Abstract
Different drying accelerators were studied to improve vegetable drying: acetone (AC), ethanol (ET), isopropanol (ISO) and acetic acid (AA). Pre-treatments were performed by immersion of pumpkin cylinders. Convective drying was performed at 40 °C and air velocity 1 m/s. Different aspects were evaluated: drying kinetics, structural changes (microstructure and macrostructure), thermal profile and viscoelastic and rehydration behaviours. The microstructure was modified by pre-treatments, being more pronounced with AC and AA. Thinner cell walls, changes on turgor and extraction of components and air were reported, affecting the mass transfer. Moreover, the microstructural changes reinforced anisotropy and also affected the macrostructure, changing the viscoelastic behaviour. All pre-treatments resulted in a super-diffusive behaviour, decreasing the drying time from 9% (ISO) to 22% (AC). Possible relations were discussed among the compounds’ physical properties, sample temperature profile, drying kinetics and equilibrium moisture. Rehydration was improved by ET and ISO, but impaired by AA. Although AC accelerates drying, it did not affect the rehydration. The viscoelasticity reflected the structure and composition, with the pre-treatments with higher structure modifications (AA and AC) losing elastic properties. In conclusion, the pre-treatments with isopropanol and ethanol showed better results, improving drying and rehydration, and are thus recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drying is an important unit operation widely used to produce stable products, with a small quantity of water, prone to preservation and industrial processing. Being a time- and energy-consuming process, drying enhancement is still necessary. For instance, different studies have been carried out in order to obtain new alternatives to improve the drying process and the dry product properties (Mujumdar and Law 2010; Fernandes et al. 2011). Among them, emerging technologies such as ultrasound (Wang et al. 2019a; Rodríguez et al. 2019), microwave (Monteiro et al. 2018; Szadzińska et al. 2018), radio frequency (Gong et al. 2020), pulsed electric field (Ostermeier et al. 2018; Liu et al. 2020), high pressure (de Oliveira et al. 2015), freezing (Vallespir et al. 2018) and different pre-treatments (Vega-Gálvez et al. 2012; Llavata et al. 2019) have been applied in various products, obtaining good results. However, most of the cited technologies still have disadvantages and challenges: for example, they are not available to all, they involve sophisticated knowledge or require specialized maintenance, and there is still no industrial-scale equipment. Therefore, simple but effective techniques are still required.
In this context, a good alternative is the use of different compounds that can accelerate the drying process, here named “drying accelerators”. Moreover, they can also improve the final product properties. In fact, some studies have already considered some organic compounds as drying accelerators, ethanol being the most applied. Ethanol improved the convective drying of pineapple slices (Corrêa et al. 2012); pumpkin (Rojas and Augusto 2018b; Rojas et al. 2020a); and banana (Corrêa et al. 2012); the microwave and convective drying of apple slices (Funebo et al. 2002); the pulsed vacuum drying of apple slices (Amanor-Atiemoh et al. 2020); the natural convective and vacuum drying of okara (Umbelina et al. 2018); fluidized bed drying of structured samples (Tatemoto et al. 2015); the ultrasound-assisted convective drying of apple slices (Rojas et al. 2020a); and the infrared drying of potato (Rojas and Augusto 2018a) and scallion (Wang et al. 2019b). Higher drying rates, when compared to the drying of control samples, were reported in all of them.
Although it is expected the compound vapour pressure is an important property during convective drying, determining its tendency to evaporate, other properties can induce other phenomena, also affecting drying. For instance, the surface tension can induce the Marangoni effect (Silva et al. 2012), which can also contribute to water removal during pre-treatment and drying. Moreover, both viscosity and density can make the fluid flow inside the product structure difficult, for example, in capillaries. The relationship among these variables is complex, and other compounds must be evaluated, considering both their possible effectiveness and as an artifice to understand the associated mechanisms.
Regarding other compounds, acetone and acetic acid started to be studied by Silva et al. (2012), and isopropanol by Tatemoto et al. (2015), both considering the drying of foods. Acetone and methanol have been used in the dehydration protocols of biological samples in laboratories (Pathan et al. 2010). However, the effect on drying kinetics has not yet been adequately described.
This study explored and better described the effects of applying acetone (AC), ethanol (ET), isopropanol (ISO) and acetic acid (AA) as pre-treatments to convective drying of pumpkin. The effect on the microstructure, drying and rehydration kinetics, and viscoelastic properties was evaluated.
Materials and Methods
Sample Preparation
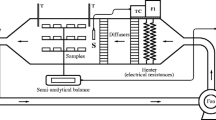
Fresh pumpkin (Cucurbita moschata Duch.) was obtained from a local market (Piracicaba, São Paulo, Brazil). Pumpkin was selected as a structural representative model for plant food due to its tissue type, principally parenchymatic and vascular tissue (Rojas and Augusto 2018b). Moreover, it can be used both as food (to be consumed directly dried and also after rehydration) and as industrial product for carotenoid extraction (as a natural pigment, pro-vitamin, etc.). In natura pumpkin cylinders (0.3 cm thick and 1.5 cm in diameter) (Fig. 1) were obtained just previously to processing from the same pulp region using a sharp stainless-steel tube considering a longitudinal cut.
Pre-treatments
The in natura pumpkin cylinders were pre-treated by immersion in ethanol (ET), isopropanol (ISO), acetone (AC) or acetic acid (AA) (up to 99% v/v) at 25 °C for 5 min using a ratio of 0.3:1 (w/v) of pumpkin cylinders:accelerator compound. The samples were removed from the compounds, drained and superficially dried with paper towels. Then, the pre-treated samples, including control treatment (CT), were dried (Fig. 1).
Microstructure Analyses
The sample microstructures were evaluated after each pre-treatment by light microscopy. The microscopy was carried out using an optical microscope (L100 Bioval, Curitiba) with a halogen lamp of 20 W coupled to a portable colour camera of 5 MP. The microstructure was analysed from the surface and internal tissues of the samples, which were cut into ~ 20-μm dishes using a microtome (CUT 4062, Slee Mainz, Germany). The images were captured after guaranteeing a representative field after several visualizations.
Drying
The convective drying was carried out in an automated tray dryer (UOP8MKII, Armfield, England) with air at 39.8 ± 0.6 °C and 1.06 ± 0.02 m/s velocity. The pumpkin cylinders were placed on a stainless-steel net to promote the circulation of warm air through the samples and promote the largest contact area. The mass of the samples was continuously measured by the weighing system of the dryer, composed by three load cells. The samples were dried until constant weight was reached.
Initial and final (after drying) moisture contents were measured by completely drying crushed samples at 105 °C in a moisture analyser (MX-50, A&D Company, Japan).
During the sample pre-treatment, two instances of mass flux occur: water outlet and accelerator compound inlet (Rojas and Augusto 2018b). Therefore, the moisture content after the pre-treatment (Mp%) (Eq. (1)) includes both the remaining water (wp) and the amount of compound (cp) which enters the sample. Consequently, the moisture content (Mp,t%) along the drying time (Eq. (2)) includes both the volatile substances, the water and the compound which are still in the sample.
Drying curves were plotted with dimensionless moisture content (MR) (Eq. (3)) as a function of drying time, where Mt is the moisture content during the drying process time (t), Meq is the equilibrium moisture and M0 is the initial moisture after pre-treatment with compounds (Mp). For the control sample, M0 is the initial moisture of the in natura (fresh) pumpkin.
In addition, along the pumpkin drying process, thermal images of each sample were recorded by using an infrared camera (Testo, Testo 865, Germany) with 0.95 of emissivity. The thermal images were analysed using the software IRSoft 4.5 (Testo SE & Co, Germany).
Rehydration
Rehydration was carried out by immersion of the dried cylinders in distilled water at 24 ± 1 °C. The ratio of dried pumpkin to water was 0.003:1 (w/w), in order to avoid limitations during rehydration in relation to water availability. The absorption of water by samples was determined by mass balance. For this, the slices were removed from the water, superficially dried with absorbent paper, weighed and returned to the water until the end of the rehydration process, when constant mass was registered.
Viscoelastic Properties: Stress-Relaxation Behaviour
The stress-relaxation analysis was used to describe the viscoelastic changes of samples after pre-treatments and processes, compared with those of the in natura pumpkin. It consists of an instantaneous deformation to a sample, maintaining the strain constant and then evaluating the related stress over the time (Rao and Steffe 1992). The stress-relaxation behaviour of the in natura and rehydrated samples (control, ethanol, ethanol + US and water + US) was evaluated using a Texture Analyser (TA.XT Plus, Stable Micro Systems Ltd., Surrey, UK) with a load cell of 50 kg-f (490.03 N) and a 35-mm aluminium cylindrical probe (P/35R). The cylinders were firstly compressed until a strain of 0.20 with 1 mm s−1 velocity. Then, the deformation was maintained constant for 20 s, the data of force (N) versus time (s) being recorded every 0.005 s to detailing analysing the relaxation curves and the model adjustment. From each treatment, 10 replicates were evaluated.
Mathematical Description of Drying, Rehydration and Stress Relaxation
The experimental drying data were fitted using the Page model (Eq. (4)) (Page 1949).
According to Simpson et al. (2017), the drying rate parameter (k, min−n) could be associated with the diffusion coefficient and the geometry of the sample, while the dimensionless drying parameter (n, dimensionless) describes the “type of diffusion” (n > 1 super-diffusion and n < 1 sub-diffusion), which can be related with the sample microstructure and mechanisms of mass transfer.
The rehydration data were fitted using Peleg’s model (Eq. (6)) (Peleg 1979), where k1 is the constant rate (describing the reciprocal of the initial absorption water rate), and k1 (min d.b−1) and k2 (d.b−1) is the constant of capacity, which is inversely related to the equilibrium moisture reached during the process.
To describe the viscoelastic behaviour, the data was fitted to the Peleg model of viscoelasticity (Peleg 1979), described in Eq. (6). The Peleg model (Eq. (6)) is an empirical model with only two parameters (I1, I2), but excellent fitting for different materials. In fact, it has already been used to successfully describe different materials. The reciprocal of I1 represents the initial decay rate, while the reciprocal of I2 represents the hypothetical asymptotic level of the normalized relaxation curve (Peleg 1979; Peleg and Normand 1983).
The models (Eqs. (4)–(6)) were fitted by nonlinear regression, whose parameters were determined by minimizing the sum of squared errors (SSE in Eq. (7)). The estimation of the percentage of explained variance (%Var), according to Eq. (8), was \( {S}_{\mathrm{cal}}^2 \) is the variance of calculated data and \( {S}_{\mathrm{exp}}^2 \) is the variance of the experimental data as used to evaluate how accurately the models fit the experimental data.
Experimental Design and Statistical Analysis
A completely randomized design (CRD) was conducted. The analysis of variance (ANOVA) was carried out with a significance level of 5%. To determine differences among averages of pre-treatments, Tukey test was used. Statistical analyses were performed using IBM SPSS Statistics 23 software (IBM SPSS, USA).
Results and Discussion
Microstructure Modifications After Pre-treatments
Figure 2 shows the microstructure of the in natura (IN) pumpkin, as well as after the pre-treatment with ethanol (ET), isopropanol (ISO), acetone (AC) and acetic acid (AA). The pumpkin tissue is mainly composed of parenchymatic and vascular tissue (xylem vessels) (Rojas and Augusto 2018c). Since the xylem vessels are rigid elements composed of cellulose, hemicellulose and lignin, they did not show structure modifications after treatments. No difference in the structure of xylem vessels was also evidenced after ethanol pre-treatments in pumpkin (Rojas and Augusto 2018b). However, Fig. 2 shows the modifications observed in parenchymatic tissue.
Microstructure modification in pumpkin tissue after pre-treatments with acetone (AC), ethanol (ET), isopropanol (ISO) and acetic acid (AA), in comparison with the in natura (IN) sample. Both external (ex, which was in contact with the accelerator compound) and internal (in, with high magnification) tissues are shown
In the in natura (IN) sample, the cells containing chromoplasts (cr) and the cell wall (cw) with air in the intercellular spaces were evidenced. After the pre-treatments, in all cases, the external surface of the pumpkin cylinders showed a contraction of the tissue with agglomerated cells. Since the external surface is in direct contact with the accelerator compounds, this was probably a result of water extraction and loss of some solutes during pre-treatment. However, in the AC treatment, these external modifications were less pronounced. The acetone probably did not exert as much effect on the outer surface as it was easily distributed into the sample.
In addition, the continuous tissue (in) close to the surface showed some changes mainly in the cell wall. In the case of ethanol and isopropanol treatments, the cells did not show significant changes in size, but their walls were thinning. This is a consequence of the extraction of intercellular air and some cell wall compounds, which was reported in previous studies with ethanol (Rojas and Augusto 2018a, b; Funebo et al. 2002). Similar effects could occur during the pre-treatments with isopropanol and acetone. In the case of the samples treated with acetone, their cells look wilted, with a wrinkled appearance. The same was observed in the treatments with acetic acid, where the cells also showed a plasticized appearance.
This is the first time the pumpkin microstructure is evaluated after pre-treatments with acetone (AC), isopropanol (ISO) and acetic acid (AA), and maybe any vegetable material with focus on further drying. Therefore, it is important to highlight the biological materials are anisotropic and became even more after pre-treatments, as well as the need to evaluate it.
The changes in the composition and structure of the cells affect the micro- and macrostructural characteristics (Aguilera and Stanley 1999). These structure modifications can be evidenced indirectly through viscoelastic property analysis. As observed in Fig. 3 and Table 4, the in natura (IN) pumpkin showed the highest stress and remained higher along the stress-relaxation time, compared to the pre-treated samples. This behaviour could be attributed to the integrity of their cell walls, the higher cell bonding and high turgor pressure in the in natura samples, which are the major contributors to determining the mechanical properties (Mayor et al. 2007; Lin and Pitt 1986; Smith et al. 2003). These characteristics were modified when pre-treatments were applied. To explain these modifications, the data were described using the Peleg model, whose parameters are shown in Table 4.
Stress-relaxation behaviour of samples after pre-treatments with acetone (AC), ethanol (ET), isopropanol (ISO) and acetic acid (AA), in comparison with the in natura (IN) sample. Continuous curves indicate the mean of experimental data, the shaded bands are the confidence intervals (95%) and the dotted curves are the Peleg model for viscoelasticity (Eq. (6))
The I2 parameter in the Peleg model is considered as “a representation of the solidity degree”: for fluids, I2 = 1, while for solids, I2→∞ (Peleg and Normand 1983). Therefore, the highest I2 values shown for the fresh control samples show the elasticity or “solidity” of these samples is higher than that for the pre-treated ones.
In a previous work (Rojas and Augusto 2018b), a correlation between the viscous and elastic characteristics with the elements and composition of the sample has been performed, taking pumpkin as a reference. The elastic characteristics have been related to the rigid structures of the microstructure, being mainly attributed to the cell wall and the internal pressure of the cells (turgor). On the other hand, the viscous characteristics have been correlated with the fluid composition (aqueous phase and air), which are compressible. In addition, according to Smith et al. (2003), the turgor of the cells depends on the cell wall integrity and their aqueous quantity and composition.
Therefore, the lower I2 in pre-treated samples makes sense since, as mentioned, modifications in the microstructure were evidenced during the pre-treatments, mainly in the cell wall. In addition, there was air and water outlet and entry of accelerator compounds inside the sample tissue. These modifications cause the cells to be weakened and lose turgidity, being easier to deform, reducing their elastic characteristics (lower I2 values). Particularly, the samples that showed the lowest I2 values were those pre-treated with AC and AA, in which higher structure modifications were also evidenced in the internal tissue cells (Fig. 2).
Figure 4 summarizes the relationship between the microstructure and the viscoelastic responses observed. It shows the characteristics of the samples before (in natura) and after the pre-treatment and how they have influenced the described viscoelastic behaviour.
The structure modifications caused by the pre-treatments can directly impact the water flow during drying and rehydration, as evaluated as follows.
Drying Kinetics
The fresh pumpkin samples presented a moisture of 9.1 ± 3 (g water/g dry matter), which after pre-treatments was not significantly (p < 0.05) modified. Just after pre-treatments, the samples were convectively dried.
The drying time needed by the control samples to achieve a moisture of 25% (in wet basis) was 426 ± 57 min. It was reduced up to 16% for ET, 9% for ISO, 22% for AC and 12% for AA. These reductions were similar to time reductions reported previously. For example, 13.4% (to achieve MR = 0.02) in apple slices pre-treated with ethanol (96% for 3 min) and convectively dried (1.5 m/s, 70 °C) (Zubernik et al. 2019); 16% in guaco leaves pre-treated with ethanol (99.5% for 5 s) and convectively dried (0.42 m/s at 50 °C) (Silva et al. 2018); 24.6% in scallion slices pre-treated with ethanol (70% for 10 min) and dried using infrared-hot air (60 °C, 2 m/s) (Wang et al. 2019b); and 30% in potato slices pre-treated with ethanol (92.8% for 3 min) and infrared dried at 100 °C (Rojas and Augusto 2018a). It is important to mention that, at greater drying accelerator concentration and longer pre-treatment time, high effects could be observed. Reductions of more than 50% were found during conventional drying of pumpkin (Rojas and Augusto 2018b; Rojas et al. 2020b) and apple samples (Rojas et al. 2020a), all pre-treated by immersion in ethanol but for longer times (from 15 to 60 min).
Figure 5 shows the dimensionless drying data for all pre-treatments, which were described by using the Page model, whose parameters are shown in Table 1. The kinetic parameter (k) showed a tendency to increase with pre-treatment application, although no significant differences (p < 0.05) were evidenced among them due to high variability.
Dimensionless moisture (MR) data along convective drying time for ethanol (a), isopropanol (b), acetone (c) and acetic acid (d) all compared to the control data. The dots are experimental MR for each pre-treatment, the vertical bars are the standard deviation and the continuous lines are the calculated values according to the Page model (Eq. (4), red line)
To date, the application of the compounds here proposed has not been studied or still needs to be understood—with the exception of the application of ethanol.
Different explanations for the drying acceleration due to ethanol application were previously proposed. Drying enhancement is attributed to water transfer mechanisms promoted by ethanol properties, such as its higher vapour pressure and lower surface tension, if compared to water properties (Corrêa et al. 2012; Silva et al. 2012). Only a few works evaluated the product structure, attributing to changes on structure and composition of cell wall permeability and then drying behaviour (Funebo et al. 2002; Rojas and Augusto 2018a, b).
Table 2 shows selected physical properties of the used drying accelerator compounds. Despite the big differences in vapour pressure, all used compounds present similar surface tension values, but which are three times lower than the surface tension of water. Therefore, the vapour pressure alone cannot describe the observed behaviour in drying curves, once their higher difference did not result in higher effects during drying. Therefore, and considering their similar behaviour during drying among pre-treatments (Fig. 5, Table 1), the effect of surface tension in drying acceleration must be considered. The mass transfer phenomenon which depends on surface tension is the Marangoni effect, which was first introduced to the drying of foods by Silva et al. (2012), being detailed by Rojas and Augusto (2018b).
Even so, the AC and AA pre-treatments showed the highest and lowest vapour pressure and, at the same time, they showed the lowest and the highest equilibrium moisture, respectively. The component properties as vapour pressure, surface tension and density are determinant to facilitate the drying process and, additionally with the viscosity, fluid flow, vaporization and vapour flow, micro- and macrostructural changes during pre-treatment and also drying can affect the drying rate and equilibrium moisture (Silva et al. 2012). However, the exact contribution of each physical property and mechanism still needs to be understood, which highlights the food drying process using drying accelerators is a complex phenomenon.
In addition, the surface temperature profiles among pre-treatments were evaluated during drying. Figure 6 shows that there were differences in the surface temperature detected in the samples throughout processing. As observed in Table 2, both ethanol and isopropanol have similar vapour pressure values; at the same time, the surface temperature of the samples pre-treated with these compounds (ET and ISO) showed similar behaviour. On the other hand, at the beginning of drying, up to approximately 30 min, the lowest observed temperature corresponds to the AC pre-treatment, while the highest temperature corresponds to the AA pre-treatment, whose compounds have the highest and lowest vapour pressure, respectively. During drying, phase changes (liquid to vapour) occur, which involves the use of energy (heat) absorbed from the surface of the samples, then impacting on the surface temperature. Therefore, a liquid with high vapour pressure, as the case of acetone, evaporates faster and at lower temperature. This explains the differences observed at the beginning of drying among temperature profiles of pre-treated samples compared to control (Fig. 6).
Up: average surface temperature profile for control (CT), ethanol (ET), isopropanol (ISO), acetone (AC) and acetic acid (AA) treatments along the drying process until 240 min (dotted curves are the experimental data and shaded bands are the confidence interval at 95%). Down: thermal images acquired along the drying time for each treatment
Upon reaching a processing time of approximately 30 min, all treatments showed similar surface temperatures, up to 120 min. From this time, some differences can again be observed among treatments, probably because at this stage the moisture content begins to have more influence on the temperature profile behaviour. In fact, from 120 min, the reduction in moisture (with respect to its initial moisture) in the pre-treated samples was greater than in the control samples: the pre-treated samples were increasingly drier than the control samples, having lost at 240 min more than 60% of their initial moisture, while the control samples showed a reduction of 20%.
Consequently, compared to the vapour pressure effect, the surface tension may have a greater effect on drying acceleration, as proposed by Silva et al. (2012). Vapour pressure may have a greater influence on both the superficial temperature profile of samples along drying and on the residual amount of the compound at the end of the drying process.
Furthermore, the n value of the Page model did not show significant changes with pre-treatments. For all cases, n indicates a super-diffusion behaviour (n > 1) (Simpson et al. 2017), as reported by Rojas and Augusto (2018b) and Rojas et al. (2020b) during pumpkin drying. Our interpretation, as in previous works, is that when n ≠ 1, other mechanisms than a purely diffusive mass transfer are significant, such as capillarity and mass flow induced by structural changes during processing. Those mechanisms occur due to the samples’ heterogeneous structure, with different tissues and cells, as well as the different changes related to the pre-treatments and processing.
Therefore, although the modifications by the pre-treatments carried out here were not enough to reflect differences in the model parameters associated with the mechanisms of drying, they hastened drying and made it possible to reduce the drying time. Once again, we highlight reductions up to 22% in drying time were achieved, which are representative values. In fact, the reduction in drying time outweighs the 5 min used during pre-treatments and, in turn, would have a direct effect on reducing energy consumption during the drying process.
In fact, the industrial drying is an energy-intensive process. Moreover, drying is considered a fuel-intensive activity, once the high-temperature industrial dryers are powered specially by propane and electricity (EIA 2018). Therefore, the energy consumption is an important issue and there is a demand for minimal environmental impact due to the combustion of fossil fuels used to provide energy for drying (Kudra and Mujumdar 2009). For example, 4–5% of the total amount of energy consumed only in the USA is related to drying processes, representing 11% of the industrial energy consumption (Sholl and Lively 2016).
As described by Rojas et al. (2020b), an estimate of the energy consumption during convective drying can be obtained by considering the product, air and process properties, being linearly related with the drying time. Therefore, the reduction in drying time here reported can also be interpreted as a direct reduction on energy consumption, highlighting once again the relevance of the obtained results.
Rehydration
After drying, the samples have experimented different modifications, which could influence the rehydration behaviour. During drying, the amount and the way in which the water left the product being dried have a great influence on the integrity of the product structure, in addition to the shrinkage and deformation achieved. All these effects cause compactness of tissue, cell wall breaking, and cell-debonding mechanism (reduction of the contact area between cells) (Lin and Pitt 1986; Mattea et al. 1989).
The rehydration behaviour (Fig. 7) was described using the Peleg model, whose parameters are shown in Table 3. It was observed that the initial absorption water rate (k1) was similar (p < 0.05) for all treatments except for the AA pre-treatment, which showed the highest k1 value, indicating a lower rehydration rate. Probably, the changes in the structure produced by this pre-treatment in the cells and tissue (Fig. 2) were abrupt and irreversible after drying, therefore decreasing its rehydration rate.
Rehydration kinetics of pumpkin cylinders convectively dried after different pre-treatments: control (CT), acetone (AC), ethanol (ET), isopropanol (ISO) and acetic acid (AA). Squares are the experimental data (average), vertical bars are the standard deviation and dotted lines are the Peleg model for hydration (Eq. (6)). The horizontal line represents the moisture of in natura pumpkin
When compared to control (CT), the ET and ISO pre-treatments improved the water retention; they showed the lowest k1 and k2 values (p < 0.05) (Table 3), indicating higher water retention. This rehydration improvement was also reported in previous works where ethanol was used as pre-treatment, such as apple (Funebo et al. 2002), pumpkin (Rojas and Augusto 2018b) or scallion (Wang et al. 2019b). Furthermore, the product final moisture of pre-treated samples was 22% (ET) and 37% (ISO) higher than the final moisture of control samples, and even 12% (ISO) higher than the moisture of the in natura pumpkin.
The AC pre-treatment did not affect the rehydration, since both rehydration curve and Peleg parameters were similar to the CT. On the other hand, the AA pre-treatment showed negative effects in relation to rehydration properties: the product final moisture was only 47% of the in natura pumpkin.
In the rehydration process, in addition to the product having good absorption and water retention, another objective is that it maintains a structure as close as possible to the original product (in natura). Therefore, the sample viscoelastic properties were evaluated after rehydration (Fig. 8 and Table 4) in order to show how the modifications of the previous processes (pre-treatments + drying + rehydration) have affected their structure.
Stress-relaxation behaviour of samples after pre-treatment, drying and rehydration. Pre-treatments: control (CT), ethanol (ET), isopropanol (ISO), acetone (AC) and acetic acid (AA). Continuous curves indicate the mean of experimental data, the shaded bands are the confidence intervals (95%) and the dotted curves are the Peleg model for viscoelasticity (Eq. (6)). See the detail at the top right for better visualization
After rehydration, the I2 decreased highly for all treatments if compared to the in natura characteristics (Table 1): the I2 value decreased in similar percentages for CT (29%), ET (32%), ISO (33%), AC (34%) and AA (29%) regarding the in natura pumpkin. This indicates after rehydration all treatments lose part of their elasticity, which is associated with all the effects mentioned in previous sections, that there were modifications in structure and composition after pre-treatments and after drying. On the other hand, it was previously reported that during rehydration the fibres and cells get more separated due to the entrance of water in the intercellular spaces (Mayor et al. 2007). Consequently, the rehydrated samples fail at lower stress and the structure modifications conferred low resistance to flow, reflecting the observed decrease in elasticity.
On the other hand, by comparing the I2 value among the samples after rehydration, it was observed that the AA pre-treated samples showed a high elastic character (high I2 value), contrary to what was observed after pre-treatment (Table 4). Probably, it was explained by the amount of absorbed water: in the AA samples occurred the low water absorption during rehydration (Fig. 7, Table 4). Therefore, the lower the amount of water inside the samples, the lower the compressibility of the samples.
As general mode, all the rehydrated samples showed a decrease in their elastic character as a consequence of the modifications in their structure and composition along processing. However, it is interesting to note that the applied pre-treatments did not negatively influence the viscoelastic characteristics when compared to those of the control treatment. Therefore, drying and rehydration could be improved through purposed pre-treatments without compromising macroscopic characteristics as reflected in the viscoelasticity of the product.
Conclusions
The application of ethanol, isopropanol, acetone and acetic acid was studied as drying accelerators of pumpkins. Pre-treatments changed the microstructure, which was different for each compound, reinforcing anisotropy. Comparing with the control sample, all pre-treatments decreased the drying time. Possible mechanisms were discussed, related to physical properties of the accelerator. The surface tension possibly has a greater effect on drying acceleration while the vapour pressure possibly has a greater influence on the sample surface temperature profiles and on the residual amount of the used compound at the end of drying. The pre-treatments with ethanol and isopropanol increased the absorbed water during rehydration, reaching 12% more water than in natura samples. On the other hand, while acetone was effective in accelerating drying, it did not affect rehydration, while acetic acid decreased the rehydration capacity. The pre-treatment, drying and rehydration effects on structure and composition were reflected in the viscoelastic properties. The treatments that showed high structure modifications (AA and AC) also showed loss of elastic properties. Ethanol seemed to be the most viable compound to be applied in food products. However, the other compounds could also be applied for other applications, such as pharmaceutical and cosmetics. Further studies are necessary to better describe the effects of treatments on possible products.
References
Aguilera, J. M., & Stanley, D. W. (1999). Microstructural principles of food processing and engineering. Springer Science & Business Media.
Amanor-Atiemoh, R., Zhou, C., Abdullaleef Taiye, M., Sarpong, F., Wahia, H., Amoa-Owusu, A., Ma, H., & Chen, L. (2020). Effect of ultrasound-ethanol pretreatment on drying kinetics, quality parameters, functional group, and amino acid profile of apple slices using pulsed vacuum drying. Journal of Food Process Engineering., 43(2), e13347.
Corrêa, J. L. G., Braga, A. M. P., Hochheim, M., & Silva, M. A. (2012). The influence of ethanol on the convective drying of unripe, ripe, and overripe bananas. Drying Technology., 30(8), 817–826.
de Oliveira, M. M., Tribst, A. A. L., de Castro Leite Jr., B. R., de Oliveira, R. A., & Cristianini, M. (2015). Effects of high pressure processing on cocoyam, Peruvian carrot, and sweet potato: changes in microstructure, physical characteristics, starch, and drying rate. Innovative Food Science & Emerging Technologies, 31, 45–53.
EIA. (2018). Energy Information Administration: today in energy. Available at https://www.eia.gov/todayinenergy/detail.php?id=32912. Accessed 5/09/2018 2018.
Fernandes, F. A. N., Rodrigues, S., Law, C. L., & Mujumdar, A. S. (2011). Drying of exotic tropical fruits: a comprehensive review. Food and Bioprocess Technology., 4(2), 163–185.
Funebo, T., Ahrné, L., Prothon, F., Kidman, S., Langton, M., & Skjöldebrand, C. (2002). Microwave and convective dehydration of ethanol treated and frozen apple – physical properties and drying kinetics. International Journal of Food Science & Technology., 37(6), 603–614.
Gong, C., Liao, M., Zhang, H., Xu, Y., Miao, Y., & Jiao, S. (2020). Investigation of hot air–assisted radio frequency as a final-stage drying of pre-dried carrot cubes. Food and Bioprocess Technology, 1–11.
INCHEM I. (2020). Internationally peer reviewed chemical safety information. In. p^pp. World Health Organization (WHO).
Kudra, T., & Mujumdar, A. S. (2009). Advanced drying technologies. CRC Press.
Lin, T. T., & Pitt, R. (1986). Rheology of apple and potato tissue as affected by cell turgor pressure. Journal of Texture Studies., 17(3), 291–313.
Liu, Y., Zeng, Y., Hu, X., & Sun, X. (2020). Effect of ultrasonic power on water removal kinetics and moisture migration of kiwifruit slices during contact ultrasound intensified heat pump drying. Food and Bioprocess Technology., 13(3), 430–441.
Llavata B, García-Pérez JV, Simal S & Cárcel JA (2019) Innovative pre-treatments to enhance food drying: a current review. Current Opinion in Food Science.
Mattea, M., Urbicain, M. J., & Rotstein, E. (1989). Computer model of shrinkage and deformation of cellular tissue during dehydration. Chemical Engineering Science, 44(12), 2853–2859.
Mayor, L., Cunha, R., & Sereno, A. (2007). Relation between mechanical properties and structural changes during osmotic dehydration of pumpkin. Food Research International, 40(4), 448–460.
Monteiro, R. L., Link, J. V., Tribuzi, G., Carciofi, B. A. M., & Laurindo, J. B. (2018). Microwave vacuum drying and multi-flash drying of pumpkin slices. Journal of Food Engineering, 232, 1–10.
Mujumdar, A. S., & Law, C. L. (2010). Drying technology: trends and applications in postharvest processing. Food and Bioprocess Technology, 3(6), 843–852.
Ostermeier, R., Giersemehl, P., Siemer, C., Töpfl, S., & Jäger, H. (2018). Influence of pulsed electric field (PEF) pre-treatment on the convective drying kinetics of onions. Journal of Food Engineering, 237, 110–117.
Page, G. E. (1949). Factors influencing the maximum rates of air drying shelled corn in thin layers. Ann Arbor: Purdue University.
Pathan, A. K., Bond, J., & Gaskin, R. E. (2010). Sample preparation for SEM of plant surfaces. Materials Today, 12, 32–43.
Peleg, M. (1979). Characterization of the stress relaxation curves of solid foods. Journal of Food Science, 44(1), 277–281.
Peleg, M., & Normand, M. D. (1983). Comparison of two methods for stress relaxation data presentation of solid foods. Rheologica Acta, 22(1), 108–113.
PubChem. (2020). Chemistry database at the National Institutes of Health (NIH). In. p^pp. National Library of Medicine, Bethesda.
Rao, M. A., & Steffe, J. F. (1992). Viscoelastic properties of foods. New York: Elsevier Applied Science.
Rodríguez, Ó., Eim, V., Rosselló, C., Femenia, A., Cárcel, J. A., & Simal, S. (2019). Application of power ultrasound on the convective drying of fruits and vegetables: effects on quality. Journal of the Science of Food and Agriculture, 99(2), 966–966.
Rojas, M. L., & Augusto, P. E. D. (2018a). Ethanol and ultrasound pre-treatments to improve infrared drying of potato slices. Innovative Food Science & Emerging Technologies, 49, 65–75.
Rojas, M. L., & Augusto, P. E. D. (2018b). Ethanol pre-treatment improves vegetable drying and rehydration: kinetics, mechanisms and impact on viscoelastic properties. Journal of Food Engineering, 233, 17–27.
Rojas, M. L., & Augusto, P. E. D. (2018c). Microstructure elements affect the mass transfer in foods: the case of convective drying and rehydration of pumpkin. LWT., 93, 102–108.
Rojas, M. L., Augusto, P. E. D., & Cárcel, J. A. (2020a). Ethanol pre-treatment to ultrasound-assisted convective drying of apple. Innovative Food Science & Emerging Technologies, 61, 102328.
Rojas, M. L., Silveira, I., & Augusto, P. E. D. (2020b). Ultrasound and ethanol pre-treatments to improve convective drying: drying, rehydration and carotenoid content of pumpkin. Food and Bioproducts Processing, 119, 20–30.
Sholl, D. S., & Lively, R. P. (2016). Seven chemical separations to change the world. Nature., 532(7600), 435–437.
Silva, M. A., Braga, A. M. P., & Santos, P. H. S. (2012). Enhancement of fruit drying: the ethanol effect. In: Proceedings of the 18th International Drying Symposium (IDS 2012), Xiamen.
Silva, M. G., Celeghini, R. M. S., & Silva, M. A. (2018). Effect of ethanol on the drying characteristics and on the coumarin yield of dried guaco leaves (Mikania laevigata SCHULTZ BIP. EX BAKER). Brazilian Journal of Chemical Engineering, 35(3), 1095–1104.
Simpson, R., Ramírez, C., Nuñez, H., Jaques, A., & Almonacid, S. (2017). Understanding the success of Page’s model and related empirical equations in fitting experimental data of diffusion phenomena in food matrices. Trends in Food Science & Technology, 62, 194–201.
Smith, A. C., Waldron, K. W., Maness, N., & Perkins-Veazie, P. (2003). Vegetable texture: measurement and structural implications. Postharvest Physiology and Pathology of Vegetables, 2, 297–329.
Szadzińska, J., Łechtańska, J., Pashminehazar, R., Kharaghani, A., & Tsotsas, E. (2018). Microwave-and ultrasound-assisted convective drying of raspberries: drying kinetics and microstructural changes. Drying Technology, 1–12.
Tatemoto, Y., Mizukoshi, R., Ehara, W., & Ishikawa, E. (2015). Drying characteristics of food materials injected with organic solvents in a fluidized bed of inert particles under reduced pressure. Journal of Food Engineering, 158(supplement C), 80–85.
ToolBox E. (2020). Vapor pressure common liquids. Available at https://www.engineeringtoolbox.com/. Accessed 04/20/2020 2020.
Umbelina, A., Luiz, J., Corrêa, G., Letícia Almeida, O., Francemir José, L., & Elias, R. (2018). The influence of ethanol and vacuum on Okara drying.
Vallespir, F., Rodríguez, Ó., Eim, V. S., Rosselló, C., & Simal, S. (2018). Freezing pre-treatments on the intensification of the drying process of vegetables with different structures. Journal of Food Engineering, 239, 83–91.
Vega-Gálvez, A., Lara, E., Flores, V., Di Scala, K., & Lemus-Mondaca, R. (2012). Effect of selected pretreatments on convective drying process of blueberries (var. O’neil). Food and Bioprocess Technology, 5(7), 2797–2804.
Wang, J., Xiao, H.-W., Ye, J.-H., Wang, J., & Raghavan, V. (2019a). Ultrasound pretreatment to enhance drying kinetics of kiwifruit (Actinidia deliciosa) slices: pros and cons. Food and Bioprocess Technology, 12(5), 865–876.
Wang, X., Feng, Y., Zhou, C., Sun, Y., Wu, B., Yagoub, A. E. A., & Aboagarib, E. A. A. (2019b). Effect of vacuum and ethanol pretreatment on infrared-hot air drying of scallion (Allium fistulosum). Food Chemistry, 295, 432–440.
Zubernik, J., Dadan, M., Cichowska, J., & Witrowa-Rajchert, D. (2019). The impact of the pre-treatment in ethanol solution on the drying kinetics and selected properties of convective dried apples. International Journal of Food Engineering, 16(1–2), 11.
Acknowledgments
The authors are grateful to the São Paulo Research Foundation (FAPESP, Brazil) for funding project n° 2019/05043-6, the GR Carvalho post-doctoral fellowship (2018/17844-0) and the I Silveira BSc scholarship (2019/19307-5); to the National Council for Scientific and Technological Development (CNPq, Brazil) for the productivity grant of PED Augusto (306557/2017-7); and to the Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica (FONDECYT, Peru) from the “Consejo Nacional de Ciencia, Tecnología e Innovación Tecnológica” (CONCYTEC, Peru) for funding project n° 409-2019-FONDECYT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Drying accelerators were evaluated to enhance the process and the properties of pumpkin.
• Pre-treatments promoted structural changes in the product, reflecting on stress-relaxation behaviour.
• The structural changes modified drying and rehydration.
• Drying time was reduced from 9 to 22% depending on the compound used.
• Ethanol and isopropanol accelerated rehydration and increased the absorbed water.
Rights and permissions
About this article
Cite this article
Carvalho, G.R., Rojas, M.L., Silveira, I. et al. Drying Accelerators to Enhance Processing and Properties: Ethanol, Isopropanol, Acetone and Acetic Acid as Pre-treatments to Convective Drying of Pumpkin. Food Bioprocess Technol 13, 1984–1996 (2020). https://doi.org/10.1007/s11947-020-02542-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02542-6