinking density.26 On the other hand, the tensile strength and Young’s modulus increased up to 2.31 and 14.18 MPa, respectively (COPUA-15), and after higher concentration of reactive diluents (COPUA-25) the values were 3.29 and 46.18 MPa, respectively, which may be due to the increases in the hardness of the film.
Swelling measurement of the COPUA
Swelling measurements were done to obtain information on the crosslink density of the coatings. Mass swelling percentage of cured free films was found to be between 5.62% and 1.23%. These low percentages indicate the hydrophobic character and the high crosslinking density of the COPUA films. These analysis results indicate that the COPUA films have excellent hydrophobicity and the results are consistent with the contact angle analysis.
Photocuring kinetics by real-time infrared spectroscopy (RT-IR)
UV photocuring kinetics of COPUA were studied by real-time infrared spectroscopy. The photoinitiator of Darocur 1173 (3 wt%) was used to initiate photopolymerization. As shown in Fig. 8a, to reduce viscosity and increase the efficiency of photopolymerization, the reactive diluent of HEA with different content (from 0% to 30%, respectively) was also added to the system. The conversion of the C=C bonds was rapidly polymerized within 20 s, and all resins reached about 100% conversion rate within 1 min. Obviously, lower concentration of HEA favors the increase in conversion rate within 20 s, because the HEA increased the distance of C=C bond during the curing process.
We further evaluated the effect of triethylene glycol diacrylate (TEGDA) and trimethylolpropane triacrylate (TMPTA) on the photopolymerization with the same content of the reactive diluent (20%). Figure 8b illustrates that photocuring rates of TEGDA were faster than that of TMPTA and HEA within 15 s, but the final conversions of double bond of TMPTA were higher than that of TEGDA and HEA. As shown in Fig. 8b, higher concentration of acrylate structure in TMPTA makes the photocuring rate slower in the initial curing process; however, it increased the final conversion of C=C bonds. At the same time, it is clearly seen that all the final conversion increased to above 98%.
Conclusions
In this study, polyfunctionality castor oil-based polyurethane acrylate was prepared from renewable castor oil, IPDI, and PETA and the molecule structure was confirmed by FTIR and 1H NMR. Bibulous rate decreased from 5.62% to 1.23%, and contact angle varied from 87.41° to 105.77°, which indicted all the samples guaranteed high hydrophobicity (≥87.41°). The thermal performance and glass transition temperature of the COPUA increased with the increasing reactive diluent content. At the same time, the samples were thermally stable below 350°C and the glass transition temperatures were in a range from 31.81 to 48.09°C. In addition, the system also had excellent tensile properties, indicating that the COPUA had formed a well-crosslinked system. CO appears to be a promising alternative raw material instead of petroleum-based polyol in the manufacture of polyurethane prepolymers. It has significant implications for the design of fully bio-based novel coating materials with desired properties.
References
Ren, Y, Pan, H, Li, L, Xia, J, Yang, Y, “Synthesis of Polyurethane Acrylate and Application to Ultraviolet-Curable Pressure-Sensitive Adhesive.” Polym. Plast. Technol. Eng., 45 495–502 (2006)
Li, K, Shen, Y, Fei, G, Wang, H, Li, J, “Preparation and Properties of Castor Oil/Pentaerythritol Triacrylate-Based UV Curable Waterborne.” Prog. Org. Coat., 78 146–154 (2015)
Wang, C, Chen, X, Chen, J, Liu, C, Xie, H, Cheng, R, “Synthesis and Characterization of Novel Polyurethane Acrylates Based on Soy Polyols.” J. Appl. Polym. Sci., 122 2449–2455 (2011)
Chen, G, Guan, X, Xu, R, Tian, J, He, M, Shen, W, Yang, J, “Synthesis and Characterization of UV-Curable Castor Oil-Based Polyfunctional Polyurethane Acrylate Via Photo-Click Chemistry and Isocyanate Polyurethane Reaction.” Prog. Org. Coat., 93 11–16 (2016)
Palanisamy, A, Rao, BS, “Photo-DSC and Dynamic Mechanical Studies on UV Curable Compositions Containing Diacrylate of Ricinoleic Acid Amide Derived from Castor Oil.” Prog. Org. Coat., 60 161–169 (2007)
Luo, A, Jiang, X, Lin, H, Yin, J, ““Thiol-ene” Photo-Cured Hybrid Materials Based on POSS and Renewable Vegetable Oil.” J. Mater. Chem., 21 12753–12760 (2011)
Su, X, Zhao, Q, Zhang, D, Dong, W, “Synthesis and Membrane Performance Characterization of Self-emulsified Waterborne Nitrocellulose Dispersion Modified with Castor Oil.” Appl. Surf. Sci., 356 610–614 (2015)
Tathe, DS, Jagtap, RN, “Biobased Reactive Diluent for UV-Curable Urethane Acrylate Oligomers for Wood Coating.” J. Coat. Technol. Res., 12 187–196 (2015)
Gao, Z, Peng, J, Zhong, T, Sun, J, Wang, X, Yue, C, “Biocompatible Elastomer of Waterborne Polyurethane Based on Castor Oil and Polyethylene Glycol with Cellulose Nanocrystals.” Carbohyd. Polym., 87 2068–2075 (2012)
Mohamed, HA, Badran, BM, Rabie, AM, Morsi, SMM, “Synthesis and Characterization of Aqueous (Polyurethane/Aromatic Polyamide Sulfone) Copolymer Dispersions from Castor Oil.” Prog. Org. Coat., 77 965–974 (2014)
Thakur, S, Karak, N, “Castor Oil-Based Hyperbranched Polyurethanes as Advanced Surface Coating Materials.” Prog. Org. Coat., 76 157–164 (2013)
Rao, BS, Palanisamy, A, “Photocuring and Thermomechanical Properties of Multifunctional Amide Acrylate Compositions Derived from Castor Oil.” Prog. Org. Coat., 67 6–11 (2010)
Xu, G, Shi, W, “Synthesis and Characterization of Hyperbranched Polyurethane Acrylates Used as UV Curable Oligomers for Coatings.” Prog. Org. Coat., 52 110–117 (2005)
Xu, Y, Dong, A, Zhao, Y, Zhang, T, Jiang, Z, Wang, S, Chen, H, “Synthesis, Characterization and Biomedical Properties of UV-Cured Polyurethane Acrylates Containing a Phosphorylcholine Structure.” J. Biomater. Sci., 23 2089–2104 (2012)
Deshmukh, PP, Mahanwar, PA, “Synthesis of Urethane Acrylate from PENTA Based Polyol and EB Curing with Varying Ratio of TMTPA.” Pigment Resin Technol., 41 284–295 (2012)
Gurunathan, T, Mohanty, S, Nayak, SK, “Isocyanate Terminated Castor Oil-Based Polyurethane Prepolymer: Synthesis and Characterization.” Prog. Org. Coat., 80 39–48 (2015)
Fang, C, Zhou, X, Yu, Q, Liu, S, Guo, D, Yu, R, Hu, J, “Synthesis and Characterization of Low Crystalline Waterborne Polyurethane for Potential Application in Water-Based Ink Binder.” Prog. Org. Coat., 77 61–67 (2014)
Liu, CG, Lei, W, Cai, ZC, Chen, JQ, Hu, LH, Dai, Y, “Use of Tung Oil as a Reactive Toughening Agent in Dicyclopentadiene-Terminated Unsaturated Polyester Resins.” Ind. Crop. Prod., 49 412–418 (2013)
Huang, YG, Pang, LX, Pang, LX, Zhong, R, Zeng, ZH, Yang, JW, “Synthesis and Properties of UV-Curable Tung Oil Based Resins Via Modification of Diels–Alder Reaction, Nonisocyanate Polyurethane and Acrylates.” Prog. Org. Coat., 76 654–661 (2013)
Mishra, AK, Mishra, RS, Narayan, R, Raju, KVSN, “Effect of Nano ZnO on the Phase Mixing of Polyurethane Hybrid Dispersions.” Prog. Org. Coat., 67 405–413 (2010)
Asif, A, Shi, WF, Shen, XF, Nie, KM, “Physical and Thermal Properties of UV Curable Waterborne Polyurethane Dispersions Incorporating Hyperbranched Aliphatic Polyester of Varying Generation Number.” Polymer, 46 (2005) 11066–11078 (1078)
Ahn, YU, Lee, SK, Lee, SK, Jeong, HM, Kim, BK, “High Performance UV Curable Polyurethane Dispersions by Incorporating Multifunctional Extender.” Prog. Org. Coat., 60 17–23 (2007)
Corcuera, MA, Rueda, L, Fernandez, B, Arbelaiz, A, Marieta, C, Mondragon, I, Eceiza, A, “Microstructure and Properties of Polyurethanes Derived from Castor Oil.” Polym. Degrad. Stab., 95 2175 (2010)
Lin, S, Huang, J, Chang, PR, Wei, S, Xu, Y, Zhang, Q, “Structure and Mechanical Properties of New Biomass-Based Nanocomposite: Castor Oil-Based Polyurethane Reinforced with Acetylated Cellulose Nanocrystal.” Carbohydr. Polym., 95 91–102 (2013)
Bai, CY, Zhang, XY, Dai, JB, Zhang, CY, “Water Resistance of the Membranes for UV Curable Waterborne Polyurethane Dispersions.” Prog. Org. Coat., 59 331–340 (2007)
Mülazim, Y, Akmakc, EC, Kahraman, MV, “Preparation of Superhydrophobic Antistatic Coatings from Branched Alternating Copolymers P (St-alt-MAn) and Carbon Nanotubes Based on Organic–Inorganic Hybrid Approach.” Prog. Org. Coat., 72 394–401 (2011)
Acknowledgments
This research was financially supported by Natural Science Foundation of Jiangsu Province (Grant No. BK20161122) and Major State Research & Development Program of China (Grant No. 2016YFD0600802).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Y., Liu, C., Shang, Q. et al. Synthesis and characterization of novel renewable castor oil-based UV-curable polyfunctional polyurethane acrylate. J Coat Technol Res 15, 77–85 (2018). https://doi.org/10.1007/s11998-017-9948-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-017-9948-z