Abstract
The alteration in human micro-flora results in an increase in the population of the pathogenic bacteria, which further gives rise to the gastrointestinal diseases and disorders. To this extent, the supplementation of food products with probiotics may eliminate the pathogenic microbiota from the adhesion sites and regulate the immune response via the stimulation of the specific genes within the human’s gastrointestinal tract (GIT). Nonetheless, due to the sensitivity of probiotics to the environmental conditions during food manufacture/storage, it is a challenge to develop probiotic products with a desirable shelf life that maintain the viability of the probiotic cells. The spray drying of bacteria is a sustainable process and enables bulk production with lower energy costs. This is also a promising way to encapsulate bacteria within various protective matrices to ensure their improved resistance during storage, technological processes, and digestive stresses. This review assembles and summarizes the scientific data on various aspects of probiotic bacteria encapsulated using conventional spray drying and incorporated into different functional food products, as well as the aspects of safety, toxicity, and regulations of adding encapsulated probiotics into functional foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The word “probiotic” comes from the Greek word “προ-βίος” meaning “for life”. Probiotics are defined as “live microorganisms which when administered in adequate amounts, confer health benefits to the host” (FAO & WHO, 2014). To achieve a healthy lifestyle, an interest in employing functional foods containing bioactive ingredients producing many health-promoting properties has been emerged in recent years. Functional foods are defined as “foods that contribute to the management of diseases or reduce the risk of their development in addition to providing essential nutrients” (Salmeron, 2017). Functional foods market comprises of the largest segment of probiotics, prebiotics, and synbiotics, further improving gut health, with probiotics alone covering about 65% of the world’s functional foods market (Spigno et al., 2015). Normally, the intestinal flora is in a constant state of flux and balance, but such a balance can be disturbed by factors including food ingredients, alcohol, antibiotics, stress, aging, and digestive disorders (Amara & Shibl, 2015). Some methods have been developed for the delivery of probiotics into the gastrointestinal tract (GIT) such as pharmaceutical supplements and food-based products. To maintain the viability and functionality of probiotics into food products, they should be able to colonize within the gut and to survive the environmental conditions of the upper GIT (Mattila-Sandholm et al., 2002). Food-based probiotic products are divided into two categories: dairy products (e.g. cheese, yogurt, ice cream, milk, acidified milk, cream), and non-dairy products (e.g. meats and meat products, bread, chocolate, fruit juices, etc.) (Trabelsi et al., 2019).
Probiotics provide beneficial effects on the health of both humans and animals. They do this by improving the intestinal health, enhancing the immune response, reducing the serum cholesterol, preventing various types of cancer, and production of many other health-promoting agents (Kerry et al., 2018; Tarrah et al., 2018). Various factors influence the viability of probiotics in food products during processing and storage; and these include pH, titratable acidity, oxygen, water activity, presence of salt, sugar, hydrogen peroxide, bacteriocins, artificial flavouring and colouring agents. Fermentation parameters (e.g. incubation temperature, heat treatment, cooling and storage conditions of the product, packing materials, production scale) and microbiological factors (e.g. probiotic strain used, rate and proportion of inoculation) are among the processing parameters (Putta et al., 2018). The health benefits of some probiotic strains have been demonstrated for the genera: Bacillus, Enterococcus, Lactobacillus, Saccharomyces, Leuconostoc, Streptococcus and Pediococcus (Fijan, 2014). Some of the numerous health benefits of probiotics (from in vitro and in vivo studies) include the improvement in lactose intolerance condition and other GIT disorders/diseases, suppression of various types of cancer, the management of cardiovascular diseases, type II diabetes, obesity, allergy, inflammatory bowel diseases, and irritable bowel syndrome (Markowiak & Śliżewska, 2017; Kumar et al., 2013).
For exerting health benefits, probiotics need to overcome the main natural barrier of the GIT (e.g. low pH and bile salts) and maintain their viability throughout the gut (Gerez et al., 2012). According to WHO, probiotic foods must comply with some requirements, such as containing at least 106 microorganisms per gram or mL when consumed, supplying an antimicrobial effect, adhesion capacity, being non-pathogenic and allowing their survival throughout the GIT, tolerating the stomach’s low pH and the high bile salts conditions in the intestine (Liu et al., 2015). Even at the time of storage, the bacteria are undesirably affected by water activity, temperature, moisture content, light, and oxygen (Pandey & Vakil, 2017). Generally, the number of live microorganisms that can reach the intestine is too low (to exert their action after their ingestion), and thus, it is very important to protect them (Mokhtari et al., 2017a, b). In order to increase the number of foods containing live probiotic, it is inevitable to increase the viability of these microorganisms using some protecting techniques such as encapsulation (Arslan-Tontul & Erbas, 2017; Rokka & Rantamäki, 2010). Encapsulation assists in achieving high cell densities, improves substrate concentration, and acts as a barrier against the release of entrapped cells. Various food grade materials that are used to encapsulate probiotics are proteins, lipids, gums, maltodextrin, carbohydrates, skimmed milk, fructo-oligosaccharides, and inulin.
Although there are many studies on the encapsulation of probiotics, there are limited review publications covering the application of final encapsulated ingredients in real food products; particularly for spray dried probiotics as a popular technique of encapsulation. As per literature, spray drying is one of the most popular, suitable, fast, and cost-effective techniques to produce powders (solid microparticles) from starting liquid raw materials. This has resulted in application of spray drying as one of the promising processes to produce dry probiotic formulations, as well as a strategy to protect and improve the viability of probiotic cells within GIT (Azam et al., 2020; Bhagwat et al., 2020). Moreover, spray drying is one of the most efficient encapsulation methods that can be used for the delivery of sensitive organisms such as probiotic bacteria. Encapsulation refers to a process where bioactive ingredients or cells are surrounded/encapsulated by a protective continuous film of wall materials (Lai et al., 2021). This review aimed to compile the available information and recent publications related to the spray drying encapsulation of the probiotics and the incorporation of such encapsulated live organisms into different functional food products.
Challenges Towards Probiotics Delivery and the Role of Encapsulation
The viability and bioactivity of probiotics decreases substantially during food processing, storage, and consumption, as well as during the passage through the GIT due to different stress conditions, heat treatment, exposure to oxygen, mechanical processing, temperature fluctuations, the physical state of the food, and the chemical micro-environment (Mokhtari et al., 2019). To achieve the highest beneficial effects, probiotics must survive throughout the GIT and retain their bioactivity at their target site (colon). However, several probiotics are incapable of delivering their targeted beneficial effects to the host for several reasons such as the presence of gastric acids and intestinal juices (Rokka & Rantamäki, 2010). Additionally, the adverse conditions such as high temperature and high/low humidity may elicit a sub-lethal effect on the microorganisms including probiotics (Saarela et al., 2000). In particular, the survivability of probiotics in food products is affected by a range of factors including pH, post-acidification (during storage) of the fermented products, hydrogen peroxide production, oxygen toxicity (oxygen permeation through packaging), storage temperatures, stability in dried or frozen forms, poor growth in some food matrices such as milk and dairy products, lack of proteases to break down milk proteins to the simpler nitrogenous substances, and compatibility with the traditional starter cultures during the fermentation. Importantly, oxygen plays a major role in the poor survival of probiotic bacteria (Kailasapathy, 2002). Therefore, after ingestion, the number of live microorganisms reaching the gut is too low to exert their action, making probiotic cell protection a necessity before their incorporation into functional foods (Pinto et al., 2015).
To this extent, microencapsulation is the most promising technique applied for enhancing the bacterial viability, since it protects the bacteria during all phases of food preparation, storage, and digestion. Encapsulation is defined as the technology for packaging solids, liquid, or gaseous materials (the so-called core) in an inert shell, capsules, that can release their contents in a controlled rate under specific conditions (Mahdavi et al., 2016; Sarabandi et al., 2020). Various encapsulation technologies have been developed for the protection and delivery of probiotics, including extrusion, emulsification, coacervation, spray drying, and freeze drying. In addition to maintaining the viability of probiotic cells, microencapsulation technology is a promising solution for the problems related to the deterioration of flavour and aroma of fermented foods during storage due to the bioactivity of living probiotic cells (Liao et al., 2017). For example, acetic acid produced by Bifidobacterium spp. during the fermentation period, gives an off-flavour (known as vinegar taint) to the fermented probiotic products such as yogurt. Microencapsulation of Bifidobacteria has been used to overcome this problem. Adhikari et al. (2000) reported that the amount of the produced acetic acid in yogurt generated by the encapsulated Bifidobacteria was considerably lower than that produced by non-encapsulated bacteria; hence, it improved the flavour properties of the fermented product. It has also been reported that the addition of microencapsulated probiotics such as L. casei, B. bifidum, L. acidophilus, and B. lactis had no significant effects on the sensorial properties of the products such as non-fermented ice cream, cream‐filled cakes, dry sausages, mayonnaises, cheese, yogurt, fermented liquid porridges (mahewu), and fruit juices (Kokott, 2004; Kailasapathy, 2006; Muthukumarasamy & Holley, 2006; Fahimdanesh et al., 2012; Zanjani et al., 2012; Homayouni et al., 2008; Krasaekoopt & Kitsawad, 2010; Ningtyas et al., 2019).
The assortment of the encapsulation method is administered by certain variables, such as the preferred size of the microparticles, processing cost, type of food in which probiotics are incorporated and release mechanisms of microparticles in food and the GIT, physicochemical properties of the core, and wall materials. Spray drying is a common and popular method for encapsulation of probiotics (Assadpour & Jafari, 2019), which will be discussed briefly in the following section.
Spray Drying Encapsulation of Probiotics; an Overview
Spray drying is an economical, conventional, and flexible method for drying liquid foods; however, there is a trend to use this technique for microencapsulation of probiotic cultures. Based on several studies, the survival of probiotic cultures during spray drying depends on many factors such as the species and strain of the probiotics used, the drying parameters (outlet air temperature, type of atomization), the drying method (hot air drying, freeze drying, spray drying, and vacuum drying), and growth medium (temperature, sugar substrates, moisture content, oxygen content/redox potential, pH) (Fu et al., 2018).
Advantages of Spray Drying for Encapsulation of Probiotics
As shown schematically in Fig. 1, spray drying involves atomization of an aqueous or oily suspension of probiotic microorganisms and wall material(s) into a drying chamber, resulting in the rapid evaporation of water (Rokka & Rantamäki, 2010). To atomize the liquid systems such as solutions, dispersions, and emulsions, different atomization devices with pressure nozzles and rotary atomizers are used (Romano et al., 2018; Rokka & Rantamäki, 2010). Air inlet and outlet temperatures, product feed, and gas flow rate are some of the parameters which control the atomization process.
Spray drying provides small capsules with average diameters of < 100 µm at comparably low costs and among various encapsulation methods for bioactive live organisms, which facilitates a greater contact surface for the nutrient’s availability. Spray drying is widely used in the food industry because of its short processing/drying time, low water activity (aw), low energy consumption, simplifying transport, flexibility, high process yield, easy storage, homogeneous distribution throughout the product and favourable applications in the development of functional foods (Assadpour & Jafari, 2019). It is highly suitable for heat sensitive compounds as total drying time is few milliseconds to few seconds (Avila-Reyes et al., 2014). Despite thermal inactivation of microorganisms, spray drying encapsulation technology has many advantages. The amount of energy used during the process is 6–10 times lower than is used during freeze drying. Spray drying is also 30–50 times cheaper (Martin et al., 2015; Šipailienė & Petraitytė, 2018). This technique of encapsulation is easy-to-scale up and can be combined with other methods such as extrusion, freeze drying, emulsification, and fluidized bed drying (Alves et al., 2016; Martín et al., 2015; Pinto et al., 2015). Table 1 shows a summary of the research related to spray drying encapsulation of probiotics.
Wall Materials Used for Spray Drying Encapsulation of Probiotics
Polysaccharides and proteins are widely used to prepare carriers/delivery systems, playing a pivotal role in their structure and stability (Choudhury et al., 2021). The wall materials in the spray dried particles can give the probiotic cells adequate protection during food processing and passage through the GIT (Arslan-Tontul & Erbaas, 2017). Generally, for the spray drying technique, water-soluble polymers such as modified starches, whey proteins, maltodextrin, β-cyclodextrin, and gum Arabic are used as the wall/coating material. Among them, gum Arabic is the most commonly used ingredient (Gharsallaoui et al., 2007). Maltodextrin (MD) can replace water in bacterial membranes, thus maintaining their structural and functional integrity (Akanny et al., 2020).
The major chemical group in the structure of gum Arabic (GA) is a highly-branched polysaccharide consisting of a galactose backbone with linked branches of arabinose and rhamnose (Hosseini et al., 2015). GA prevents the complete dehydration of cell components and stabilizes bacterial cells during drying and storage (Arepally & Goswami, 2019). Lactose, milk proteins, and reconstituted skimmed milk were reported as the effective matrices for the protection of the cellular structure, cell viability, and functions of probiotics during spray drying encapsulation (Huang et al., 2017). Soy protein isolate, whey protein isolate, and casein have also been used for the encapsulation of probiotics by spray drying (Khem et al., 2016a, b; Liu et al., 2018). These protein matrices, with a high glass transition temperature, slow down the degradation of the cells by retarding the molecular mobility in the cytoplasm. In the case of polymers that are commonly used as wall materials for encapsulation, the glassy state corresponds to a rigid solid, while the supercooled state is observed to be of a rubbery or viscoelastic nature for low molecular weight materials. It is important to note the details of dextrose equivalence (DE) for maltodextrin provided by the manufacturers, since lower DE corresponds to a higher molecular weight, and hence, higher glass transition temperature, which is favourable (Seimons et al., 2020).
Stability of Spray Dried Probiotics
During the storage, the oxidation and subsequent saturation of membrane lipids exert a negative impact on the cell viability of probiotics. The peroxidation of lipids leads to a decrease in the ratio of unsaturated to saturated fatty acids in the membrane lipids of lactic acid bacteria, which is caused by reactive oxygen species (ROS). Furthermore, the products of lipid peroxidation have been shown to induce damage to the bacterial cell wall, cell membrane, and DNA during storage. This oxidative damage during spray drying can be efficiently lowered by the addition of antioxidants (Rodklongtan & Chitprasert, 2017). For example, ascorbic acid can function as a biological antioxidant in living cells through electron donation to quench radicals (Cuddihy et al., 2008). The addition of ascorbic acid as an antioxidant to skim milk for spray drying of Lactococcus lactis subsp. cremoris ASCC930119 has resulted in > 10% higher viability (Ghandi et al., 2012). In another report (Huang et al., 2017), the addition of ascorbic acid and monosodium glutamate protected Lactobacillus bulgaricus cells, but only during the storage at 4 °C. At 20 °C, the death rate of the culture was even higher in the presence of these compounds than in the control sample (Huang et al., 2017). This could be explained by the pro-oxidant properties of ascorbic acid as a metal ions reducer, in addition to its antioxidant function as a radical scavenger.
Rodklongtan and Chitprasert (2017) investigated the combined effects of holy basil essential oil (HBEO) and inlet temperature on the lipid peroxidation and survival of Lactobacillus reuteri KUBAC5 during spray drying. The addition of HEBO resulted in an encapsulation efficiency of 42.52–47.73% in skim milk at the inlet temperature of 130 °C and HBEO concentration of 6 mg/mL. The incorporation of HEBO resulted in the reduction of lipid peroxidation, thermal stress, oxidative stress, and cell death during spray drying. This is because the stability of the spray dried microorganisms is increased due to the presence of carbohydrates in the dehydration media which increases their capacity to create amorphous structures that inhibits or slows down the deterioration processes (Romano et al., 2018). Zhang et al. (2016) investigated the effects of different parameters such as heat adaptation, media, and outlet temperature on the viability of Lactobacillus salivarius NRRL B-30514. An improvement in the viability of this organism was observed by the addition of drying media (sucrose, lactose, and trehalose) followed by a higher mortality rate with the increase of outlet temperature. In vitro studies have described that probiotics can be protected from the stress in the course of digestion using a suitable spray drying medium. Páez et al. (2013) conducted an in vivo study using spray dried Lactobacillus paracasei A13, Lactobacillus acidophilus A9, and Lactobacillus casei with 20% (w/v) skimmed milk. The administration of spray dried probiotic powders to mice for 5 and 10 days showed a significant proliferation in the number of Immunoglobulin A (IgA)-producing cells in the small intestine, when compared with the non-encapsulated probiotic cultures.
Challenges Toward Spray Drying Encapsulation of Probiotics
Despite the advantages of the spray drying technique, the viability of probiotics and their activity in the final product could be reduced by the temperature conditions of the spray drying process. In this regard, to retain the activity and viability of probiotics, the maximum and minimum air inlet temperature reported in the literature is 170 and 100 °C, respectively, while the air outlet temperature varies between 105 and 45 °C (Gbassi & Vandamme, 2012). Besides the progressive effects on probiotic survival, there are several factors that contribute to the loss of probiotic viability during spray drying and storage.
These include airflow rate, dehydration, inlet and outlet drying temperature conditions, the concentration of the probiotics in the suspension, the carrier materials used in the process, storage temperature, heat and dehydration stresses, osmotic and oxidative stress and packaging conditions (Broeckx et al., 2017; Sosnik & Seremeta, 2015). However, the level of inactivation and mechanism also depends upon the strain/species, growth conditions, or medium and stage of growth. Drying and heat stresses are mainly responsible for the decrease in viability of the probiotics (Huang et al., 2017).
At higher temperatures during spray drying, the probiotic cells get damaged and formation of cellular pores and leakage of the intracellular substances occur (Anekella & Orsat, 2013). Spray drying at higher outlet temperatures induce greater viability losses due to more dehydration resulting from exposure of micro-particles to higher temperatures (Peighambardoust et al., 2011; Pispan et al., 2013). It has been suggested that the viability of bacteria during spray drying is inversely proportional to the outlet temperature and not directly related to the inlet temperature of the dryer (Ranadheera et al., 2015). This can be explained by the theory that any increase of outlet temperature directly increases the temperature that the droplets are subjected to; moreover, the time needed to decrease the outlet air temperature prolongs the drying duration (Arslan et al., 2015). In addition, high-temperature also results in phase change and stress, which damages proteins and cell membranes. So, using thermos-protectants like trehalose has been reported to reduce the thermal stress, and further viability of cells can be enhanced by adding prebiotics, granular starch, and soluble fibres (such as inulin, gum acacia, and polydextrose). Simultaneously, the rate of survival of probiotics during storage or drying operation after incorporation of fibres, coating, etc., is also dependent upon the strain and operating conditions while spray drying (Ermis, 2021). Generally, water-soluble coating materials are used in spray drying. Several protectants are added to media containing probiotics since the stress induced by high-temperature drying may decline the viability of the cells (Ziaee et al., 2019).
Adoption of suitable protectants during spray drying conferred protection to the encapsulated cells (Khem et al., 2016a, b), which can be attributed to mechanisms of enhancing the intrinsic stress tolerance of probiotic cells, providing extracellular protection on cells as physical shield and having favourable drying kinetics (Zheng et al., 2016). Additional thermo-protection can be achieved through the addition of free radical scavengers or by reducing water mobility through the cell membranes and the cell wall, thus modulating dehydration upon heating (Ying et al., 2012). On the other hand, lowering of inlet temperature results in higher post-encapsulation viability but greater moisture and water activity which adversely affects the prolonged storage. However, the thermal and osmotic damage to the probiotic cells should be minimized by carefully optimizing operating conditions and the composition carrier media (Behboudi-Jobbehdar et al., 2013). In addition to this, utilization of optimized lab conditions or pilot scale to industrial scale is challenging. The primary reason for this is the shorter residence time of the lab-scale dryer due to the small chamber height; whereas, on an industrial scale, the drying chamber height is more prominent (Ziaee et al., 2019). Moreover, the method of atomization also varies from lab-scale to industrial scale. Usually, industrial-scale uses either pressure nozzle or rotary disk, whereas, at lab scale, two-fluid nozzles for atomization are used (Broeckx et al., 2016). These parameters affect the drying droplet size and time of spray drying, due to which variation in moisture content and temperature were observed that affects the inactivation mechanism of probiotics.
To overcome these hurdles and correlate the conditions, Siemons et al. (2021) suggested that single droplet drying be used to know the insight of the process. It was difficult to quantify in situ probiotic inactivation as well as changes in bacterial cells. Hence, it is beneficial to use single droplet drying under representative drying conditions. Experiments for single droplet drying can be performed differently by suspending the droplet in conditioned and moving air to achieve the closest spray drying experimental resemblance. Nedovic et al. (2011) stated that around 80 to 90% of the industrial encapsulates are produced using spray drying technology. On the other hand, the limitation of this technique is that it requires a high initial investment due to the high cost of its auxiliary parts like atomizer, etc. Moreover, controlling the particle size of the spray-dried powder is very challenging. Additionally, uneven drying might happen due to the drying chamber’s variable temperature zones (Arslan-Tontul & Erbas, 2017). Because spray drying produces powders with particle sizes in the micrometre range (apart from nano-spray drying, which is not used for encapsulation of probiotics due to their large size), the particles would have a smoother mouthfeel than microbeads and so, allowing the encapsulated probiotics to be added to a wider range of food products (Yonekura et al., 2014). Considerations should be given to pre-drying, and post-drying stages during spray drying for retaining the viability of encapsulated probiotics at recommended levels (Huang et al., 2017).
Application of Spray Dried Encapsulated Probiotics in Functional Foods
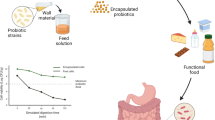
A schematic illustration of spray dried probiotics in food products is represented in Fig. 2. The incorporation of the encapsulated probiotics into functional food products ensures that the strains of these live organisms maintain their expected characteristics and viability (106 to 108 CFU/g daily at the time of the consumption) during the production and storage at the specified storage temperature (freezing, refrigeration, room temperature) (Terpou et al., 2019). Probiotics must survive in the physiological conditions of GIT, including the acidic pH of the stomach, enzymatic degradation, and the presence of bile salts in the small intestine (Aragón-Rojaset al., 2018). Various research groups have focused on the negative effects of high temperature in spray drying process and improving the resistance and stability of encapsulated cells, by the addition of prebiotics, mucilages, gums and soluble fibres to the encapsulating material as thermal protectors (Rodrigues et al., 2020). Application of encapsulated probiotics by spray drying in different food products will be discussed in the following sections.
Dairy Products
Presently, dairy products such as yogurt, fermented sour milk, and cheese remain at the forefront of probiotic food development. Even though fermented dairy foods are one of the most popular and traditional ways to provide probiotics to people, several non-dairy, non-traditional, and convenient probiotic products (e.g. capsules) have been developed and commercialized in several countries (Ranadheera et al., 2017). The incorporation of the encapsulated probiotics into dairy foods may aid in tolerating harsh GIT conditions better than that of non-dairy carrier foods, due to the buffering capacity of milk and milk fat, which possesses a protective effect by reducing the direct exposure of probiotics to the harsh conditions (Ranadheera et al., 2010). Dairy foods rich in milk fat (e.g. cream, cheese, and ice cream), were found to be more effective in enhancing the survivability and bile acid tolerance of probiotics (Ranadheera et al., 2013). The dairy industry covers about 33% of the functional food market in this area (Granato et al., 2010). Table 2 shows the microencapsulated probiotics incorporated into dairy food products by spray drying technique. Among dairy products, the application of probiotics has been widely explored in cheese and fermented milk, which will be discussed below.
Cheese
Gardiner et al. (2002) developed a milk powder product containing probiotic Lactobacillus paracasei NFBC 338 using spray drying (with a viable count of 109 CFU/g) and used this powder for the manufacture of Cheddar cheese. After three months of ripening, a count of 7.7 × 107 CFU/g probiotics in the product was reported, without any adverse effects on the texture, flavour, functionality, or appearance of the cheese. In another study by Radulovic et al. (2017), the highest viability of spray dried Lactobacillus Plantarum 564 cells (> 8 log units/g) in soft goat cheese throughout eight weeks of storage indicated the possibility of this application in soft cheese production with a longer storage period. Spray drying was efficient in maintaining the number of Lactobacillus Plantarum 564 strain on a higher level compared to the free cell counts. The survival of non-encapsulated and encapsulated L. rhamnosus in the functional cream cheese was also studied over 35 days of storage at 4 °C, and the structural and textural properties of the functional cream cheese were investigated. L. rhamnosus in both forms remained viable (> 106 CFU/g) in the cream cheese throughout the storage period. Probiotic cream cheese with β-glucan and phytosterol emulsions (spray dried) showed less reduction in their viable counts after 35 days of refrigerated storage. The addition of probiotics either in the non-encapsulated or encapsulated forms did not significantly change the pH, moisture, protein, or fat content of the experimental cheese (Ningtyas et al., 2019).
Milk Beverages
Spray-dried Lactobacillus casei ATCC393 was added into a fermented milk product, and it was found that spray drying encapsulation resulted in an increase in the survival rate of this probiotic during the refrigerated storage of the fermented milk, when compared with the free form of the bacteria (Dimitrellou et al., 2016). In another study, a mixture of probiotic cultures including Lactobacillus acidophilus LA-5, Bifidobacterium animalis subsp. lactis BB-12, and novel potential probiotic Propionibacterium jensenii 702 was suspended in a reconstituted (20% w/v) goat’s milk, and then spray dried in a mini spray dryer (inlet temperature of 195 ˚C and outlet temperature of 85 ˚C). The spray dried powder was stored in airtight glass jars at two different temperatures (4 and 30 ˚C) for 24 weeks. The powder quality and the probiotic viability after spray drying and the subsequent storage were measured. Although there was a significant reduction in the viability of probiotics in the reconstituted goat’s milk, all three probiotics were able to maintain their satisfactory viability levels (106–108 CFU/g) after spray drying (Ranadheera et al., 2015).
Another study aimed to evaluate the survivability and safety of Lactobacillus plantarum HM47 strain supplemented in milk chocolate during storage and transit throughout the GIT of mice (Nambiar et al., 2018). The milk chocolate was supplemented with microencapsulated Lactobacillus plantarum HM47 (isolated from human breast milk). Water activity (aw), pH, and sensory attributes of the milk chocolates containing L. Plantarum HM47 were analysed. The HM47 were found to be viable up to 180 days of storage at 25 °C (> 8 log CFU/g), and the overall acceptability results suggested that the addition of the encapsulated probiotic had no significant negative effects (P > 0.05) on the sensorial attributes of the milk chocolate (Nambiar et al., 2018).
Yogurt
The encapsulation of Lactococcus lactis Gh1, spray dried using gum Arabic and Synsepalum dulcificum, when incorporated into a functional yogurt retained viability of 107 CFU/mL, compared with non-encapsulated cells which presented viability of 105 CFU/mL. In the simulated gastric juice (pH = 1.5), the viability was 1.11 × 106 CFU/mL after 2 h (Fazilah et al., 2019). Picot & Lacroix (2004) successfully encapsulated Bifidobacterium strains (B. breve R070 and B. longum R023) in whey protein-based microcapsules using spray drying. B. breve R070 exhibited a high survival rate during spray drying and encapsulated cells showed higher viability than unencapsulated cells during 28 days of storage in low pH yogurts and the simulated gastrointestinal environment.
Bakery Products
Spray drying technique is widely utilized to encapsulated probiotics in food. However, the spray drying process is associated with high cell mortality resulting from simultaneous dehydration and thermal inactivation microorganisms. Besides, the dimension of microencapsulation produced by the spray-drying process usually has micron size (Lieu et al., 2017) decreasing the survival rate of probiotic supplemented in the bakery during baking (Dong et al., 2020). In this context, Malmo et al. (2013) prepared a potentially probiotic chocolate soufflé using Lactobacillus reuteri DSM 17,938 cells which were microencapsulated by spray drying in alginate matrix and further coated with chitosan. Microencapsulation led to a survival rate of 10% after baking a chocolate soufflé.
However, when Lactobacilli were encapsulated by spray-coating and added to cookie fillings, the cells did not show a satisfactory viability during storage, and it was found that the water activity had a greater effect on the viability levels than coating (Belvis et al., 2006). Recently, Arslan-Tontul et al. (2019) aimed to incorporate single and double-layered microcapsules containing Saccharomyces boulardii, Lactobacillus acidophilus, and Bifidobacterium bifidum, produced by spray drying and spray chilling, in cake products. In one treatment, encapsulated probiotics were added after baking to three different types of cakes (cream-filled, marmalade-filled, and chocolate-coated), and in another treatment (plain cake), the microcapsules were injected into the centre of the cake mix and baked at 200 °C for 20 min. After baking of plain cakes, the count of S. boulardii and L. acidophilus, as determined in the double-layered microcapsules, produced by spray chilling was 2.9 log CFU/g. The survivability rates of S. boulardii and L. acidophilus were also determined as 67.4 and 70.7% in this type of microcapsules, respectively. However, there was no viable B. bifidum detected after baking. The free forms of these probiotics did not survive in any cake formulation. Single-layered microcapsules produced by spray chilling provided a better protective effect on the probiotics in cream-filled and marmalade-filled cake samples during the 3-month storage at 4 °C. This study showed that a combination of spray chilling and spray drying microencapsulation techniques (double-layered microcapsules) could increase the survivability of probiotic microorganisms after the cake baking process. During the storage, the cake samples had a near-neutral pH value, and the textural properties deteriorated due to staling. However, staling had a limited effect on the sensorial attributes of the cakes and the samples could be readily consumed after the storage for 90 days (Arslan-Tontul et al., 2019).
Fruits and Vegetables
Table 3 shows the research related to the spray dried encapsulated probiotics which have been incorporated into non-dairy food products. So far, the development of the probiotic juices using spray drying has not been carried out extensively and systematically.
Dias et al. (2018) developed a probiotic passion fruit juice as a novel non-dairy product by the incorporation of microencapsulated Bifidobacterium animalis ssp. lactis BB-12, which was encapsulated by spray drying using maltodextrin and inulin as the wall materials. The results showed that the viability was in the range of 8.08–8.41 CFU/g. According to Ying et al. (2013), an increase of 2 logCFU/100 mL within 1–2 weeks of the storage was reported for an apple juice containing microencapsulated probiotics L. rhamnosus GG, using whey protein and resistant starch matrices as the carrier material. Prior to that, Saarela et al. (2006) reported that the viability of L. rhamnosus in another apple juice product could be sustained by the addition of oat flour containing 20% β-glucan. Acerola nectar juice was prepared using probiotic culture of B. animalis microencapsulated by spray drying using cellulose acetate phthalate, and the results confirmed the substantial survivability of 8 log CFU per portion (200 mL per nectar) for 30 days, when stored under the refrigeration conditions of 5 ˚C (Antunes et al., 2013). Similarly, encapsulated L. casei cells (spray dried by maltodextrin) when added into a bitter gourd juice powder, showed the highest viability counts over a storage period of four weeks. The capsules produced with maltodextrin and gum Arabic showed a higher count, when compared with the capsules produced with a mixture of both maltodextrin and gum Arabic (Kalal et al., 2017).
The addition of spray dried Pediococcus acidilactici HA-6111–2 or and Lactobacillus plantarum 299v into an orange juice product showed that the powder with aw value of 0.4, presented no loss of microbial viability (Barbosa et al., 2015). A decrease in the microbial viability from 9.5 to 5 log CFU/mL in raspberry juice incorporated with spray dried Lactobacillus acidophilus and L. rhamnosus was shown when the inlet temperature increased from 100 ˚C to 130 ˚C (Anekella & Orsat, 2013). Considering this information on the incorporation of the spray dried probiotics in fruit juice products, it is important to carry out the required corresponding clinical studies, to understand the release behaviour of the microencapsulated probiotics into the body.
Other Food Products
Four thermotolerant lactic acid bacteria (LAB) were encapsulated in Acacia gum (using spray drying) and inoculated into cooked meat batters (Pérez-Chabela et al., 2013), and it was reported that the inoculation of spray dried LAB enhanced the initial LAB count with a concomitant Enterobacteria reduction. These results suggest that the spray drying encapsulation is an effective way to protect thermotolerant LAB in cooked meat batters (Pérez-Chabela et al., 2013). The microencapsulation of Lactobacillus casei in synbiotic mayonnaise using whey protein, maltodextrin, and galacto-oligosaccharides showed viability of 1.55 to 3.27 log CFU/g as compared with the free cells, in which the viability significantly decreased by about 4 log CFU/g after six weeks of storage. Whey protein showed a more protective effect than maltodextrin during the spray drying process (Lieu et al., 2017).
Safety, Toxicity, and Regulations of Adding Encapsulated Probiotics Into Functional Foods
Like any other food ingredients, probiotics are subject to the regulations contained in the general food law, according to which they should be safe for human and/or animal health. In the USA, microorganisms used for consumption purposes should follow the GRAS (Generally Regarded as Safe) guidelines, regulated by the FDA (Food and Drug Administration). In Europe, EFSA (the European Food Safety Authority) introduced the term of QPS (Qualified Presumption of Safety) for these types of products. The QPS concept involves some additional criteria for the safety assessment of the bacterial supplements, including the history of safe usage and absence of the risk of acquired resistance to antibiotics (Markowiak and Śliżewska, 2017).
According to the suggestions of the WHO, FAO, and EFSA, in their selection process, probiotic strains must meet both safety and functionality criteria, as well as those related to their technological usefulness. Probiotic characteristics are not associated with the genus or species of a microorganism, but with few and specially selected strains of some particular species (Hill et al., 2014). The safety of a strain is defined by its origin, the absence of association with pathogenic cultures, and the antibiotic resistance profile. Functional aspects define their survival in the GIT and their immunomodulatory effect. Probiotic strains must meet the requirements associated with the technology of their production, which means they must be able to survive and maintain their properties throughout the storage and distribution processes (Lee et al., 2009).
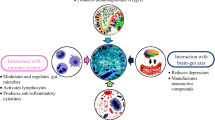
The schematic illustration in Fig. 3 represents the release and safety of probiotics in GIT. Various types of bacteria are used as probiotics for human consumption; thus, the safety of such microorganisms is tied to the specific microbes intended for use. The safety of probiotics depends on the deliberation of possible susceptibility of the consumer, the dose and duration of the consumption, and both the manner and frequency of the administration (Sanders et al., 2010). Unlike other food or drug ingredients, probiotics are exceptional as they are alive when administered, and possess the potential for infectivity or in situ toxin production. The presence of transferable antibiotic resistance genes, which comprises a theoretical risk of transfer to a less innocuous member of the gut microbial community, must also be considered. Genetic stability of the probiotics over time, deleterious metabolic activities, and the potential for pathogenicity or toxicogenicity must be assessed depending on the characteristics of the genus and species of the microbe being used. In addition, the immunological effects must be considered, especially in certain vulnerable populations, including infants with undeveloped immune function. Only a few reports about the negative effects of probiotics supplemented to humans have been published, meaning that their significance is yet to be better understood with a more complete understanding of the mechanisms of the probiotic interaction with the host and colonizing microbes.
According to a 2002 report released by WHO and FAO, “probiotics may theoretically be responsible for four types of side effects such as systemic infections, deleterious metabolic activities, excessive immune stimulation in susceptible individuals and gene transfer”. Further, it has been recommended that the new probiotic strains should be evaluated for safety by testing for antibiotic resistance, toxin production, and haemolytic potential, assessing metabolic activities such as d-lactate production and bile salt de-conjugation. Human studies should be conducted to evaluate their side effects and post-market surveillance of the consumers, and ideally, the administration in the immune compromised animals to determine the infectivity of the probiotic organism in this type of host should be investigated (Doron & Snydman, 2015). The use of readily available and low-cost genomic sequencing technologies to assure the absence of genes of concern is advisable for the candidate probiotic strains. However, there is a scarcity of information in this field of probiotic safety as the required studies are yet to be designed to particularly assess the safety contrasted with the long history of the safe use of many of these microbes in foods (Sanders et al., 2010).
Conclusion and Future Aspects
Probiotics are beneficial microbial supplements and when ingested in sufficient levels, they can improve the intestinal microbial balance of the host. Several factors such as acidic conditions and the exposure to the environmental conditions (e.g. oxygen, temperature, and pH) can negatively affect the viability of probiotics. Nowadays, there is an increasing trend towards the application of probiotics in functional food products. To fulfil the criteria of having 106 CFU/mL at the time of consumption of functional food products, microencapsulation of probiotics is gaining an increasing interest in parallel to the growing demand for probiotic fermented foods. Large-scale production and application of the encapsulated probiotics would allow a better in vivo assessment for the survival of the consumed probiotics and their beneficial effects on human health. Spray drying is an efficient and available encapsulation technique that can be applied to proliferate the resistance of various strains of probiotics and facilitate the incorporation of live probiotics into various food products. It is a low-cost technique with high process yield, and more time-efficiency, which produces the end products with desirable moisture contents.
To date, little attention has been paid to the effects of spray drying devices on the viability of probiotics in powders. The main influence of different devices on probiotic powders is probably the residence time of particles in the drying chamber: The longer the residence time, the longer the bacteria are exposed to stress and consequently the poorer the viability. Another factor worth noting is that the industrial scale spray dryers are normally equipped with pneumatic devices to enable the continuous collection and cooling of the powders, thus maximizing the cell viability. Several spray dried probiotic powders have been added into dairy and non-dairy-based functional foods, but there still exists some challenges regarding the viability of spray dried encapsulated probiotics in vivo, as well as the corresponding regulations in most countries around the globe. Challenges faced during the encapsulation of probiotics by spray drying method need to be considered. Spray dried encapsulated probiotics can have the great potential for formulation of functional foods, and their commercial application would benefit both industries and consumers.
Data Availability
No datasets were generated or analysed during the current study.
References
Adhikari, K., Mustapha, A., Grün, I. U., & Fernando, L. (2000). Viability of microencapsulated Bifidobacteria in set yogurt during refrigerated storage. Journal of Dairy Science, 83(9), 1946–1951.
Agudelo, J., Cano, A., González-Martínez, C., & Chiralt, A. (2017). Disaccharide incorporation to improve survival during storage of spray dried Lactobacillus rhamnosus in whey protein-maltodextrin carriers. Journal of Functional Foods, 37, 416–423. https://doi.org/10.1016/j.jff.2017.08.014
Akanny, E., Bourgeois, S., Bonhommé, A., Commun, C., Doleans-Jordheim, A., Bessueille, F., & Bordes, C. (2020). Development of enteric polymer-based microspheres by spray drying for colonic delivery of Lactobacillus rhamnosus GG. International Journal of Pharmaceutics, 584, 119414.
Alves, N. N., Messaoud, G. B., Desobry, S., Costa, J. M. C., & Rodrigues, S. (2016). Effect of drying technique and feed flow rate on bacterial survival and physicochemical properties of a non-dairy fermented probiotic juice powder. Journal of Food Engineering, 189, 45–54.
Amara, A. A., & Shibl, A. (2015). Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharmaceutical Journal, 23(2), 107–114.
Anekella, K., & Orsat, V. (2013). Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT-Food Science and Technology, 50(1), 17–24.
Antunes, A. E. C., Liserre, A. M., Coelho, A. L. A., Menezes, C. R., Moreno, I., Yotsuyanagi, K., & Azambuja, N. C. (2013). Acerola nectar with added microencapsulated probiotic. LWT-Food Science and Technology, 54(1), 125–131.
Aragón-Rojas, S., Quintanilla-Carvajal, M. X., & Hernández-Sánchez, H. (2018). Multifunctional role of the whey culture medium in the spray drying microencapsulation of lactic acid bacteria. Food Technology and Biotechnology, 56(3), 381–397.
Arepally, D., & Goswami, T. K. (2019). Effect of inlet air temperature and gum Arabic concentration on encapsulation of probiotics by spray drying. LWT-Food Science and Technology, 99, 583–593.
Arslan-Tontul, S., Erbas, M., & Gorgulu, A. (2019). The Use of probiotic-loaded single-and double-layered microcapsules in cake production. Probiotics and Antimicrobial Proteins, 11(3), 840–849.
Arslan, S., Erbas, M., Tontul, I., & Topuz, A. (2015). Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT-Food Science and Technology, 63(1), 685–690.
Arslan-Tontul, S., & Erbas, M. (2017). Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT-Food Science and Technology, 81, 160–169.
Assadpour, E., & Jafari, S. M. (2019). Advances in spray drying encapsulation of food bioactive ingredients: From microcapsules to nanocapsules. Annual Review of Food Science and Technology, 10, 103–131.
Avila-Reyes, S. V., Garcia-Suarez, F. J., Jiménez, M. T., San Martín-Gonzalez, M. F., & Bello-Perez, L. A. (2014). Protection of L. rhamnosus by spray drying using two prebiotics colloids to enhance the viability. Carbohydrate Polymers, 102, 423–430.
Azam, M., Saeed, M., Pasha, I., & Shahid, M. (2020). A prebiotic-based biopolymeric encapsulation system for improved survival of Lactobacillus rhamnosus. Food Bioscience, 37, 100679.
Barajas-Álvarez, P., González-Ávila, M., & Espinosa-Andrews, H. (2022). Microencapsulation of Lactobacillus rhamnosus HN001 by spray drying and its evaluation under gastrointestinal and storage conditions. LWT, 153, 112485.
Barbosa, J., Borges, S., Amorim, M., Pereira, M. J., Oliveira, A., Pintado, M. E., & Teixeira, P. (2015). Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. Journal of Functional Foods, 17, 340–351.
Behboudi-Jobbehdar, S., Soukoulis, C., Yonekura, L., & Fisk, I. (2013). Optimization of spray drying process conditions for the production of maximally viable microencapsulated L. acidophilus NCIMB 701748. Drying Technology, 31(11), 1274–1283.
Belvis, J., Tompkins, T. A., Wallace, T. A., Casavant, L., Fortin, C., & Caron, C. (2006). Stability of probiotic bacteria in food stuffs. In CIFST Meeting, Montréal, May (Vol. 30).
Bhagwat, A., Bhushette, P., & Annapure, U. S. (2020). Spray drying studies of probiotic Enterococcus strains encapsulated with whey protein and maltodextrin. Beni-Suef University Journal of Basic and Applied Sciences, 9(1), 1–8.
Broeckx, G., Vandenheuvel, D., Claes, I. J., Lebeer, S., & Kiekens, F. (2016). Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. International Journal of Pharmaceutics, 505(1–2), 303–318.
Broeckx, G., Vandenheuvel, D., Henkens, T., Kiekens, S., van den Broek, M. F., Lebeer, S., & Kiekens, F. (2017). Enhancing the viability of Lactobacillus rhamnosus GG after spray drying and during storage. International Journal of Pharmaceutics, 534(1–2), 35–41.
Bustamante, M., Oomah, B. D., Rubilar, M., & Shene, C. (2017). Effective Lactobacillus plantarum and Bifidobacterium infantis encapsulation with chia seed (Salvia hispanica L.) and flaxseed (Linumusitatissimum L.) mucilage and soluble protein by spray drying. Food Chemistry, 216, 97–105.
Chaikham, P., Kemsawasd, V., & Seesuriyachan, P. (2017). Spray drying probiotics along with maoluang juice plus Tiliacoratriandra gum for exposure to the in vitro gastrointestinal environments. LWT-Food Science and Technology, 78, 31–40.
Choudhury, N., Meghwal, M., & Das, K. (2021). Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Frontiers, 2(4), 426–442.
Cuddihy, S. L., Parker, A., Harwood, D. T., Vissers, M. C., & Winterbourn, C. C. (2008). Ascorbate interacts with reduced glutathione to scavenge phenoxyl radicals in HL60 cells. Free Radical Biology and Medicine, 44(8), 1637–1644.
De Castro-Cislaghi, F. P., Carina Dos Reis, E. S., Fritzen-Freire, C. B., Lorenz, J. G., & Sant’Anna, E. S. (2012). Bifidobacterium Bb-12 microencapsulated by spray drying with whey: Survival under simulated gastrointestinal conditions, tolerance to NaCl, and viability during storage. Journal of Food Engineering, 113(2), 186–193.
Dias, C. O., de Almeida, J. D. S. O., Pinto, S. S., de Oliveira Santana, F. C., Verruck, S., Müller, C. M. O., Prudêncio, E. S., & Amboni, R. D. D. M. C. (2018). Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Bioscience, 24, 26–36.
Dimitrellou, D., Kandylis, P., Petrović, T., Dimitrijević-Branković, S., Lević, S., Nedović, V., & Kourkoutas, Y. (2016). Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT-Food Science and Technology, 71, 169–174.
Dong, L. M., Luan, N. T., & Thuy, D. T. K. (2020). The viability of encapsulated Lactobacillus plantarum during cupcake baking process, storage, and simulated gastric digestion. Journal of Microbiology, Biotechnology and Food Sciences, 9(6), 1157–1161.
Doron, S., & Snydman, D. R. (2015). Risk and safety of probiotics. Clinical Infectious Diseases, 60(suppl_2), S129-S134.
Eratte, D., McKnight, S., Gengenbach, T. R., Dowling, K., Barrow, C. J., & Adhikari, B. P. (2015). Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate–gum Arabic complex coacervates. Journal of Functional Foods, 19, 882–892.
Ermis, E. (2021). A review of drying methods for improving the quality of probiotic powders and characterization. Drying Technology, 1–18.
Fahimdanesh, M., Mohammadi, N., Ahari, H., Zanjani, M. K., Hargalani, F. Z., & Behrouznasab, K. (2012). Effect of microencapsulation plus resistant starch on survival of Lactobacillus casei and Bifidobacterium bifidum in mayonnaise sauce. African Journal of Microbiology Research, 6(40), 6853–6858.
Fazilah, N. F., Hamidon, N. H., Ariff, A. B., Khayat, M. E., Wasoh, H., & Halim, M. (2019). Microencapsulation of Lactococcus lactis Gh1 with Gum Arabic and Synsepalumdulcificum via Spray Drying for Potential Inclusion in Functional Yogurt. Molecules, 24(7), 1422.
Fijan, S. (2014). Microorganisms with claimed probiotic properties: An overview of recent literature. International Journal of Environmental Research and Public Health, 11(5), 4745–4767.
Fu, N., Huang, S., Xiao, J., & Chen, X. D. (2018). Producing powders containing active dry probiotics with the aid of spray drying. In Advances in Food and Nutrition Research (Vol. 85, pp. 211–262). Academic Press.
Gardiner, G. E., Bouchier, P., O’Sullivan, E., Kelly, J., Collins, J. K., Fitzgerald, G., Collins, J. K., & Stanton, C. (2002). A spray dried culture for probiotic Cheddar cheese manufacture. International Dairy Journal, 12(9), 749–756.
Gbassi, G. K., & Vandamme, T. (2012). Probiotic encapsulation technology: From microencapsulation to release into the gut. Pharmaceutics, 4(1), 149–163.
Gerez, C. L., Font de Valdez, G., Gigante, M. L., & Grosso, C. R. F. (2012). Whey protein coating bead improves the survival of the probiotic Lactobacillus rhamnosus CRL 1505 to low pH. Letters in Applied Microbiology, 54(6), 552–556.
Ghandi, A., Powell, I. B., Howes, T., Chen, X. D., & Adhikari, B. (2012). Effect of shear rate and oxygen stresses on the survival of Lactococcus lactis during the atomization and drying stages of spray drying: A laboratory and pilot scale study. Journal of Food Engineering, 113(2), 194–200.
Gharsallaoui, A., Roudaut, G., Chambin, O., Voilley, A., & Saurel, R. (2007). Applications of spray drying in microencapsulation of food ingredients: An overview. Food Research International, 40(9), 1107–1121.
Granato, D., Branco, G. F., Cruz, A. G., Faria, J. D. A. F., & Shah, N. P. (2010). Probiotic dairy products as functional foods. Comprehensive Reviews in Food Science and Food Safety, 9(5), 455–470.
Guerin, J., Petit, J., Burgain, J., Borges, F., Bhandari, B., Perroud, C., Desobry, S., Scher, J., & Gaiani, C. (2017). Lactobacillus rhamnosus GG encapsulation by spray drying: Milk proteins clotting control to produce innovative matrices. Journal of Food Engineering, 193, 10–19.
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., Morelli, L., Canani, R. B., Flint, H. J., & H.J., Salminen, S., Calder, P. C. & Sanders, M.E. (2014). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11(8), 506–514.
Homayouni, A., Azizi, A., Ehsani, M. R., Yarmand, M. S., & Razavi, S. H. (2008). Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chemistry, 111(1), 50–55.
Hosseini A, Jafari SM, Mirzaei H, Asghari A, Akhavan S (2015) Application of image processing to assess emulsion stability and emulsification properties of Arabic gum.Carbohydrate Polymers, 126,1–8.
Huang, S., Vignolles, M. L., Chen, X. D., Le Loir, Y., Jan, G., Schuck, P., & Jeantet, R. (2017). Spray drying of probiotics and other food-grade bacteria: A review. Trends in Food Science & Technology, 63, 1–17.
Joint FAO/WHO Working Group. (2014). Guidelines for the evaluation of probiotics in food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food (p. 2002). Ontario, Canada.
Kailasapathy, K. (2002). Microencapsulation of probiotic bacteria: Technology and potential applications. Current Issues in Intestinal Microbiology, 3(2), 39–48.
Kailasapathy, K. (2006). Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT-Food Science and Technology, 39(10), 1221–1227.
Kalal, A. Y., Hiregoudar, S., Nidoni, U., Ramachandra, C. T., Naik, N., & Roopabai, R. S. (2017). Nanoencapsulation of Lactobacillus casei in Bitter Gourd Juice using Spray Drying. Advances in Bioresearch, 8(6).
Kalita, D., Saikia, S., Gautam, G., Mukhopadhyay, R., & Mahanta, C. L. (2018). Characteristics of synbiotic spray dried powder of litchi juice with Lactobacillus plantarum and different carrier materials. LWT-Food Science and Technology, 87, 351–360.
Kearney, N., Meng, X. C., Stanton, C., Kelly, J., Fitzgerald, G. F., & Ross, R. P. (2009). Development of a spray dried probiotic yoghurt containing Lactobacillus paracasei NFBC 338. International Dairy Journal, 19(11), 684–689.
Kerry, R. G., Patra, J. K., Gouda, S., Park, Y., Shin, H. S., & Das, G. (2018). Benefaction of probiotics for human health: A review. Journal of Food and Drug Analysis, 26(3), 927–939.
Khem, S., Bansal, V., Small, D. M., & May, B. K. (2016a). Comparative influence of pH and heat on whey protein isolate in protecting Lactobacillus plantarum A17 during spray drying. Food Hydrocolloids, 54, 162–169.
Khem, S., Small, D. M., & May, B. K. (2016b). The behaviour of whey protein isolate in protecting Lactobacillus plantarum. Food Chemistry, 190, 717–723.
Kingwatee, N., Apichartsrangkoon, A., Chaikham, P., Worametrachanon, S., Techarung, J., & Pankasemsuk, T. (2015). Spray drying Lactobacillus casei 01 in lychee juice varied carrier materials. LWT-Food Science and Technology, 62(1), 847–853.
Kokott, S. (2004). Microencapsulation and supply of Bifidobacterium lactis DSM 10140 in fermented traditional African beverages (Doctoral dissertation, Cape Peninsula University of Technology) MTech (Food Technology) Available from: http://hdl.handle.net/20.500.11838/824. Accessed 2020 April 9.
Krasaekoopt, W., & Kitsawad, K. (2010). Sensory characteristics and consumer acceptance of fruit juice containing probiotics beads in Thailand. Assumption University Journal of Technology, 14(1), 33–38.
Kumar, M., Rakesh, S., Nagpal, R., Hemalatha, R., Ramakrishna, A., Sudarshan, V., Ramagoni, R., Shujauddin, M., Verma, V., Kumar, A., Tiwari, A., Singh, B., & Rajesh Kumar, R. (2013). Probiotic Lactobacillus rhamnosus GG and Aloe vera gel improve lipid profiles in hypercholesterolemic rats. Nutrition, 29(3), 574–579.
Kumari, R., & Seth, D. (2016). Optimization of microencapsulation of probiotics in carambola fruit juice by spray drying. In: International Conference on Emerging Technologies in Agricultural and Food Engineering (ETAE 2016), IIT Kharagpur, December 27–30.
Lai, K., How, Y., & Pui, L. (2021). Microencapsulation of Lactobacillus rhamnosus GG with flaxseed mucilage using co-extrusion technique. Journal of Microencapsulation, 38(2), 134–148.
Lee, Y. K. (2009). Selection and maintenance of probiotic microorganisms. In Y. K. Lee & S. Salminen (Eds.), Handbook of Probiotics and Prebiotics (pp. 177–187). Wiley-VCH.
Liao, L. K., Wei, X. Y., Gong, X., Li, J. H., Huang, T., & Xiong, T. (2017). Microencapsulation of Lactobacillus casei LK-1 by spray drying related to its stability and in vitro digestion. LWT-Food Science and Technology, 82, 82–89.
Lieu, M. D., Dang, T. K. T., & Nguyen, T. H. (2017). Viability of microencapsulated Lactobacillus casei in synbiotic mayonnaise. Food Research, 1(6), 234–239.
Liu, H., Gong, J., Chabot, D., Miller, S. S., Cui, S. W., Zhong, F., & Wang, Q. (2018). Improved survival of Lactobacillus zeae LB1 in a spray dried alginate-protein matrix. Food Hydrocolloids, 78, 100–108.
Liu, H., Gong, J., Chabot, D., Miller, S. S., Cui, S. W., et al. (2015). Protection of heat-sensitive probiotic bacteria during spray drying by sodium caseinate stabilized fat particles. Food Hydrocolloids, 51, 459–467.
Maciel, G. M., Chaves, K. S., Grosso, C. R. F., & Gigante, M. L. (2014). Microencapsulation of Lactobacillus acidophilus La-5 by spray drying using sweet whey and skim milk as encapsulating materials. Journal of Dairy Science, 97(4), 1991–1998.
Mahdavi, S. A., Jafari, S. M., Assadpoor, E., & Dehnad, D. (2016). Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. International Journal of Biological Macromolecules, 85, 379–385.
Malmo, C., La Storia, A., & Mauriello, G. (2013). Microencapsulation of Lactobacillus reuteri DSM 17938 cells coated in alginate beads with chitosan by spray drying to use as a probiotic cell in a chocolate soufflé. Food and Bioprocess Technology, 6(3), 795–805.
Markowiak, P., & Śliżewska, K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients, 9(9), 1021.
Martín, M. J., Lara-Villoslada, F., Ruiz, M. A., & Morales, M. E. (2015). Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innovative Food Science & Emerging Technologies, 27, 15–25.
Mattila-Sandholm, T., Myllarinen, P., Crittenden, R., Mogensen, G., Fonden, R., & Saarela, M. (2002). Technological challenges for future probiotic food. International Dairy Journal, 12(2), 173–182.
Moayyedi, M., Eskandari, M. H., Rad, A. H. E., Ziaee, E., Khodaparast, M. H. H., & Golmakani, M. T. (2018). Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. Journal of Functional Foods, 40, 391–399.
Mokhtari, S., Jafari, S. M., & Khomeiri, M. (2019). Survival of encapsulated probiotics in pasteurized grape juice and evaluation of their properties during storage. Food Science and Technology International, 25(2), 120–129.
Mokhtari, S., Jafari, S. M., Khomeiri, M., Maghsoudlou, Y., & Ghorbani, M. (2017a). The cell wall compound of Saccharomyces cerevisiae as a novel wall material for encapsulation of probiotics. Food Research International, 96, 19–26.
Mokhtari, S., Khomeiri, M., Jafari, S. M., Maghsoudlou, Y., & Ghorbani, M. (2017b). Descriptive analysis of bacterial profile, physicochemical and sensory characteristics of grape juice containing Saccharomyces cerevisiae cell wall-coated probiotic microcapsules during storage. International Journal of Food Science & Technology, 52(4), 1042–1048.
Muthukumarasamy, P., & Holley, R. A. (2006). Microbiological and sensory quality of dry fermented sausages containing alginate-microencapsulated Lactobacillus reuteri. International Journal of Food Microbiology, 111(2), 164–169.
Nambiar, R. B., Sellamuthu, P. S., & Perumal, A. B. (2018). Development of milk chocolate supplemented with microencapsulated Lactobacillus plantarum HM47 and to determine the safety in a Swiss albino mice model. Food Control, 94, 300–306.
Nedovic, V., Kalusevic, A., Manojlovic, V., Levic, S., & Bugarski, B. (2011). An overview of encapsulation technologies for food applications. Procedia Food Science, 1, 1806–1815.
Ningtyas, D. W., Bhandari, B., Bansal, N., & Prakash, S. (2019). The viability of probiotic Lactobacillus rhamnosus (non-encapsulated and encapsulated) in functional reduced-fat cream cheese and its textural properties during storage. Food Control, 100, 8–16.
Nunes, G. L., de Araújo Etchepare, M., Cichoski, A. J., Zepka, L. Q., Lopes, E. J., Barin, J. S., Marlon, É., de Moraes Flores, E. M., & de Bona da Silva, C., & de Menezes, C. R. (2018). Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT-Food Science and Technology, 89, 128–133.
Páez, R., Lavari, L., Audero, G., Cuatrin, A., Zaritzky, N., Reinheimer, J., & Vinderola, G. (2013). Study of the effects of spray drying on the functionality of probiotic lactobacilli. International Journal of Dairy Technology, 66(2), 155–161.
Paim, D. R., Costa, S. D., Walter, E. H., & Tonon, R. V. (2016). Microencapsulation of probiotic jussara (Euterpe edulis M.) juice by spray drying. LWT-Food Science and Technology, 74, 21–25.
Pandey, K. R., & Vakil, B. V. (2017). Encapsulation of probiotic Bacillus coagulans for enhanced shelf life. Jornal of Appied Biology and Biotechnology, 5, 57–65.
Peighambardoust, S. H., Tafti, A. G., & Hesari, J. (2011). Application of spray drying for preservation of lactic acid starter cultures: A review. Trends in Food Science & Technology, 22(5), 215–224.
Pérez-Chabela, M. L., Lara-Labastida, R., Rodriguez-Huezo, E., & Totosaus, A. (2013). Effect of spray drying encapsulation of thermotolerant lactic acid bacteria on meat batter properties. Food and Bioprocess Technology, 6(6), 1505–1515.
Petreska-Ivanovska, T., Petrushevska-Tozi, L., Grozdanov, A., Petkovska, R., Hadjieva, J., Popovski, E., & Mladenovska, K. (2014). From optimization of synbiotic microparticles prepared by spray drying to development of new functional carrot juice. Chemical Industry and Chemical Engineering Quarterly, 20(4), 549–564.
Picot, A., & Lacroix, C. (2004). Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. International Dairy Journal, 14(6), 505–515.
Pinto, S. S., Verruck, S., Vieira, C. R., Prudêncio, E. S., Amante, E. R., & Amboni, R. D. (2015). Influence of microencapsulation with sweet whey and prebiotics on the survival of Bifidobacterium-BB-12 under simulated gastrointestinal conditions and heat treatments. LWT-Food Science and Technology, 64(2), 1004–1009.
Pispan, S., Hewitt, C. J., & Stapley, A. G. F. (2013). Comparison of cell survival rates of E. coli K12 and L. acidophilus undergoing spray drying. Food and Bioproducts Processing, 91(4), 362–369.
Prakash, K. S., Bashir, K., & Mishra, V. (2017). Development of synbiotic litchi juice drink and its physiochemical, viability and sensory analysis. Journal of Food Processing and Technology, 8(12).
Putta, S., Yarla, N. S., Lakkappa, D. B., Imandi, S. B., Malla, R. R., Chaitanya, A. K., & Aliev, G. (2018). Probiotics: Supplements, food, pharmaceutical industry. In Therapeutic, Probiotic, and Unconventional Foods (pp. 15–25). Academic Press.
Quintana, G., Gerbino, E., & Gómez-Zavaglia, A. (2018). Valorization of okara oil for the encapsulation of Lactobacillus plantarum. Food Research International, 106, 81–89.
Radulović, Z., Miočinović, J., Mirković, N., Mirković, M., Paunović, D., Ivanović, M., & Seratlić, S. (2017). Survival of spray-dried and free-cells of potential probiotic Lactobacillus plantarum 564 in soft goat cheese. Animal Science Journal, 88(11), 1849–1854.
Rajam, R., & Anandharamakrishnan, C. (2015). Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT-Food Science and Technology, 60(2), 773–780.
Ranadheera, C. S., Vidanarachchi, J. K., Rocha, R. S., Cruz, A. G., & Ajlouni, S. (2017). Probiotic delivery through fermentation: dairy vs. non-dairy beverages. Fermentation, 3(4), 67.
Ranadheera, R. D. C. S., Baines, S. K., & Adams, M. C. (2010). Importance of food in probiotic efficacy. Food Research International, 43(1), 1–7.
Ranadheera, C. S., Evans, C. A., Adams, M. C., & Baines, S. K. (2013). Production of probiotic ice cream from goat’s milk and effect of packaging materials on product quality. Small Ruminant Research, 112(1–3), 174–180.
Ranadheera, C. S., Evans, C. A., Adams, M. C., & Baines, S. K. (2015). Microencapsulation of Lactobacillus acidophilus LA-5, Bifidobacterium animalis subsp. lactis BB-12 and Propionibacterium jensenii 702 by spray drying in goat's milk. Small Ruminant Research, 123(1), 155–159.
Rodklongtan, A., & Chitprasert, P. (2017). Combined effects of holy basil essential oil and inlet temperature on lipid peroxidation and survival of Lactobacillus reuteri KUB-AC5 during spray drying. Food Research International, 100, 276–283.
Rodrigues, F. J., Cedran, M. F., Bicas, J. L., & Sato, H. H. (2020). Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications-a narrative review. Food Research International, 137(109682.10), 1016.
Rokka, S., & Rantamäki, P. (2010). Protecting probiotic bacteria by microencapsulation: Challenges for industrial applications. European Food Research and Technology, 231(1), 1–12.
Romano, N., Mobili, P., Zuñiga-Hansen, M. E., & Gómez-Zavaglia, A. (2018). Physico-chemical and structural properties of crystalline inulin explain the stability of Lactobacillus plantarum during spray drying and storage. Food Research International, 113, 167–174.
Saarela, M., Mogensen, G., Fonden, R., Mättö, J., & Mattila-Sandholm, T. (2000). Probiotic bacteria: Safety, functional and technological properties. Journal of Biotechnology, 84(3), 197–215.
Saarela, M., Kirkajarvi, I., Alkomi, H. L., Sigvart-Mattila, P., & Matto, J. (2006). Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk. International Dairy Journal, 16, 1477–1482.
Salmeron, I. (2017). Fermented cereal beverages: From probiotic, prebiotic and synbiotic towards Nanoscience designed healthy drinks. Letters in Applied Microbiology, 65(2), 114–124.
Sanders, M. E., Akkermans, L. M., Haller, D., Hammerman, C., Heimbach, J. T., Hörmannsperger, G., & Huys, G. (2010). Safety assessment of probiotics for human use. Gut Microbes, 1(3), 164–185.
Sarabandi, K., Gharehbeglou, P., & Jafari, S. M. (2020). Spray drying encapsulation of protein hydrolysates and bioactive peptides: Opportunities and challenges. Drying Technology, 38(5–6), 577–595.
Siemons, I., Vaessen, E. M. J., van Peski, S. O., Boom, R. M., & Schutyser, M. A. I. (2021). Protective effect of carrier matrices on survival of Lactobacillus plantarum WCFS1 during single droplet drying explained by particle morphology development. Journal of Food Engineering, 292, 110263.
Šipailienė, A., & Petraitytė, S. (2018). Encapsulation of probiotics: Proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms. Probiotics and Antimicrobial Proteins, 10(1), 1–10.
Sosnik, A., & Seremeta, K. P. (2015). Advantages and challenges of the spray drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Advances in Colloid and Interface Science, 223, 40–54.
Spigno, G., Garrido, G. D., Guidesi, E., & Elli, M. (2015). Spray drying encapsulation of probiotics for ice-cream application. Chemical Engineering Transactions, 43, 49–54.
Surnis, S. A., Huparikar, K. B., & Pramod, A. K. (2016). Microencapsulation of Probiotics (Lactobacillus Casei and Bifidobacterium Longum) in Pineapple Jam by Spray Drying and its Comparitive Study. International Journal of Engineering Research & Technology, 5(3), 675–677.
Tarrah, A., De Castilhos, J., Rossi, R. C., Duarte, V. D. S., Ziegler, D., Corich, V., & Giacomini, A. (2018). In vitro probiotic potential and anti-cancer activity of newly isolated folate-producing Streptococcus thermophilus strains. Frontiers in Microbiology, 9, 2214.
Terpou, A., Papadaki, A., Lappa, I. K., Kachrimanidou, V., Bosnea, L. A., & Kopsahelis, N. (2019). Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients, 11(7), 1591.
Trabelsi, I., Slima, S. B., Ktari, N., Triki, M., Abdehedi, R., Abaza, W., HafedhMoussac, H., Abdeslam, A., & Salah, R. B. (2019). Incorporation of probiotic strain in raw minced beef meat: Study of textural modification, lipid and protein oxidation and color parameters during refrigerated storage. Meat Science, 154, 29–36.
Verruck, S., de Carvalho, M. W., de Liz, G. R., Amante, E. R., Vieira, C. R. W., Amboni, R. D. D. M. C., & Prudencio, E. S. (2017). Survival of Bifidobacterium BB-12 microencapsulated with full-fat goat’s milk and prebiotics when exposed to simulated gastrointestinal conditions and thermal treatments. Small Ruminant Research, 153, 48–56.
Vivek, K., Mishra, S., & Pradhan, R. C. (2020). Characterization of spray dried probiotic Sohiong fruit powder with Lactobacillus plantarum. LWT-Food Science and Technology, 117, 108699.
Ying, D., Sanguansri, L., Weerakkody, R., Bull, M., Singh, T. K., & Augustin, M. A. (2016). Effect of encapsulant matrix on stability of microencapsulated probiotics. Journal of Functional Foods, 25, 447–458.
Ying, D., Schwander, S., Weerakkody, R., Sanguansri, L., Gantenbein-Demarchi, C., & Augustin, M. A. (2013). Microencapsulated Lactobacillus rhamnosusGG in whey protein and resistant starch matrices: Probiotic survival in fruit juice. Journal of Functional Foods, 5(1), 98–105.
Ying, D., Sun, J., Sanguansri, L., Weerakkody, R., & Augustin, M. A. (2012). Enhanced survival of spray-dried microencapsulated Lactobacillus rhamnosus GG in the presence of glucose. Journal of Food Engineering, 109(3), 597–602.
Yonekura, L., Sun, H., Soukoulis, C., & Fisk, I. (2014). Microencapsulation of Lactobacillus acidophilus NCIMB 701748 in matrices containing soluble fibre by spray drying: Technological characterization, storage stability and survival after in vitro digestion. Journal of Functional Foods, 6, 205–214.
Zanjani, M. A. K., Tarzi, B. G., Sharifan, A., Mohammadi, N., Bakhoda, H., & Madanipour, M. M. (2012). Microencapsulation of Lactobacillus casei with calcium alginate-resistant starch and evaluation of survival and sensory properties in cream-filled cake. African Journal of Microbiology Research, 6(26), 5511–5517.
Zhang, Y., Lin, J., & Zhong, Q. (2015). The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Research International, 71, 9–15.
Zhang, Y., Lin, J., & Zhong, Q. (2016). Effects of media, heat adaptation, and outlet temperature on the survival of Lactobacillus salivarius NRRL B-30514 after spray drying and subsequent storage. LWT-Food Science and Technology, 74, 441–447.
Zheng, X., Fu, N., Huang, S., Jeantet, R., & Chen, X. D. (2016). Exploring the protective effects of calcium-containing carrier against drying-induced cellular injuries of probiotics using single droplet drying technique. Food Research International, 90, 226–234.
Ziaee, A., Albadarin, A. B., Padrela, L., Femmer, T., O’Reilly, E., & Walker, G. (2019). Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. European Journal of Pharmaceutical Sciences, 127, 300–318.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interests
All authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, R., Rashidinejad, A. & Jafari, S.M. Application of Spray Dried Encapsulated Probiotics in Functional Food Formulations. Food Bioprocess Technol 15, 2135–2154 (2022). https://doi.org/10.1007/s11947-022-02803-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02803-6