Abstract
High-density, non-thermal, glow-discharge atmospheric-pressure plasma was used for graft polymerization of a vapor deposited fluorocarbon mixture of 1,1,2,2-tetrahydroperfluorodecyl acrylate and 1,1,2,2-tetrahydroperfluorododecyl acrylate on undyed cotton fabrics, which furnished a highly durable nanolayer water and oil repellent finish. In this study, monomer vapor was deposited onto cotton fabrics on single and double sides of the fabrics. The influence of monomer flow rate and plasma preactivation was studied. The surface of the cotton treated with fluorocarbons is evaluated using the standard AATCC Test Methods. Surface chemistry and morphology of the treated cotton were characterized using FTIR, XPS, SEM, and TOF–SIMS. Plasma-assisted graft polymerization of fluorocarbon in the presence of the crosslinker pentaerythritol triacrylate (10:1 molar ratio of monomer: crosslinker) resulted in a polyfluorocarbon nanolayer on cotton, which was hydrophobic and durable to one accelerated laundering, which is equivalent to 10 home launderings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plasma is the fourth state of the matter and is generated when electromagnetic energy is coupled to a process gas producing a neural medium composed of electrons, neutral, positive, and negative particles.1 The three major types of atmospheric pressure plasma are corona discharge, dielectric barrier discharge (DBD), and atmospheric pressure glow discharge (APGD).2 Plasmas generated by corona discharge are non-uniform and require narrow spacing between electrodes. The free electron density in corona discharge is ~108 electrons/cm3, and these types of plasmas have a relatively low power density of <0.1 W/cm3. Plasmas generated by DBD have electron density of 1010 electrons/cm3 and a power density of ~0.1 W/cm3. However, plasmas generated by glow discharge have the highest electron and power density of all at 1012 electrons/cm3 and >10 W/cm3, respectively. Moreover, plasmas generated by glow discharge are classified as non-thermal plasma because the temperature of the electrons is much higher than that of ions and neutral species. Because electrons are enormously light, they move much faster than the other species present and have no heat capacity.3 Atmospheric non-thermal high-density plasma produced by glow discharge is composed of a mixture of highly reactive species, e.g., ions, radicals, photons, electrons, and excited state species. The ratio of the number of electrons to the number of ions and neutrals mainly depends on the geometry of the radio frequency (RF) electrode, gas flow rate, power input (Watts), and frequency (MHz). Surface treatment of materials depends on all the aforementioned parameters and plasma exposure time.
Treatment of textile substrates with plasmas has until recently been limited to batch processes involving the use of vacuum plasma devices, limiting its applicability in textiles industry. Atmospheric pressure plasma devices, developed to overcome the shortcomings of vacuum plasma devices, save significant capital cost and provide significantly higher throughput for many treatment processes. The use of atmospheric pressure plasma to alter surface properties of materials including textile substrates has been increasing rapidly in recent years and attracting more commercial interests. The great benefit of using this plasma technique, especially non-thermal glow-discharge plasma, which is the type of plasma for the present work, is that only the topmost polymeric stratum of material is modified, causing homolytic bond fission and generating free radicals on the polymer surface by abstracting hydrogen free radicals1 while maintaining the bulk properties intact.4 Moreover, this plasma technique is an environmentally friendly process because it uses 90% less chemicals and requires no waste treatment.5,6
Plasma treatment of textile substrates can be divided into three main different paradigms. The first paradigm involves modifications of the surface properties of the material using only noble gases with no monomers or chemicals involved. The second paradigm is the deposition of a thin polymer film on the surface of the material with no chemical reaction between the polymer and the first few top nanolayers of the materials.7 The third paradigm is plasma-induced graft polymerization on the surface of the materials by either activating the surface of the materials prior to monomer deposition and/or plasma treatment after monomer deposition. It was reported that atmospheric pressure plasma treatments were employed in surface energy and chemical modifications, etching, and plasma polymerization.8–12 Furthermore, developed atmospheric pressure plasma systems have reached a level where they can be used to treat textiles continuously at atmospheric pressures and room temperature. These plasma systems have been used to produce chemical coatings,13–18 modify surface energies,19–26 and enhance desizing27–30 and dyeing properties.31
The main objective of this work was to achieve highly durable water and oil repellent finishes on cotton fabrics using plasma-induced graft polymerization. In the current study, THPFDA fluorocarbon monomer was used to achieve water and oil repellent cotton. The surface chemistry and topographical changes in cotton surface were studied using FTIR, SEM, XPS, and TOF–SIMS. The durability of cotton fabrics was evaluated following plasma treatment using the AATCC standard test methods.
Experimental
Materials and chemicals
A well-prepared undyed cotton twill fabric was used in this work. Fluorocarbon monomer THPFDA, a mixture of 1,1,2,2-tetrahydroperfluorodecyl acrylate (70–90%) and 1,1,2,2-tetrahydroperfluorododecyl (10–30%) acrylate, was used in this work (Fig. 1a). THPFDA was obtained from Daikin America as TG-10 and was used as received. Pentaerythritol triacrylate (PETA) was purchased from Sigma-Aldrich and used as received as a crosslinker.
Atmospheric pressure plasma jet system (APJeT)
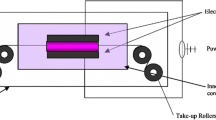
Plasma polymerization was carried out using the e-Rio™ Atmospheric Pressure Plasma System Model APPR-D300-13 (APJeT, Inc. Santa Fe, New Mexico) (Fig. 1b). It is a glow-discharge plasma reactor having dimensions of 33 cm by 12.5 cm powered by an RF (13.56 MHz) power supply and matching network. It can operate in in situ or downstream mode depending on the configuration of the electrodes. In the in situ mode of operation, the sample stage is grounded so that it becomes the ground electrode and forms parallel plate electrodes with the RF-powered electrode. In the downstream mode, the substrate is passed under the plasma volume at a certain distance that can be adjusted externally with the use of a synchronized adjustment screw. All plasma experiments in this study were carried out in the downstream mode. Applied plasma power of 675 W was used to generate helium plasma at a flow of 40 L/min. The distance between substrate surface and electrode was kept at 3 mm.
In the case of THPFDA, the liquid monomer was pumped into the evaporation chamber of the APPR where it was vaporized in an argon atmosphere at 165°C. The monomer vapors were then pushed with the aid of argon flow (0.6 L/min) into the 175°C applicator and allowed to condense on the room temperature fabric immediately prior to entering the plasma chamber. Unless otherwise stated, all fabric samples (12″ × 12″) were exposed to plasma for 30 s.
Undyed fabric one-sided treatment
THPFDA was vapor deposited, using a 1-mL/min flow rate, onto one side of the cotton fabric which then plasma treated for 30 s.
Undyed fabric double-sided treatment
THPFDA was vapor deposited using flow rates 1–5 ml/min, on both sides of the cotton fabric to provide different %SAO (solid add-on). Each side was then exposed to plasma for 30 s.
Test methods
The following AATCC Test Methods32 were used to evaluate the water and oil repellency of the treated fabrics: Test Method 118, “Oil Repellency: Hydrocarbon Resistance Test”; Test Method 193, “Aqueous Liquid Repellency: Water/Alcohol Solution Resistance Test”; and Test Method 61(2A conditions), “Colorfastness to Laundering, Home and Commercial: Accelerated.”
Film thickness measurements using field emission SEM
A cross-sectional sample was prepared by embedding the sample in Spurr’s epoxy and then sectioning with a Leica Ultracut microtome. The surface was then plasma etched using oxygen plasma for 7 min to give some surface relief in a Tegal Corporation Barrel Asher. A thin AuPd coating was sputtered on the surface to prevent charging. The sample was then observed in a JEOL 6400FESEM.
FTIR/ATR measurements
Attenuated Total Reflectance Fourier Transform Infrared spectral measurements were performed on a Nicolet Nexus 470 bench and OMNIC software Version 7.2 using an OMNI-ATR-Sampler with a germanium crystal. All spectra were collected using 32 scans and 4 cm−1 resolution.
XPS (X-ray photoelectron spectroscopy)
Surface chemical changes of the PET nonwovens were analyzed by a Perkin Elmer PHI 5400 XPS. The X-ray source was MgO (1253.6 eV) with a 45° take-off angle. The possible scanning area varied from 200 microns in diameter to 3–10 mm, and the scanning depth was about 1–10 nm. The references of XPS spectra were 285 eV for C1s, observed in hydrocarbon polymers.
Time-of-flight secondary ion mass spectrometry (TOF–SIMS)
TOF–SIMS analyses were conducted using a TOF–SIMS V (ION TOF, Inc. Chestnut Ridge, NY) instrument equipped with a Bi m+ n (n = 1 to 5, m = 1, 2) liquid metal ion gun. The instrument vacuum system consists of a load lock for sample loading and an analysis chamber, separated by the gate valve. The analysis chamber pressure is maintained below 5.0 × 10−9 mbar to avoid contamination of the surfaces to be analyzed. For the mass spectral images acquired in this study, a 256 by 256 pixel image of a 500 by 500 µm area was acquired using a Bi3 + primary ion beam. An electron gun was used to prevent charge buildup on the insulting sample surfaces. The total accumulated primary ion dose for an image acquisition was less than 1 × 1013 ions/cm2, an amount of ions which are within the static SIMS regime. The static SIMS condition dictates that less than 1% of the sample surface has been impacted by the primary ion beam during a given analysis ensuring that ejected secondary ions originate from an undamaged portion of the surface. Secondary ions were extracted into a TOF mass spectrometer with post acceleration to improve detection sensitivity. The combination of primary ion pulse width used and the TOF analyzer tuning combined to provide a mass resolution of approximately 100 m/Dm at 29AMU. The negative secondary ion mass spectra obtained were calibrated using C−, O−, OH−, and C − n .
Cross sections of the cotton fabric were prepared as follows. The cotton fabric was folded (threefold), mounted in a clamp constructed of Lego blocks, and cut with a new, cleaned razor blade. The cotton fabric was folded in a manner that would present the cross section of the fabric for TOF SIMS analysis with a top-bottom-bottom-top-top-bottom structure (T-B-B-T-T-B; Fig. 1c). 400 × 400 μm regions of the as acquired 500 × 500 μm TOF SIMS images are presented in the results section of this work.
Results and discussion
One-side treatment
Complete plasma-induced graft polymerization of THPFDA at a flow rate of 1 mL/min was achieved after plasma exposure for 30 s. It was also found that substrates exposed to plasma (10 s) prior to monomer deposition provided slightly higher oil and water repellency possibly due to free radicals already being generated on the fiber surface when the monomers were vapor deposited, which possibly led to more uniform and efficient graft polymerization. This was also consistent with the results obtained from graft polymerization of a mixture of THPFDA and pentaerythritol triacrylate (10:1 molar ratio). PETA was used to enhance the durability of the fluorocarbon finish.
This can be explained through a free-radical chain growth graft polymerization mechanism, which is initiated by free radicals from the plasma medium. The same free radical mechanism applies to both the monomers with and without crosslinker. The only difference is that in the presence of a crosslinker, a crosslinked network of fluorocarbon crosslinker is formed with itself and with the fabric, which enhanced the durability of the fluorocarbon on cotton.

It was shown from the ATR/FTIR analysis of the carbonyl peak, 1738.1 cm−1, of two samples, one with (Fig. 2a) and one without surface preactivation (Fig. 2b), that the degree of polymerization of the sample with surface preactivation is much greater than that the sample without surface preactivation, which was inferred from the area under the peak of the carbonyl peak, 59.8 for the sample with surface preactivation versus 17 for the sample without surface preactivation. Moreover, FTIR/ATR analysis showed the disappearance of the vinyl group of THPFDA (1637 cm−1), which substantiates complete polymerization of the monomer. Both Figs. 2a and 2b show only the peak corresponding to the carbonyl of the ester group with no signs of the vinyl group.
(a) FTIR spectrum of sample AA zooming in at the peak corresponding to the carbonyl of the ester group (area under the peak = 59.8). The rest of the spectrum was omitted for clarity. (b) FTIR spectrum of sample AB zooming in at the peak corresponding to the carbonyl of the ester group (area under the peak = 17)
Table 1 shows results of the AATCC 118 and 193 tests performed on cotton treated with the above mixture with and without surface preactivation. The data show clearly that the AATCC 118 and 193 ratings were higher for the sample that had surface preactivation. Consistently, SEM of AA, samples pretreated with plasma prior to monomer deposition, and AB samples, no plasma pretreatment, (Figs. 3a–c) showed clearly that the surface of the nanolayer in AA is much smoother and pores free than that of AB, which confirms that surface preactivation with plasma conferred better and uniform graft polymerization. Figure 4 shows a uniform graft polymerized nanolayer (171.2 nm) generated using 1 mL/min THPFDA flow rate, measured using field emission SEM.
Although cotton was plasma treated on one side at 1 mL flow rate of THPFDA, XPS analyses were performed on both sides of the fabric to determine whether plasma-induced graft polymerization of THPFDA propagated from one side of the fabric all the way through to the other side. Figure 5a shows the XPS analysis for the front side of cotton showing F at 54%. On the other hand, Fig. 5b shows the XPS analysis for the back side showing F at 30%. Hence, one-side treatment was enough to render both sides hydrophobic, with one slightly more hydrophobic than the other.
Moreover, TOF–SIMS was performed on both sides of the treated cotton sample to determine F− distributions. Figure 6a shows an optical image of the sample (left) showing the orientation of the fabric in the TOF SIMS and F− image (right). The brighter area corresponds to higher F− secondary ion intensity and so higher F content, see color bar secondary ion intensity scale to the right of image; max pixel intensity = 137. The TOF-SIMS image of F− (m/z 19) clearly shows the spatial distribution of F− on the cross section of the fluorocarbon coated cotton fabric. Higher F content is profound on the top of the cotton fabric.
Figure 6b shows an overlay image of F− (green, m/z 19) on cotton (C3H2O -2 , m/z 71) fragment of cotton (red), which shows clearly that F− is more prevalent on the top of the treated cotton than the bottom.
Double-sided cotton treatment with THPFDA
THPFDA monomer was vapor deposited at different flow rates (1–5 ml/min) to furnish different %SAO, consecutively to both sides of cotton fabric followed by a 30-s helium plasma exposure on each side of the fabric to graft polymerize the repellent finish (THPFDA). The durability of the repellent finish was evaluated by subjecting the treated fabric to two cycles of an accelerated laundry procedure (AATCC Test Method 61-2A); each cycle of the accelerated procedure is equivalent to five conventional home laundry cycles. The repellency of the finish in each case was determined with AATCC Test Method 193 on a scale of 0–8 with 8 the highest water/alcohol repellency, and the results are given in Table 2. It is clear from the results shown in Table 2 that after 10 home laundry cycles, good durability plasma-induced graft polymerization of THPFDA on cotton was achieved using as low as 5.8% SAO of THPFDA. Although the performance and durability of plasma-induced graft polymerization of THPFDA on cotton was found to be comparable to conventional pad-dry-cure repellent finish, the durability of fluorocarbon finishes was only achieved when double-sided treatment was used. One possible explanation is that with the one-side plasma treatment, the other side of the cotton fabric is open for water molecules to infiltrate the cellulose structure, forming new hydrogen bonds with the hydroxyl groups of polymer chains, and causing chain sliding, which could have eventually led to breaking the bonding between the fluorocarbon finish and topmost polymeric stratum of materials. On the other hand, with the double-sided fluorocarbon treatment at 5.8% SAO, there was enough monomer on both sides and within the fabric structure for free radicals of fluorocarbon polymer chains to propagate from the side and reach out to the other side so that a polymer network was formed, which made the fluorocarbon finish durable. Figure 7 shows the proposed mechanism of chain sliding when H2O molecules infiltrate cellulose chains breaking existing intermolecular hydrogen bonds and new hydrogen bonds are formed between polymer chains followed drying. Figure 8 shows a schematic representation of the plasma-induced graft polymerization process sequence for THPFDA on cotton.
Conclusions
Plasma-induced graft polymerization of THPFDA on cotton was achieved with high durability, which is unprecedented. Hence, this plasma approach can be a viable alternate to conventional repellent finishing. It was demonstrated that the degree of polymerization of the sample with surface preactivation is much greater than the sample without surface preactivation. It was found using XPS that one-side treatment was enough to render both sides hydrophobic, with one side slightly more hydrophobic than the other. TOF-SIMS analysis confirms the presence of higher F content on the top of the plasma grafted cotton fabric. PETA crosslinker addition into the monomer mixture was found to enhance the durability of the fluorocarbon finish. Additional work is needed to demonstrate commercial feasibility.
Abbreviations
- DBD:
-
Dielectric barrier discharge
- APGD:
-
Atmospheric pressure glow discharge
- RF:
-
Radio frequency
- THPFDA:
-
Tetrahydroperfluorodecyl acrylate and tetrahydroperfluorododecyl acrylate
- APPR:
-
Atmospheric pressure plasma reactor
- FTIR:
-
Fourier transform infrared spectrometer
- XPS:
-
X-ray photoelectron spectroscope
- TOF–SIMS:
-
Time-of-flight secondary ion mass spectrometry
- SEM:
-
Scanning electron microscope
- AATCC:
-
American Association of Textile Chemists and Colorists
- SAO:
-
Solid add-on
References
Baddour, RF, Timmins, RS, The Application of Plasmas to Chemical Processing. The MIT Press, Cambridge (1967)
Shishoo, R, “The potential of plasma technology in the textile industry.” In: Shishoo, R (ed.) Plasma Technologies for Textiles, pp. xv–xxx. Woodhead Publishing Limited and CRC Press LLC, Cambridge and NW (2007)
Selwyn, GS, Herrmann, HW, Park, J, Henins, I, “Materials Processing Using an Atmospheric Pressure, RF-Generated Plasma Source.” Contrib. Plasma Phys., 41 (6) 610–619 (2001)
Poll, HU, Schladitz, U, Schreiter, S, “Penetration of Plasma Effects Into Textile Structures.” Surf. Coat. Technol., 142–144 489–493 (2001)
Yip, J, Chan, K, Sin, KM, Lau, KS, “Low Temperature Plasma-Treated Nylon Fabrics.” J. Mater. Process. Technol., 123 (1) 5–12 (2002)
Lieberman, Michael A, Lichtenberg, AJ, Principles of Plasma Discharges and Materials Processing. Wiley, New York (1994)
Vohrer, U, Müller, M, Oehr, C, “Glow-Discharge Treatment for the Modification of Textiles.” Surf. Coat. Technol, 98 1128–1131 (1998)
Hwang, YJ, Qiu, Y, Zhang, C, Jarrard, B, Stedeford, R, Tsai, J, Park, YC, McCord, MJ Adhes, “Effects of Atmospheric Pressure Helium/Air Plasma Treatment on Adhesion and Mechanical Properties of Aramid Fibers.” Sci. Technol., 17 847–860 (2003)
Janca, J, Stahel, P, Buchta, J, Subedi, D, Krcma, F, Pryckova, J, “A Plasma Surface Treatment of Polyester Textile Fabrics Used for Reinforcement of Car Tires.” Plasmas Polym., 6 (1–2) 15–26 (2001)
Babayan, SE, Jeong, JY, Schütze, A, Tu, VJ, Maryam, M, Selwyn, GS, Hicks, RF, “Deposition of Silicon Dioxide Films with a Non-Equilibrium Atmospheric-Pressure Plasma Jet.” Plasma Sources Sci. Technol., 10 (4) 573 (2001)
Qiu, Y, Zhang, C, Hwang, YJ, Bures, BL, McCord, M, “The Effect of Atmospheric Pressure Helium Plasma Treatment on the Surface and Mechanical Properties of Ultrahigh-Modulus Polyethylene Fibers.” J. Adhes. Sci. Technol., 16 (1) 99–107 (2002)
Goossens, O, Dekempeneer, E, Vangeneugden, D, Van de Leest, R, Leys, C, “Application of Atmospheric Pressure Dielectric Barrier Discharges in Deposition, Cleaning and Activation.” Surf. Coat. Technol., 142–144 474–481 (2001)
Ward, LJ, Badyal, JPS, Goodwin, AJ, Merlin, PJ, “Solventless Coupling of Perfluoroalkylchlorosilanes to Atmospheric Plasma Activated Polymer Surfaces.” Polymer, 46 (12) 3986–3991 (2005)
Herbert, T, “Atmospheric Pressure Plasma Liquid Deposition.” Int. Dyer, 191 (5) 12–13 (2006)
Gawish, SM, Matthews, SR, Wafa, DM, Breidt, F, Bourham, MA, “Atmospheric Plasma-Aided Biocidal Finishes for Nonwoven Polypropylene Fabrics. I. Synthesis and Characterization.” J. Appl. Polym. Sci., 103 (3) 1900–1910 (2007)
Wafa, DM, Breidt, F, Gawish, SM, Matthews, SR, Donohue, KV, Roe, RM, Bourham, MA, “Atmospheric Plasma-Aided Biocidal Finishes for Nonwoven Polypropylene Fabrics. II. Functionality of Synthesized Fabrics.” J. Appl. Polym. Sci., 103 (3) 1911–1917 (2007)
Wang, X, McCord, MG, “Grafting of Poly(N-Isopropylacrylamide) Onto Nylon and Polystyrene Surfaces by Atmospheric Plasma Treatment Followed with Free Radical Graft Copolymerization.” J. Appl. Polym. Sci., 104 (6) 3614–3621 (2007)
Hove, TV, “Depositing Nano-sized Coatings by Means of AS Coating Star as an Aerosol Assisted Large Area Cold Atmospheric Plasma Technology.” UNITEX, 15 (4) 38–47 (2007)
Tomiji Wakida, ST, Niu, Shouhua, Kawamura, Haruo, Sato, Yukihiro, Lee, Muncheul, Uchiyama, Hiroshi, Inagaki, Hideo, “Surface Characteristics of Wool and Poly(Ethylene Terephthalate) Fabrics and Film Treated with Low-Temperature Plasma Under Atmospheric Pressure.” Textile Res. J., 63 433–438 (1993)
Hartmann, U, “Pretreatment with Atmospheric Plasma.” Flock, 28 (4) 6–7 (2002)
Matthews, SR, Hwang, YJ, McCord, MG, Bourham, MA, “Investigation into Etching Mechanism of Polyethylene Terephthalate (PET) Films Treated in Helium and Oxygenated-Helium Atmospheric Plasmas.” J. Appl. Polym. Sci., 94 (6) 2383–2389 (2004)
Leroux, F, “Atmospheric Plasma: An Issue for Textiles.” Ind. Textile, 1372 46–47 (2005)
Samanta, K, Jassal, M, Agrawal, AK, “Atmospheric Pressure Glow Discharge Plasma and its Applications in Textile.” Indian J. Fibre Textile Res, 31 (1) 83–88 (2006)
Swedberg, J, “Don’t Forget your Coating.” Ind. Fabric Prod. Rev., 91 (6) 54 (2006)
Wolf, RA, Mullertz, C, “Nonwoven Surface Treatment Through Atmospheric Plasma and Photografting.” Nonwovens World, 15 (4) 41–44 (2006)
Wang, XCQ, “Effect of Atmospheric Pressure Plasma Jet Treatment on Wettability of Two Sides of Wool Fabric.” Wool Textile J., 1 23–27 (2007)
Cai, ZS, Hwang, YJ, Park, YC, Zhang, CY, McCord, M, Qiu, YP, “Preliminary Investigation of Atmospheric Pressure Plasma-Aided Desizing for Cotton Fabrics.” AATCC Rev., 2 (12) 18–21 (2002)
Cai, Z, Qiu, Y, Hwang, YJ, Zhang, C, McCord, M, “The Use of Atmospheric Pressure Plasma Treatment in Desizing PVA on Viscose Fabrics.” J. Ind. Textile., 32 (3) 223–232 (2003)
Cai, Zaisheng, Qiu, Yiping, Zhang, Chuyang, Hwang, YJ, Mccord, M, “Effect of Atmospheric Plasma Treatment on Desizing of PVA on Cotton.” Textile Res. J., 73 (8) 670–674 (2003)
Cai, Z, Qiu, Y, “The Mechanism of Air/Oxygen/Helium Atmospheric Plasma Action on PVA.” J. Appl. Polym. Sci., 99 (5) 2233–2237 (2006)
Wakida, T, Tokino, S, Niu, Shouhua, Lee, M, Uchiyama, H, Kaneko, M, “Dyeing Properties of Wool Treated with Low-Temperature Plasma Under Atmospheric Pressure.” Textile Res. J., 63 (8) 438–442 (1993)
AATCC Technical Manual, American Association of Textile Chemists and Colorists. Research Triangle Park, NC, USA (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Shafei, A., Helmy, H., Ramamoorthy, A. et al. Nanolayer atmospheric pressure plasma graft polymerization of durable repellent finishes on cotton. J Coat Technol Res 12, 681–691 (2015). https://doi.org/10.1007/s11998-015-9665-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-015-9665-4