Abstract
Rice is an important component of many ready-to-eat frozen and refrigerated products. Changes in temperature during storage or handling of frozen cooked rice may lead to thermal transitions, ice recrystallization, and subsequent adverse changes in physicochemical properties. In this study, we explored the kinetics of starch retrogradation over a 12-week storage in frozen cooked rice of two varieties: 8% amylose and 0% amylose. Cooked rice was frozen at − 32 ± 1 °C for 5 days to achieve a glassy state and then subjected to temperature fluctuations of varying amplitudes to achieve different physical states (e.g., glassy, rubbery, and thawed states). The temperature fluctuation refers to regular/cyclic change from storage temperature to preset temperature. Starch retrogradation enthalpy was measured using differential scanning calorimetry. There was significant (p < 0.05) increase in retrogradation enthalpy with the 12-week storage time, in both varieties of rice. Among the treated samples, with increased degree of temperature fluctuation, there was significant (p < 0.05) increase in retrogradation enthalpy: rice with temperature that fluctuated to the thawed state showed the highest increase in retrogradation enthalpy. A lower degree of starch retrogradation was observed in 8% amylose rice, indicating a lower susceptibility to changes in physicochemical properties as a result of retrogradation during frozen storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Throughout the world, higher incomes and lifestyle changes have led to an increase in the consumption and purchase of frozen meals, impacting the quality of frozen meals, from manufacture to consumer, through cold chain distribution. An increase in the number of people joining the workforce, single-person households, and multi-income families and the expansion of food service have contributed to the growth of frozen food industry, leading to a variety of available product categories and items (Yam and Lai 2004).
Temperature fluctuations in cold distribution chains may cause repeated thawing and refreezing in a frozen food. It may go through state and phase transitions, altering the mobility of water and ratio of ice and unfrozen water present in the food. It is important to consider whether reformulating a food is necessary to reduce quality loss from temperature fluctuations. Since freezing involves conversion of water into ice crystals and ice is less dense than water, freezing causes food to expand. Therefore, subsequent thawing and refreezing may lead to changes in physical, chemical, and biological properties of food from tissue damage, and shifts in pH due to changes in solute concentration and precipitation of intracellular constituents. Temperature fluctuation can also cause surface desiccation and textural changes.
Cooked rice is a common component of many foods available to consumers in frozen and refrigerated forms. In studies on starch-based foods, Shi and Gao (2016) reported a decrease in digestibility in gelatinized wrinkled pea starch gel (30.8% amylose content) in 21 days (d). This was due to an increase in the slowly digesting starch (SDS) component at storage temperature cycles between 4 and 30 °C for 1 day each. Early-stage amylopectin retrogradation favors SDS formation (Zhang et al. 2008). The digestibility of starch in the human small intestine can range from rapid to indigestible (Englyst et al. 1992). SDS is the structure of starch with slow digestibility and is an intermediate fraction. This is evident in native maize starch, which causes slow and sustained glucose levels in the blood upon consumption (Lehmann and Robin 2007). The rate of starch retrogradation may impact food quality including digestibility (Wang et al. 2015) and viscoelastic properties such as storage modulus (Lu et al. 2009).
Xia et al. (2018) reported a loss of stability to alpha-amylase activity in recrystallized cassava starch microspheres (SM). SM show better stability to alpha-amylase activity when stored at 30 °C for 20 days with no temperature fluctuation than those that had undergone temperature fluctuations. Therefore, starches subject to temperature fluctuations are more susceptible to enzymatic hydrolysis.
Studies have also examined freeze–thaw cycles in order to modify starches with desired properties, as an alternative to chemical and enzymatic approaches. Szymońska et al. (2003) observed slight changes in the water holding capacity and water solubility of modified rice starch upon repeated freezing and thawing. In fact, the first freeze–thaw cycle induced pronounced changes in the granular starch properties.
Amylose and amylopectin contents may influence starch retrogradation, which also affect other properties of rice (Miles et al. 1985). In a study on high-amylose rice starch (20% amylose) that had undergone temperature fluctuation between 4 and 25 °C for up to five cycles over 10 days, both control and treated rice with high gelatinization temperatures (up to 95 °C) stored for 8 days or longer demonstrated high retrogradation enthalpy, which in turn improved the texture of rice noodles (Denchai et al. 2019). Ding et al. (2019) reported the effect of moisture content during gelatinization (30–42%) and storage conditions (isothermal at 4 °C, 15 °C, and 30 °C; cycled at − 18/30 °C and 4/30 °C) on the retrogradation of rice starch (23.4% amylose). They concluded that starch gelatinized at 42% moisture content (favoring amylopectin recrystallization) and stored at 4 °C showed the highest total retrogradation enthalpy (8.44 J/g). Rice starch gel (65% w/v water) showed a higher rate of starch retrogradation (approximately 0.6 day−1) at T = Tg′ + 15 °C than at storage temperatures below Tg′ (0.15 day−1 at T = Tg′ − 7 °C) when stored at 5 °C for 28 days (Hsu and Heldman 2005). This study highlights the importance of glassy state storage and its influence on starch retrogradation rate. The relationship between glass transition and starch retrogradation for rice starch gel has been reported at storage temperature above Tg′. These studies highlight how the degree of temperature fluctuation, number of temperature cycles, and product storage conditions impact quality changes in frozen and refrigerated starches.
Each food has unique characteristic thermal transitions that depend upon its chemical composition. Depending on the thermal transition temperatures and the degree of temperature fluctuation, food may undergo phase transitions. Kumar et al. (2019), Syamaladevi et al. (2012), and Zhang et al. (2017) reported that the degree of temperature fluctuations experienced by a frozen product and the consequent thermal transition impact the degree of change in relevant physical and chemical properties. Although studies have reported starch retrogradation in rice during frozen, refrigerated, and room temperature storage, systematic studies on the impact of thermal transitions on starch retrogradation are lacking. This study examines how changes in the degree of starch retrogradation of rice during frozen storage depend on state/phase transitions induced by temperature fluctuations.
Several researchers have reported studies on rice starch retrogradation. Yu et al. (2012a) have reported the retrogradation enthalpy and degree of retrogradation of starches from rice of varying varieties, while characterizing the physico-chemical properties of starch and flour from varying rice cultivars. Xiao et al. (2013) have shown that green tea polyphenols (at 10% and 15% concentrations) can inhibit rice starch retrogradation up to 20 days at 4 °C. Li et al. (2012) report that high hydrostatic pressures (0.1 to 600 MPa) can lower retrogradation tendency in rice starch, compared with native starch. Some other studies on retrogradation are with a focus on impact of freezing process and frozen and refrigerated storage conditions. Yu et al. (2012b) have reported the impacts of low and ultra-low temperature freezing on retrogradation properties of rice amylopectin during frozen storage. The researchers recommend freezing of rice amylopectin at temperatures of − 20 °C, − 30 °C, − 60 °C, and − 100 °C, followed by storage at − 18 °C for 5 months, to be acceptable with respect to amylopectin retrogradation enthalpy. While these studies have focused on the extracted rice starches/rice starch fractions, Yu et al. (2009) have studied the impact of amylose content on starch retrogradation in cooked rice during refrigerated storage (4 °C). They observe that rice with high amylose content (up to 36%) has the highest retrogradation enthalpy, while that with intermediate amylose content (24 to 28%) had a slow rate of retrogradation. Yu et al. (2010) have observed that freezing at 1.45 °C/min and storage at − 18 °C can retard starch retrogradation in cooked rice for 7 months. Studies in cooked rice are usually closer to understanding those challenges faced in commercial food products. However, these studies do not explore the impact of fluctuating temperatures during frozen storage of cooked rice, which has been focused in the present study. In this study, we have compared the starch retrogradation in cooked rice at constant and fluctuating temperatures during frozen storage. The temperature fluctuation protocol has been designed considering the thermal transition temperatures of cooked rice that are relevant to food freezing. As a result, we get an understanding of temperature fluctuation with focus on state and phase transitions in frozen cooked rice. We also examined starch retrogradation in two varieties of cooked rice, with varying starch compositions, 8% amylose (92% amylopectin) and 0% amylose (100% amylopectin). These varieties are commonly used in commercial sushi and other rice-based ready-to-eat recipes. Findings from this study elucidate the role of temperature fluctuation and amylose/amylopectin content in influencing starch retrogradation enthalpy along with details of kinetics of rice starch retrogradation in these samples.
Materials and Methods
Gelatinization Enthalpy of Rice Grains
Rice grains were obtained from Banzai Sushi (Seattle, WA). The compositions were as follows: 8% amylose, 92% amylopectin, or rice AM; 0% amylose, 100% amylopectin, or rice AP (Pacific International Rice Mills, Woodland, CA). Gelatinization enthalpy was measured using differential scanning calorimetry (DSC, Q2000 V24.10, TA Instruments, New Castle, DE). Rice grains were ground to a fine powder using a Vitamix Classic Blender E310 (Vitamix Corporation, Cleveland, OH) with a particle size of 63–106 μm (ATM Sonic Sifter, ATM Corporation, Milwaukee, WI). The moisture content of the rice powder was determined using an oven-dry method (Reeb and Milota 1999) in a hot air oven at 105 °C for 24 h. Moisture contents were 0.11 ± 0.00 kg water/kg rice powder (N = 3) (rice AM) and 0.11 ± 0.01 kg water/ kg rice powder (N = 3) (rice AP).
To measure the gelatinization enthalpy, the procedure of Yu et al. (2012b) was adapted as follows. About 4–5 mg of each of the powdered rice grains was placed in pre-weighed aluminum pans (TA Instruments, New Castle, DE), and distilled water (17–18 mg, rice AM; 40–41 mg, rice AP) was added to each pan. The amount of distilled water added was predetermined and was the minimum amount required to detect the peak of gelatinization in the DSC thermogram. The amount of water required for the two rice samples was different and higher than reported in the literature. During starch gelatinization, starch granules lose their integrity and form a molecular network—the detection of this process by instrumental approaches is based on the water content, (Donald 2001) cultivar, and variety (Yamin et al. 1997).
Different moisture contents were used for the two rice samples. Pans were sealed hermetically and set to equilibrate for 24 h (h) at room temperature (~ 23 °C). Pans were then subjected to heating using a differential scanning calorimeter (DSC) (Q2000 V24.10, TA Instruments, New Castle, DE) and with an MDSC option under the following conditions: modulated (amplitude ± 0.159 °C, sampling interval every 60 s), heat only, scan rate of 1 °C/min, and temperature ramp from 25 to 90 °C. In the DSC, the temperature change is linear (°C/min), resulting in a single heat flow rate signal as output (a sum of all heat flow occurring at any time/temperature point). As a result, small transitions are difficult to be detected. Modulated DSC or MDSC has an additional sinusoidal/modulated heating rate, where the sample’s heat capacity is measured. From the difference in the heat flow and the heat capacity, the kinetic component is calculated. This enables detection of small transitions that may be overlooked by the DSC thermogram. The kinetic events can be separated from those occurring due to changes in heat capacity.
Thermal Transitions in Cooked Rice
Rice grains were cooked as instructed by the supplier in an electric rice cooker (SR-G06FGE, 0.6 L, 310 W, 220 V, 60 Hz, Panasonic Corporation of North America, Newark, NJ). Rice grains (450 g) were washed with distilled water three times and then drained using a domestic kitchen strainer. Water for cooking was then added in the ratio of 1:1.5 (rice grains to water). Upon completion of the 20-minute (min) cooking time, the cooker was switched off. The cooked rice was left in the rice cooker for another 25 min to cool to room temperature (~ 23 °C). Three different rice batches were cooked, and three samples were chosen from each cooked batch.
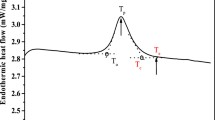
The moisture content of the cooked rice was measured using the oven dry method (Reeb and Milota 1999) and was 0.68 ± 0.03 kg water/kg cooked rice (N = 3) for rice AM and 0.52 ± 0.03 kg water/kg cooked rice (N = 3) for rice AP. Thermal transition temperatures of significance in freezing were determined using DSC. About 18–23 mg of cooked rice was equilibrated at 25 °C for 1 min, cooled to − 25 °C at a rate of 5 °C/min, held isothermally for 5 min, and finally heated to 25 °C at a rate of 5 °C/min. \( {T}_{\mathrm{m}}^{\prime } \) (onset of melting (or end point of freezing)) and Tf (initial freezing points) were calculated from the DSC thermograms (Kumar et al. 2018). These values were − 9.4 ± 0.1 °C and − 2.1 ± 0.0 °C, respectively (N = 3) (rice AM) and − 10.0 ± 0.3 °C and − 2.5 ± 0.3 °C, respectively (N = 3) (rice AP) (Fig. 1). \( {T}_{\mathrm{g}}^{\prime } \) was not measurable. Other researchers have reported that the \( {T}_{\mathrm{g}}^{\prime } \) and the \( {T}_{\mathrm{m}}^{\prime } \) values can coincide for products that are rich in starch (Chung et al. 2002), since the starch chain mobility may be suppressed in some foods. The specific heat capacity of the cooked rice was found to be 3.21 ± 0.08 J/g K (N = 3) for rice AM and 3.25 ± 0.29 J/g K (N = 3) for rice AP.
Temperature Fluctuation During Storage
The cooked rice AM and cooked rice AP underwent temperature fluctuations during storage. The test method was adapted from the Kumar et al. (2019) method for potatoes. The temperature was set to fluctuate using ambient air at room temperature (~ 23 °C) as the heat transfer medium. The time required for each temperature fluctuation cycle of the frozen cooked rice was determined experimentally. The cooked rice was placed on a tray. Rice from around the corners/edges of the tray was not used for the study, as some of these areas had lost moisture and appeared dry. The cooked rice was sealed in batches of 20 g in low-density polyethylene bags (Ziploc®, SC Johnson, Racine, WI) and frozen to − 32 ± 1 °C in a temperature-controlled cold storage room for 5 days. Rice grains of 20-g batches were packed in a single layer. The temperature of the cooked rice was measured by inserting an external K-type thermocouple (Omega Engineering Inc., Norwalk, CT) at the geometric center of each bag of rice. During freezing, the thermocouple was tightly held at the geometric center of the pack. During thawing, the position of thermocouple was checked to make sure it was placed tight and correct. Three rice samples from three cooked batches were used in this measurement. The time required to attain each of the target thermal transition states was then determined. The times thus recorded were used throughout the study to induce thermal transitions. The come-up time for rice AM and rice AP to attain a target temperature differed due to the differences in the moisture content of cooked rice.
Typically, one temperature fluctuation cycle consisted of the following stages: (i) freezing the cooked rice to attain glassy state − 32 ± 1 °C and held in storage for 5 days at − 32 ± 1 °C, (ii) raising the temperature of the frozen cooked rice to a target temperature to attain the targeted physical state (i.e., the glassy state with lower degree of temperature fluctuation, the glassy state with higher degree of temperature fluctuation, and the rubbery state or the thawed state) for a predetermined time, and (iii) refreezing the treated cooked rice to attain the glassy state at − 32 ± 1 °C. Immediately upon completion of the cycle time, the cooked rice in each state was held for 24 h of storage at the glassy state (constant at − 32 ± 1 °C).
The temperature of the cooked rice was adjusted, similar to reports by Kumar et al. (2019), based on the thermal transition temperatures obtained in the DSC, to attain different physical states: (i) treatment A, rice stored in the glassy state with no temperature fluctuation (T = constant at − 32 ± 1 °C); (ii) treatment B, rice fluctuated to the glassy state with lower degree of temperature fluctuation (\( T<<{T}_{\mathrm{m}}^{\prime } \), from − 32 to − 25 °C); (iii) treatment C, rice fluctuated to the glassy state with higher degree of temperature fluctuation (\( T<{T}_{\mathrm{m}}^{\prime } \), from − 32 to − 14 °C); (iv) treatment D, rice fluctuated to the rubbery state (\( {T}_{\mathrm{m}}^{\prime }<T<{T}_{\mathrm{f}} \), from − 32 to −8 °C); and (v) treatment E, rice fluctuated to the thawed state (T > Tf, from − 32 to − 2 °C). Based on the thermal transition temperatures of rice AM and rice AP, target temperatures for fluctuation were chosen. As a result, rice AM and rice AP will reach the suggested physical state, due to the temperature fluctuation.
The study was conducted over 12 weeks, with temperature fluctuating every 24 h. The analysis was performed every 2 weeks. Cooked rice that did not undergo any temperature fluctuation treatments for the 12-week storage study was held at a constant temperature (treatment A, rice stored at − 32 ± 1 °C, glassy state) as a control.
Kinetics of Starch Retrogradation in Cooked Rice During Frozen Storage
For rice AM and rice AP, retrogradation enthalpy of the cooked rice upon storage was measured using DSC with an MDSC option. About 20–25 mg of the frozen cooked rice was placed in pre-weighed aluminum pans (TA Instruments, New Castle, DE) and hermetically sealed. Pans were then subjected to heating under the following conditions: modulated (amplitude ± 0.159 °C, sampling interval every 60 s), heat only, scan rate 1 °C/min, and temperature ramp from 25 to 90 °C. The enthalpy of retrogradation was calculated from the DSC thermograms.
In this study, frozen cooked rice grains that were subjected to thermal transition–based temperature fluctuations were directly subjected to DSC measurements, without any pre-treatment. Other authors report pre-treatments to rice to remove the water content, such as the use of solvents and drying (Yu et al. 2010). In these studies, the dried rice was reconstituted with suitable amounts of water, to enable detection of retrogradation enthalpy by DSC. However, we performed DSC measurements directly with freeze-thawed rice. At the moisture content of the frozen cooked rice between 65 and 71%, we were able to detect the retrogradation enthalpy by DSC. Therefore, this avoided the need for any drying/reconstitution by water. A representative DSC thermogram is shown in Fig. 3 (rice AP, week 8).
The frozen cooked rice was sampled every 2 weeks during the 12-week study. Retrogradation enthalpy was measured (N = 2) from the DSC data. The kinetics of starch retrogradation were calculated as reported by Lai et al. (2000) and modeled using the Avrami equation for spherical crystals (Wunderlich 1997):
where θ is the fraction of crystallization resting to take place; ΔH0 is the retrogradation enthalpy (J/g) of the cooked rice, at time t = 0 week; ΔHt is the retrogradation enthalpy (J/g) of the cooked rice at time t week; ΔH∞ is the retrogradation enthalpy (J/g) of the cooked rice at time t = ∞ week, obtained by plotting 1/ΔHt vs. 1/t (Mita 1992); K is constant (week−1) denoting nucleation, linear crystal growth rate, and crystal geometry; and n is the Avrami exponent, denoting geometry, nucleation type, and nature of crystal growth.
The Avrami equation was applied assuming that the ΔHt values due to crystal melting are proportional to the total crystal volume. Equation (1) was linearized as follows (Lai et al. 2000):
By plotting logt vs. log(− ln θ), the slope gives the n value and the intercept gives the value of logK.
The degree of starch retrogradation (DR) was calculated as follows (Wang et al. 2016):
For each time point and treatment, the retrogradation enthalpy was determined. The gelatinization enthalpy of a sample is constant, and these values were determined in this study (Section: “Degree of Starch Retrogradation”). From these 2 values, the degree of retrogradation was calculated.
Scanning Electron Microscopy
For rice AM, upon completion of temperature fluctuation experiments, cooked rice was freeze-dried (FreeZone 2.5 L Freeze Dry System Lyophilizer, Labconco Corporation, Kansas City, MO) at a pressure of 0.79 Pa and a collector temperature of − 53 °C. Scanning electron microscopy (SEM) was used to quantify the pore size and size distribution of the freeze-dried cooked rice. Sublimation of ice during freeze-drying results in porous structures which are a representation of the ice crystals in the frozen product (Syamaladevi et al. 2012). The freeze-dried cooked rice was analyzed using an environmental scanning electron microscope (ESEM; Field Emission Instruments Co., Hillsboro, OR) with magnifications at ×100. Prior to scanning in the ESEM, the freeze-dried cooked rice was mounted on suitable stubs in a background of carbon black and sputter-coated with a layer of gold, at approximately 10 nm thickness, at a power supply of 10 mA, and an exposure time of 3 min (Hummer 6.6 Sputter System, Anatech USA, Hayward, CA), to avoid charge accumulation during imaging.
For this experiment, multiple samples were collected from different regions of the freeze-dried cooked rice to obtain a representative microstructure. Micrographs of the freeze-dried cooked rice were analyzed for geometric characteristics of the pores using ImageJ software (v. 1.51r, Image Processing and Analysis System, National Institute of Health, Bethesda, MD) (Schneider et al. 2012). The images were analyzed and the median pore equivalent diameter was calculated as reported by Kumar et al. (2019). The SEM images of treated cooked rice were collected every 4 weeks during the 12-week study. Based on the data obtained in the analysis of micrographs obtained for rice AM, the SEM analysis of pores for rice AP was not performed. This decision will be explained in detail in “Results and Discussion.”
Statistical Analysis
For rice AM and rice AP, the obtained data on retrogradation and pore parameters were analyzed for statistical significance using JMP Statistical Discovery™ statistical software (v. 14.1.0, SAS Institute, Cary, NC). A value of p < 0.05 was selected as statistically significant for the ANOVA. Tukey’s HSD method was used to estimate significant differences among the patterns of temperature fluctuations and the different storage times at p < 0.05.
Results and Discussion
Degree of Starch Retrogradation
In the presence of water and heat, the intermolecular bonds in starch undergo a breakdown. The starch granules dissolve in water, which acts as a plasticizer. This process is known as starch gelatinization—which happens in rice grains during cooking. The enthalpy of gelatinization is quantified from DSC thermogram, which is a constant value for a given type of starch. When the cooked starch undergoes cooling, there is starch retrogradation: realignment of amylose and amylopectin chains (Wang et al. 2015). Gelatinization occurs only during the cooking of rice. The DSC thermogram for gelatinization enthalpy of rice AP is shown in Fig. 2. Retrogradation, on the other hand, occurs during the entire storage period of cooked rice and the enthalpy of retrogradation depends upon storage temperature and time. Figure 3 shows the DSC thermogram for retrogradation enthalpy of rice AP at week 8.
The gelatinization enthalpy was found to be 7.06 ± 0.68 J/g (N = 3) (rice AM) (dry basis) and 3.51 ± 0.41 J/g (N = 3) (rice AP) (dry basis). The gelatinization enthalpy of rice AM (less waxy) is more than that of rice AP (waxier). This could be because of the differences in the amylopectin crystalline order in the rice samples (Tester and Morrison 1990). For rice AM, there was an overall influence of storage time upon the starch retrogradation enthalpy (Table 1). The enthalpy of retrogradation has been correlated with the weight percentage of long- (between 16 and 100 units) and short- (≤ 15 units) chain fractions of glucose units of amylopectin (Lai et al. 2000).
During starch retrogradation, there is an initial cooling of the starch paste which has formed during heating/gelatinization. This cooling process involves gelation of the amylose molecules, followed by the ordering of the amylopectin molecules (Wang et al. 2015). After week 10, the amylopectin molecules further reorder themselves into short and long glucose chain fractions, thereby leading to a significant increase in retrogradation enthalpy (p < 0.05). During initial storage, there was predominant occurrence of amylose gelation, rather than amylopectin recrystallization. As a result, the retrogradation enthalpies, especially in the first weeks, are very low (Table 1). This is indicated by onset of significant increase in starch retrogradation enthalpy starting form week 4 (p < 0.05).
For storage times ≥ 4 weeks, cooked rice AM stored at constant temperature (treatment A) exhibited higher retrogradation enthalpy than frozen cooked rice AM subject to temperature fluctuations (treatments B, C, D, and E) (Table 1), contrary to expectations. Usually, temperature fluctuation is expected to lead to an increase in retrogradation enthalpy, similar to how it impacts several other properties of frozen foods. Starch does not easily retrograde under constant temperature freezing: molecular mobility of the starch chains is restricted. Another study also reported lower retrogradation enthalpy in frozen starch-based products subjected to temperature fluctuation in comparison with control products that did not undergo temperature fluctuation. For example, Vernon-Carter et al. (2016) reported a reduction in retrogradation enthalpy of corn starch gels when subjected to four cycles of temperature fluctuation between − 20 and 90 °C. The control corn starch gels, with no temperature fluctuation, had a retrogradation enthalpy of 8.4 ± 0.5 J/g, while the test corn starch subject to 4 temperature fluctuations had a retrogradation enthalpy of 5.4 ± 0.5 J/g. Increase in the number of freeze–thaw cycles has also resulted in firmer gels and more resistant starch content.
Singh et al. (2003) attributed retrogradation enthalpy to the presence of an insoluble material (Lai et al. 2000). Temperature fluctuation induces a partial disruption of these insoluble materials, leading to a reduction of retrogradation enthalpy. In this study, we observed similar trends, attributed to the disruption of insoluble materials during temperature fluctuation. It is suggested that the composition of the rice may have an influence upon formation of the residual crystallites.
The degree of temperature fluctuation/thermal transition plays a role in influencing the retrogradation enthalpy (Table 1). Cooked rice contains the following proximate composition: 6.17 to 7.00% (crude protein), 0.2 to 0.5% (crude fiber), 0.15 to 0.25% (crude fat), and 69 to 80% (crude starch) (FAO 1993). When the product temperature was closer to \( {T}_{\mathrm{g}}^{\prime } \), there was less possibility of reordering of amylopectin molecules into short- and long-chain fractions of glucose units, due to reduced molecular mobility associated with the glassy state (Syamaladevi et al. 2012). Repeated freeze–thaw cycles have been shown to enable further ordering of the amylopectin structure, resulting in higher retrogradation enthalpy values. For example, Wang et al. (2008) subjected 10% Chinese water chestnut starch gels to freezing at − 20 °C for 22 h, followed by thawing at 30 °C for 1.5 h. Retrogradation enthalpy was found to increase with the number of freeze–thaw cycles: one freeze–thaw cycle resulted in a retrogradation enthalpy of 0.45 J/g, while two, four, and seven cycles resulted in retrogradation enthalpies of 1.08 J/g, 2.97 J/g, and 3.3 J/g, respectively. The residual crystallites formed during frozen storage melt and contribute to the DSC endotherm. These crystallites do not contribute to the retrogradation enthalpy when measured by other methods, such as Raman spectroscopy (Wang et al. 2016). These crystallites are not observed at week 0: At week 0, samples A, B, C, D, and E will not be any different. We can observe their degradation in later time points, based on degree of temperature fluctuation.
In this study, the increased degree of temperature fluctuation impacted the starch retrogradation. In treatment E, the phase transition due to the large temperature fluctuation resulted in complete melting of the ice crystals, causing maximum molecular mobility and therefore higher retrogradation enthalpy. Samples with less temperature fluctuation showed lower enthalpies, with the exception of the treatment A samples, as previously discussed.
In rice AM, DR values over time suggest that there was more reordering of the amylopectin molecules into short- and long-chain fractions of glucose units over the storage study (Table 3). This reordering occurred even during short storage times. There was a progressive increase in DR values at 12-week storage with increased degree of temperature fluctuation, through treatments B (7.58%), C (9.39%), D (10.7%), and E (16.0%). When stored at a constant temperature (treatment A), the DR was 61% for rice AM, at the end of 12 weeks. The DR values in this study imply that there was higher retrogradation in rice AM when subjected to treatment A, compared with those subject to temperature fluctuations.
Similar to rice AM, rice AP stored at constant temperature (treatment A) for 12 weeks also showed higher retrogradation enthalpy compared with rice sample subjected to temperature fluctuation (Table 1). When subjected to treatment E (the highest degree of temperature fluctuation), a DR of more than 100% was observed after 12 weeks. During storage of cooked rice (where starch is gelatinized), new starch crystallites, known as “residual crystallites,” were formed. When analyzed by DSC, these crystallites melt increasing the total enthalpy beyond the initial enthalpy of gelatinization, influencing the DR values. These crystallites do not contribute to the retrogradation enthalpy when measured by other methods, such as Raman spectroscopy (Wang et al. 2016). Additionally, there is an ordered reassociation of the adjacent double helices of gelatinized starch during storage, resulting in the formation of amylose and amylopectin crystallites (Hoover and Senanayake 1996). The melting of the amylopectin crystallites results in DSC endotherm peaks (Karim et al. 2000). With increased amylopectin content (or in the absence of amylose), such as in rice AP, large amounts of crystallites are easily formed with a slight increase in molecular mobility due to temperature fluctuation. Therefore, amylopectin content and instrumentation technique likely contributed to the high retrogradation enthalpy and DR values in rice AP compared with rice AM.
The rice samples used for gelatinization and for retrogradation did not have the same amounts of water content. The moisture contents of cooked rice were 0.68 ± 0.03 kg water/kg cooked rice AM and 0.52 ± 0.03 kg water/kg cooked rice AP, while moisture contents of rice AM and rice AP in the DSC pan for gelatinization were 0.77 to 0.83 kg water/kg gelatinized rice and 0.89 to 0.91 kg water/kg gelatinized rice. Initially, we attempted to use the same level of moisture contents estimated in cooked rice (0.68 and 0.52 kg water/kg rice) to determine starch gelatinization enthalpy. However, we were not able to obtain clear thermal transitions, i.e., endothermic peaks; this was probably due to sensitivity of the instrument. It has been reported that the gelatinization enthalpy of starch linearly increases with an increasing level of moisture content up to 0.60 kg water/kg. The increase in gelatinization enthalpy reaches to a constant value after a moisture content of 0.75 kg water/kg (Altay and Gunasekaran 2006; Spigno and de Faveri 2004; Resio and Suarez 2001). We believe that the gelatinization enthalpy determined at higher moisture contents of 0.77–0.83 and 0.89–0.91 kg water/kg rice will not be significantly different than the gelatinization enthalpy at moisture contents of 0.52 and 0.68 kg water/kg rice.
Kinetics of Starch Retrogradation
According to Lai et al. (2000), in linear plots of logt vs. log(− ln θ), the inflection points indicate changes in the mechanisms of starch retrogradation. Generally, an initial change in the slope during short storage times (of up to 1 week) reflects the possibility of both amylose and amylopectin recrystallization (Lai et al. 2000). In rice AM and rice AP, in the linear plots of log t vs. log(− ln θ) (Fig. 4a, b), we do not see any specific point of inflection. Regardless of the amylose/amylopectin contents, there is no change in the mechanism of retrogradation, which also indicates a low rate of retrogradation (Lai et al. 2000). Adding more datapoints may elucidate the points of inflection, a change in the slope, or a change in mechanisms of starch retrogradation.
The values of kinetic parameters n and K are dependent upon the geometry of the crystallites and the type of nucleation during retrogradation. Hence, their interdependence can explain the retrogradation mechanism in a system (Wunderlich 1997). However, the Avrami approach does not consider several factors, such as changes in starch crystal volume, nonlinear growth rate, and changes in starch crystal morphology that may occur in the system during storage (Wunderlich 1976). The actual values must be verified by microscopic rather than thermal approaches. Therefore, rather than comparing the absolute values of the Avrami equation kinetic parameters, we compared the ranges of values and trends in these parameters to gain information on the retrogradation mechanism in rice AM and rice AP.
For rice AM, the plot of n vs. logK values (Fig. 5a) showed a negative linear dependence similar to the reports of Lai et al. (2000). This negative linear dependence may also be due to sample concentration, storage condition, and the presence of non-starch components in cooked rice (Lai et al. 2000). These insoluble materials do not completely degrade during sample preparation and continue to influence the retrogradation kinetics during storage. Furthermore, a lower storage temperature would cause a higher n value (Jouppila et al. 1998) with transformation from amylose to amylopectin retrogradation, as seen in rice AM (Table 2). In addition, a narrow range of n values, as observed for rice AM (1.71 to 0.97), indicated diffusion-controlled conditions in retrogradation during long-term storage due to high starch concentration. In general, the n value is correlated with amylogram parameters and is used to evaluate the sensory quality of starchy foods (Armero and Collar 1998).
For rice AP, due to the absence of amylose, lower storage temperatures did not result in higher n values (Fig. 5b). A plot of n vs. logK values showed a negative linear dependence. A narrow range of n values (0.97 to 0.60) (lesser range when compared with values for rice AM) indicates a higher chance of diffusion-controlled reactions (Lai et al. 2000). Table 3 shows the DR values as influenced by thermal transition for the 12-week storage period for rice AM and rice AP.
Pore Perimeter and Pore Perimeter Distribution During Frozen Storage
SEM micrographs of freeze-dried, amylose-rich cooked rice showed the presence of pores, representing ice crystals in the frozen cooked rice AM (Fig. 6, pores marked in red for visibility). As rice AM samples underwent temperature fluctuations and passed through multiple phase transitions, pore sizes increased (23.7% in treatment a, 45.2% in treatment e; after 12 weeks). This increase in pore size was discernible at week 8 when subject to treatments b, c, and d, and at week 4 when subject to treatment e. Rice subject to treatment a (glassy state) did not show a visible change in pore size during 12-week storage study.
Overall, higher temperature fluctuations and longer storage times resulted in a greater increase in pore size (Table 4). After the 12-week storage study, frozen cooked rice AM subject to treatment E (highest degree of temperature fluctuation) showed a 45% increase in median pore diameter. This increase is less than that reported for other foods undergoing thawing or a change to the rubbery state, such as unblanched potatoes (74% increase, Kumar et al. 2019), salmon (82.4% increase, Syamaladevi et al. 2012), and mango (188% increase, Zhang et al. 2018). As a result, the SEM analysis of pores for rice AP was not performed. Starch-rich foods may be less susceptible to the impact of temperature fluctuation, with respect to the increase in ice crystal size.
Conclusions
Thermal transitions influenced the retrogradation enthalpy of frozen cooked rice during storage. Retrogradation enthalpy was influenced more by storage time than by the degree of temperature fluctuation. There was a breakdown of otherwise stable insoluble material during temperature fluctuation when cooked rice was stored in the glassy state. This was clearly reflected in the measured DR. The increased amylopectin content in rice AP (or the absence of amylose) resulted in higher DR values with time. Rice AM exhibited less change in DR values. Findings demonstrate that it is important to consider starch amylose and amylopectin contents when formulating frozen foods containing cooked rice, since this can impact food quality in long-term storage.
Data Availability
Data available upon request.
References
Altay, F., & Gunasekaran, S. (2006). Influence of drying temperature, water content, and heating rate on gelatinization of corn starches. Journal of Agricultural and Food Chemistry, 54(12), 4235–4245.
Armero, E., & Collar, C. (1998). Crumb firming kinetics of wheat breads with anti-staling additives. Journal of Cereal Science, 28(2), 165–174.
Chung, H. J., Lee, E. J., & Lim, S. T. (2002). Comparison in glass transition and enthalpy relaxation between native and gelatinized rice starches. Carbohydrate Polymers, 48(3), 287–298.
Denchai, N., Suwannaporn, P., Lin, J., Soontaranon, S., Kiatponglarp, W., & Huang, T. (2019). Retrogradation and digestibility of rice starch gels: the joint effect of degree of gelatinization and storage. Journal of Food Science, 84(6), 1400–1410.
Ding, L., Zhang, B., Tan, C. P., Fu, X., & Huang, Q. (2019). Effects of limited moisture content and storing temperature on retrogradation of rice starch. International Journal of Biological Macromolecules, 137, 1068–1075.
Donald, A. M. (2001). Plasticization and self-assembly in the starch granule. Cereal Chemistry, 78(3), 307–314.
Englyst, H. N., Kingman, S. M., & Cummings, J. H. (1992). Classification and measurement of nutritionally important starch fractions. European Journal of Clinical Nutrition, 46, S33–S50.
FAO (1993). Rice in human nutrition. Document prepared in collaboration with FAO by Juliano, B. O., international Rice research institute. FAO, Rome.
Hoover, R., & Senanayake, S. P. J. N. (1996). Composition and physicochemical properties of oat starches. Food Research International, 29(1), 15–26.
Hsu, C., & Heldman, D. R. (2005). Influence of glass transition temperature on the rate of rice starch retrogradation during low-temperature storage temperature storage. Journal of Food Process Engineering, 28(5), 506–525.
Jouppila, K., Kansikas, J., & Roos, Y. H. (1998). Factors affecting crystallization and crystallization kinetics in amorphous corn starch. Carbohydrate Polymers, 36(2-3), 143–149.
Karim, A. A., Norziah, M. H., & Seow, C. C. (2000). Methods for the study of starch retrogradation. Food Chemistry, 71(1), 9–36.
Kumar, P. K., Bhunia, K., Tang, J., Rasco, B., Thakkar, P. S., & Sablani, S. S. (2018). Thermal transition and thermo-physical properties of potato (Solanum tuberosum L.) var Russet Brown. Journal of Food Measurement and Characterization, 12(3), 1572–1580.
Kumar, P. K., Bhunia, K., Tang, J., Rasco, B., Thakkar, P. S., & Sablani, S. S. (2019). State/phase transitions induced by ice recrystallization and its influence on the mechanical properties of potatoes (Solanum tuberosum L.) var. Russet Brown. Journal of Food Engineering, 251, 45–56.
Lai, V. M., Lu, S., & Lii, C. (2000). Molecular characteristics influencing retrogradation kinetics of rice amylopectins. Cereal Chemistry, 77(3), 272–278.
Lehmann, U., & Robin, F. (2007). Slowly digestible starch – its structure and health implications: a review. Trends in Food Science and Technology, 18(7), 346–355.
Li, W., Bai, Y., Mousaa, S. A. S., Zhang, Q., & Shen, Q. (2012). Effect of high hydrostatic pressure on physicochemical and structural properties of rice starch. Food and Bioprocess Technology, 5(6), 2233–2241.
Lu, Z.-H., Sasaki, T., Li, Y.-Y., Yoshihashi, T., Li, L.-T., & Kohyama, K. (2009). Effect of amylose content and rice type on dynamic viscoelasticity of a composite rice starch gel. Food Hydrocolloids, 23(7), 1712–1719.
Miles, M. J., Morris, V. J., Orford, P. D., & Ring, S. G. (1985). The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohydrate Research, 135(2), 271–281.
Mita, T. (1992). Structure of potato starch pastes in the ageing process by the measurement of their dynamic moduli. Carbohydrate Polymers, 17(4), 269–276.
Reeb, J. E. & Milota, M. R. (1999). Moisture content by the oven dry method for industrial testing, WDKA, May 1999, 1(1–3).
Resio, A. C., & Suarez, C. (2001). Gelatinization kinetics of amaranth starch. International Journal of Food Science and Technology, 36(4), 441–448.
Schneider, C. A., Rasband, W. S., & Eliceiri, K. W. (2012). NIH image to ImageJ: 25 years of image analysis. Nature Methods, 9(7), 671–675.
Shi, M., & Gao, Q. (2016). Recrystallization and in vitro digestibility of wrinkled pea starch gel by temperature cycling. Food Hydrocolloids, 61, 712–719.
Singh, N., Singh, J., Kaur, L., Sodhi, N. S., & Gill, B. S. (2003). Morphological thermal and rheological properties of starches from different botanical sources. Food Chemistry, 81(2), 219–231.
Spigno, G., & de Faveri, D. M. (2004). Gelatinization kinetics of rice starch studied by non-isothermal calorimetric technique: influence of extraction method, water concentration and heating rate. Journal of Food Engineering, 62(4), 337–344.
Syamaladevi, R. M., Manahiloh, K. N., Muhunthan, B., & Sablani, S. S. (2012). Understanding the influence of state/phase transitions on ice recrystallization in Atlantic salmon (Salmo salar) during frozen storage. Food Biophysics, 7(1), 57–71.
Szymońska, J., Krok, F., Komorowska-Czepirska, E., & Rbilas, K. (2003). Modification of granular potato starch by multiple deep-freezing and thawing. Carbohydrate Polymers, 52(1), 1–10.
Tester, R. F., & Morrison, W. R. (1990). Swelling and gelatinization of cereal starches. I. Effects of amylopectin, amylose, and lipids. Cereal Chemistry, 67(6), 551–557.
Vernon-Carter, E. J., Bello-Pérez, L. A., Lobato-Calleros, C., Hernández-Jaimes, C., Meraz, M., & Alvarez-Ramirez, J. (2016). Morphological, rheological and in vitro digestibility characteristics of gelatinized starch dispersion under repeated freeze-thaw cycles. Starch, 68(1-2), 84–91.
Wang, L., Yin, Z., Wu, J., Sun, Z., & Xie, B. (2008). A study on freeze–thaw characteristics and microstructure of Chinese water chestnut starch gels. Journal of Food Engineering, 88(2), 186–192.
Wang, S., Li, C., Copeland, L., Niu, Q., & Wang, S. (2015). Starch retrogradation: a comprehensive review. Comprehensive Reviews in Food Science and Food Safety, 14(5), 568–585.
Wang, S., Li, C., Zhang, X., Copeland, L., & Wang, S. (2016). Retrogradation enthalpy does not always reflect the retrogradation behavior of gelatinized starch. Scientific Reports, 6, 1–10.
Wunderlich, B. (1976). The growth of crystal. In Macro-molecular physics (Vol. 2, pp. 115–347). New York: Academic Press.
Wunderlich, B. (1997). Thermal characterization of polymeric materials (Vol. 1). New York: Academic Press.
Xia, H., Kou, T., Liu, K., Gao, Q., & Fang, G. (2018). Recrystallization kinetics of starch microspheres prepared by temperature cycling in aqueous two-phase system. Carbohydrate Polymers, 198, 233–240.
Xiao, H., Lin, Q., Liu, G.-Q., Wu, Y., Wu, W., & Fu, X. (2013). Inhibitory effects of green tea polyphenols on the retrogradation of starches from different botanical sources. Food and Bioprocess Technology, 5, 2177–2181.
Yam, K. L., & Lai, C. C. (2004). Microwaveable frozen foods or meals. In Handbook of frozen foods (pp. 577–589). New York: Marcel Dekker Inc..
Yamin, F. F., Svendsen, L., & White, P. J. (1997). Thermal properties of corn starch extraction intermediates by differential scanning calorimetry. Cereal Chemistry, 74(4), 407–411.
Yu, S., Ma, Y., & Sun, D.-W. (2009). Impact of amylose content on starch retrogradation and texture of cooked milled rice during storage. Journal of Cereal Science, 50(2), 139–144.
Yu, S., Ma, Y., & Sun, D.-W. (2010). Effects of freezing rates on starch retrogradation and textural properties of cooked rice during storage. LWT - Food Science and Technology, 43(7), 1138–1143.
Yu, S., Ma, Y., Menager, L., & Sun, D.-W. (2012a). Physicochemical properties of starch and flour from different rice cultivars. Food and Bioprocess Technology, 5(2), 626–637.
Yu, S., Ma, Y., Zheng, X., Liu, X., & Sun, D.-W. (2012b). Impacts of low and ultra-low temperature freezing on retrogradation properties of rice amylopectin during storage. Food and Bioprocess Technology, 5(1), 391–400.
Zhang, G. Y., Sofyan, M., & Hamaker, B. R. (2008). Slowly digestible state of starch: mechanism of slow digestion property of gelatinized maize starch. Journal of Agricultural and Food Chemistry, 56(12), 4695–4702.
Zhang, Y., Zhao, J.-H., Ding, Y., Nie, Y., Xiao, H.-W., Zhu, Z., & Tang, X.-M. (2017). Effects of state/phase transitions on the quality attributes of mango (Mangifera indica L.) during frozen storage. International Journal of Food Science and Technology, 52(1), 239–246.
Zhang, Y., Zhao, J.-H., Ding, Xiao, H.-W., Sablani, S. S., Nie, Y., Wu, S.-J., & Tang, X.-M. (2018). Changes in the vitamin C content of mango with water state and ice crystals under state/phase transitions during frozen storage. Journal of Food Engineering, 222, 49–53.
Acknowledgments
The authors wish to thank Phil Sinz, Banzai Sushi, Des Moines, WA, for the rice; Ashutos Parhi, Department of Biological Systems Engineering, Washington State University (WSU), Pullman, WA, for the micrographs; Dr. Valerie Lynch-Holm, Franceschi Microscopy and Imaging Center, School of Biological Sciences, WSU, Pullman, WA, for the Scanning Electron Microscopy facility; and Frank Younce, School of Food Science, WSU, Pullman, WA, for the freezer facilities.
Code Availability
Not applicable.
Funding
This work was financially supported by the United States Department of Agriculture (USDA) Grant No. 2015-6701-23074 and Hatch project 1016366.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, P.K., Joyner, H.S., Tang, J. et al. Kinetics of Starch Retrogradation in Rice (Oryza sativa) Subjected to State/Phase Transitions. Food Bioprocess Technol 13, 1491–1504 (2020). https://doi.org/10.1007/s11947-020-02488-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02488-9