Abstract
A number of novel freezing systems have been developed that claim to improve the quality of frozen foods by enhancing supercooling in the food prior to ice nucleation and consequently controlling ice crystal formation. One of these is the Cells Alive System (CAS) produced by ABI of Japan, which applies oscillating magnetic fields (OMF) during freezing. This study was carried out to investigate what effect applying OMF (0.04 to 0.53 mT) during freezing had on the freezing characteristics of pork loin samples when compared to freezing under the same conditions without OMF. Overall, the results of this study clearly indicate that freezing under the OMF conditions used in these experiments had no significant effect on the freezing characteristics of pork, in comparison with freezing under the same conditions without OMF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freezing is a well-established food preservation process capable of producing high quality nutritious foods with a long storage life. However, freezing is not suitable for all foods, and freezing can cause physical and chemical changes in some foods that are perceived as reducing the quality of either the thawed material or the final product. There is a great interest in methods of improving the freezing process. Many of these innovations have been reviewed by James et al. (2015b). Among these innovations are oscillating magnetic fields (OMF) (Owada and Kurita 2001; Owada 2007).
OMF are used by the Cells Alive System (CAS), a novel patented (Owada and Kurita 2001; Owada 2007) freezing process marketed by the Japanese company ABI (ABI Corporation Ltd., Chiba, Japan). CAS is not a refrigeration process in itself, but is claimed to improve existing freezing processes (both in terms of process speeds and product quality). The patent literature (Owada and Kurita 2001; Owada 2007) claims that OMF acts on polarised water molecules to delay the formation of ice crystals. Whether this is due to engendering of supercooling or through the movement of the molecules during the ice formation process is unclear. However, the claimed consequence is that most of the ice crystals form at the same time, thus restricting their size, resulting in less cellular damage during freezing. There is a common belief that small ice crystals result in a superior quality in foods (James et al. 2015b, c). The effects of electric and magnetic fields on freezing have been reviewed by Woo and Mujumdar (2010); James et al. (2015b, c), and Otero et al. (2016). There is evidence that water, being a diamagnetic material, can be magnetised in a magnetic field. However, Wowk (2012) questioned the claims made in papers using CAS, given the very small (<1 mT) field strengths used.
There is very little scientific published data on OMF-assisted freezing of foods (James et al. 2015a; Otero et al. 2016), although a number of papers have been published on OMF-assisted freezing of biological samples (such as teeth) using ABI equipment (Kaku et al. 2010; Abedini et al. 2011; Kaku et al. 2012) and even on whole-organism survival of frozen small animals like Drosophila (Naito et al. 2012).
The aim of this short-targeted study was to investigate what effect freezing under CAS conditions, using a CAS system designed and constructed in consultation with ABI, had on the characteristics of the freezing curve and drip losses, texture and colour of samples extracted from pork loin when compared to freezing under the same conditions without CAS. Pork meat was used as test material because it is reported that “as much as 50% or more of pork produced has unacceptably high purge or drip loss” (Huff-Lonergan and Lonergan 2005) and gives rise to a considerable amount of drip loss after thawing. In common with other studies, such as Ngapo et al. (1999), small cylindrical samples were used to both minimise product variation and the effect of thermal gradient on ice formation which occurs in large samples. All trials were carried out using an air temperature of ca. −30 °C and an air speed of ca. 1 to 2 m s−1.
Materials and Methods
Samples
Fresh pork loin was obtained from a local supermarket and stored in chilled conditions, at a mean (SD) temperature of −1.4 (1.0) °C, for a period between 1 and 5 days. When required, several cylindrical samples were extracted from each pork loin using a cork borer with an inner diameter of approximately 30 mm. The longitudinal axis of the cylinder followed the fibre direction to minimise damage to fibres and potentially reduce drip losses. Care was taken to avoid obvious pieces of fat and connective tissue. After cutting, each meat cylinder was wrapped in plastic film to minimise evaporation of water from the surfaces. The wrapped pork samples were then equalised for 24 h prior to freezing, in a catering refrigerator running at a mean (SD) temperature of −0.9 (0.8) °C and then cut to a length of approximately 22 mm. A total of 40 cylinder-shaped pork samples were used throughout the trials. The mean (SD) diameter, length, and mass of the samples were 30.3 (1.0) mm, 21.8 (0.9) mm, and 13.6 (1.3) g, respectively.
Freezing Trials
An experimental batch air blast-freezer, with and without CAS being applied was used (as used by James et al. 2015a). The freezer had been designed and constructed in consultation with ABI (ABI Corporation Ltd., Chiba, Japan), and the magnetic coils and control system supplied and commissioned by ABI. The CAS freezer was equipped with both static and oscillating magnetic field generators to assist the freezing process. The air temperature was set to −30 °C (providing a mean (SD) air temperature of −29.2 (0.6) °C across the product) with CAS settings of off (control), 10, 50, and 100%. Unfortunately, details of the CAS settings were not provided by ABI. Two samples, placed at two different fixed positions on a grid, were used in each trial. Previous studies had shown that these positions were those with the highest and the lowest magnetic field strengths at the different CAS settings. The RMS values of OMF strength measured using a gaussmeter (GM07, Magnetic Instruments Ltd., Falmouth, UK) at the different CAS settings were 0, 0.53, 0.29 and 0.15 mT for the highest field point (position 1), and 0, 0.18, 0.09 and 0.04 mT for the lowest field point (position 2), respectively. Air velocities of 1 to 2 m s−1 in both positions were measured using a vane anemometer (AV-2, Airflow Instruments, High Wycombe, UK). The surface heat transfer coefficient at the two positions was also determined using the method described by Cowell and Namor (1974).
Bare wire T type (copper constantan) thermocouples (1 mm diameter), previously calibrated to an accuracy of ± 0.5 °C against a standard platinum resistance probe, were used to measure temperatures in the geometric centre of the cylindrical pork loin samples. Temperatures were recorded to a resolution of 0.2 °C, every 30 s using a datalogger (Diligence N2014, Comark Instruments (Norwich), UK) until temperature readings at the centre of both samples were below −27 °C. A total of five replicate experiments, with two samples in each, were carried out for each condition.
Freezing Curve Analysis
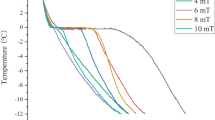
From each freezing curve, a typical example being shown in Fig. 1, data on the potential occurrence of supercooling, ‘characteristic freezing time’, and ‘completion of freezing rate’ were extracted. ‘Characteristic freezing times’ (Jiang and Lee 2005) were calculated as the time to cool the thermal centre of the samples from the initial freezing point temperature to −7 °C. ‘Completion of freezing rate’ was calculated as the rate of cooling as the thermal centre of the samples cooled from −10 to −15 °C.
Drip Losses Analysis
After freezing, the samples were wrapped in food grade polyethylene film to prevent evaporation and stored in a chest freezer for between 21 and 27 days. After storage samples were taken out of the chest freezer, the polyethylene film was removed, and the sample was wrapped in a piece of absorbent paper, before placing then in a zipped plastic bag to prevent evaporation losses. Both the bag and paper were weighed together prior to use and again together with the pork sample and the inserted thermocouple, to a resolution of 0.001 g, using a calibrated scale (RC2022, Sauter GmbH, Balingen, Germany). Samples were then thawed in a domestic refrigerator at a mean (SD) temperature of 2.6 (0.9) °C.
The samples remained in the refrigerator until their centre temperatures were above 0 °C, which took between 17 and 21 h, after which they were removed. Immediately after removal, the bags, pieces of absorbent paper, meat sample and thermocouples were weighed. Each thermocouple was then removed from the sample, and the weight of the sample and thermocouple was weighed individually. The drip loss was expressed as the percentage of mass loss according to eq. 1:
Where M b and M a are the masses (g) of the samples before and after the storage period, respectively, and the overall measurement uncertainty is less than 0.1%.
Colour Analysis
The surface colour of samples was measured using the Commission Internationale de L’Éclairage (1976) (CIE) colour space (L*, a* and b*) with a chroma meter (CR-400, Konica Minolta Corp, Tokyo, Japan). Values were initially measured on the bases of each cylindrical pork loin sample immediately before being frozen in the CAS freezer after calibration with a standard white reflector. The repeatability of the instrument is claimed to be within ΔE*ab0.07 standard deviation (when the white calibration plate is measured 30 times at intervals of 10 s) and inter-instrument agreement within 0.6.
The measurements were repeated on each sample after freezing and after thawing. Values measured after freezing/thawing were subtracted from the values measured before freezing to calculate ΔL*, Δa* and Δb*, the variations in value.
Texture Analysis
After the thawing and the colour measurements, the texture of each sample was determined using a texture meter (TVT-150, TEXVOL Instruments, Stockholm, Sweden). The texture test consisted of a compression trial performed using a 25-mm-diameter piston, applying a compression rate of 40% and a hold time of 30 s.
For each trial, a series of values were obtained: density (g/ml), hardness (g), force A (g), force B (g) and elasticity (%). Force A was the maximum force exerted during compression and force B the force exerted at the end of 30 s, just before the withdrawal of the piston. Elasticity was calculated by dividing force B by force A and expressing it as a percentage.
Statistical Analysis
The statistical analysis of the data was performed using the software IBM SPSS Statistics v. 23.0.0.0 for Windows (IBM Corp., Armonk, NY, USA). Trial results were split into two groups, depending on the positions 1 and 2, and evaluated separately in order for them to be independent from differences in heat transfer coefficient. Levene’s test was carried out to evaluate the homogeneity of variances. In cases where the hypothesis of equal variances was not rejected (significance level of p > 0.05), then a one-way analysis of variance (ANOVA) was performed, otherwise, Welch’s test was chosen to evaluate the equality of means among groups, the OMF strength being the studied factor in both cases. The potential differences between the applied fields, with a significance level of p < 0.05, was established for the previously mentioned independent variables: characteristic freezing time, completion of freezing rate, percentage of drip loss, variation of colour parameters (ΔL*, Δa* and Δb*), density, hardness, force A, force B and elasticity. If any significant differences were observed, a post-hoc test, either Tukey-b or Tamhane’s T2, depending on Levene’s test results, was carried out.
Results and Discussion
Freezing Curve Analysis
The mean (SD) values of surface heat transfer coefficients calculated by means of the cooling curve of copper blocks for positions 1 and 2 were 22.3 (1.0) W m−2 K−1 and 16.1 (1.1) W m−2 K−1, respectively. These surface heat transfer coefficients are quite low for an air-blast freezer, and the air velocities of 1 to 2 m s−1 that were measured at this point and were likely to be due to how the magnetic coils in the chamber deflected the air flow.
No significant degree of supercooling was observed in any of the samples irrespective of the application or absence of OMF during the freezing process.
The characteristic freezing time and the completion of freezing rate are shown plotted against the magnetic field strength in Figs. 2 and 3, respectively, and the mean and standard deviation of these variables are shown in Table 1. There appeared to be no relationship between magnetic field strength and characteristic freezing time or completion of freezing rate. The OMF did not appear to have any significant effect (p > 0.05) on the characteristic freezing time or the completion of freezing rate as shown in Table 2. In all cases, as would be expected given the differences in surface heat transfer coefficients, samples in position 1 cooled and froze faster than those in position 2, irrespective of magnetic field strength. Mean characteristic freezing times were 16.3 and 18.8 min at positions 1 and 2, respectively, and completion of freezing rates were −0.036 and −0.028 °C s−1, respectively.
Drip Loss Analysis
An increase in the drip from meat after freezing and thawing compared with that in chilled meat is the most common quality deterioration associated with the freezing of meat. One of the most important claims of the CAS patents (Owada and Kurita 2001; Owada 2007) is that OMF prevents cells from being damaged, due to the generation of smaller ice crystals, and thus reduces drip. Drip losses in the present work ranged from 1.7 to 4.6% (Fig. 4), which is within the usual range reported for pork (Pérez Chabela and Mateo-Oyague 2006). The results indicated no clear effect of OMF on drip loss, or between drip losses at different field strengths (Fig. 4). No significant difference (p > 0.05) between OMF strengths for each of the positions was shown, as can be seen in Table 2. The average drip loss from samples frozen in position 2 was slightly higher than that from samples frozen in position 1 indicating that there was a slight effect of freezing rate on drip loss. It should be noted that although drip loss is of great monetary importance and can make meat less attractive, it has not been shown to influence the final eating quality after cooking, except in extreme cases (Pérez Chabela and Mateo-Oyague 2006).
Colour Analysis
Pork can naturally be rather variable in colour, and colour changes during freezing can affect perceived meat quality. To check for any bias in the colour of samples prior to freezing, the values of L*, a*, and b* were plotted (data not shown in this paper) against magnetic field intensity and no bias was observed. Means (SD) for these colour parameters, before freezing and after thawing, are shown in Table 3. The application of the different OMF applied during freezing did not cause any significant (p > 0.05) effect on the variation of colour characteristics, as shown in Table 2.
Texture Analysis
If the application of OMF resulted in less cell damage, it could be expected to affect the texture of the thawed samples. The mean (SD) values of density, hardness, force A, force B and elasticity against magnetic field intensity are shown in Table 4. No significant difference (p > 0.05) showing a clear relationship between magnetic field intensity and any of the texture parameters was found, as shown in Table 2.
Overall
Overall, this study found that the application of OMFs during air-blast freezing had no effect, whichever their strength or frequency, on the freezing time or quality attributes of pork loin. Similar results were reported by Suzuki et al. (2009) and Watanabe et al. (2011) who did not find any effect of OMFs on the microstructure, drip losses, colour, texture and sensory evaluation of frozen radish, sweet potato, spinach, yellow tail fish and tuna. Yamamoto et al. (2005) also found that the application of OMF (1.5–2 mT at 20, 30, and 40 Hz) during freezing had no effect on the drip, cooking losses or the rupture stress and strain of chicken breasts frozen in a ABI CAS freezer. Similarly, Otero et al. (2017) did not find any effect of OMFs (<2 mT, 6–59 Hz) on freezing time, drip loss, water-holding capacity, toughness or whiteness of crab sticks.
Not all published studies on OMF have been negative. Studies at the Korea Food Research Institute have reported benefits for OMF frozen meats. Kim et al. (2013a, b) reported reduced total freezing times and improved quality attributes in beef, pork and chicken, and Ku et al. (2014) and Choi et al. (2015) reported similar findings for pork and beef, respectively. However, unfortunately, none of these studies compared like with like. The air blast freezer used for the magnetic resonance freezing was different to the air blast freezer used to freeze the control samples, as were the operating air temperatures. The ABI CAS freezer used was operated at −55 °C, while the conventional air blast freezer used as the control was operated at −45 °C (although in the case of the experiments carried out by Kim et al. (2013b), the published temperature curves indicate that actual temperatures in the conventional air blast were nearer −40 than −45 °C). We agree with Otero et al. (2016) conclusions on this work; since the air temperatures used in the two systems compared were very different, and no details of the air speeds or surface heat transfer coefficient used in the different systems were given, it is not possible to deduce whether the improvements detected by these authors were produced by the OMF or by the different freezing conditions applied.
In the author’s opinion, although we have yet to see any clear advantages of OMF-assisted freezing, we do not believe that studies to date completely invalidate ABI’s CAS system as a method of enhancing the overall freezing of foods; however, they do suggest that weak OMF may not affect all foods. It is possible that any effect will depend on a complex combination of food, freezing rate and magnetic field frequency. Similar observations have been made in published studies using ABI equipment to freeze human tissues (Nakagawa et al. 2012). We also agree with Otero et al. (2017) that further investigations into OMF should investigate a wider range of OMF strength and frequency than those currently employed in commercial CAS freezers. Combined pulsed electric field (PEF) and static magnetic field (SMF) technology, as demonstrated by Mok et al. (2015), may also show greater promise than OMF.
Conclusions
Overall, the results of this study clearly indicate that the application of weak OMF during air-blast freezing (1) does not enhance supercooling in pork loin, under the conditions used in these trials; (2) has no significant (p > 0.05) effect on the characteristic freezing time or completion of freezing rate after freezing of pork loin; (3) has no significant (p > 0.05) effect on drip loss from pork loin after thawing; (4) has no significant (p > 0.05) effect on colour parameters L*, a* and b* after thawing; (5) has no significant (p > 0.05) effect on texture parameters analysed using a compression test after thawing, namely density, hardness, force A, force B or elasticity has confirmed any dependence on the applied OMF. The OMF strengths and frequency ranges covered those employed in commercial CAS freezers. It is possible that other strengths and frequency ranges may have more potential for assisting food freezing.
References
Abedini, S., Kaku, M., Kawata, T., Koseki, H., Kojima, S., Sumi, H., Motokawa, M., Fujita, T., Ohtani, J., Ohwada, N., & Tanne, K. (2011). Effects of cryopreservation with a newly-developed magnetic field programmed freezer on periodontal ligament cells and pulp tissues. Cryobiology, 62, 181–187.
Choi, Y. S., Ku, S. K., Jeong, J. Y., Jeon, K. H., & Kim, Y. B. (2015). Changes in ultrastructure and sensory characteristics on electro-magnetic and air blast freezing of beef during frozen storage. Korean Journal for Food Science of Animal Resources, 35, 27–34.
Commission Internationale de L’Éclairage (1976). CIE Colorimetry — Part 4: 1976 L*a*b* Color Space. Commission Internationale de L’Éclairage.
Cowell, N. D., & Namor, M. S. S. (1974). Heat transfer coefficients in plate freezing—effect of packaging materials. Proc. of I.I.R. Meetings of Commissions B1, C1 and C2. Bressanone, Italy, Sept., 17–20.
Huff-Lonergan, E., & Lonergan, S. M. (2005). Mechanisms of water-holding capacity of meat: the role of post-mortem biochemical and structural changes. Meat Science, 71, 194–204.
James, C., Purnell, G., & James, S. J. (2015a). A review of novel and innovative freezing technologies. Food and Bioprocess Technology, 8, 1616–1634.
James, C., Reitz, B. G., & James, S. J. (2015b). The freezing characteristics of garlic bulbs (Allium sativum L.) frozen conventionally or with the assistance of an oscillating weak magnetic field. Food and Bioprocess Technology, 8(3), 702–708.
James, C., Purnell, G., & James, S. J. (2015c). Can magnetism improve the storage of foods. New Food, 18(2), 40–43.
Jiang, S. T., & Lee, T. C. (2005). Freezing seafood and seafood products: principles and applications. In Y. H. Hui (Ed.), Handbook of food science, technology, and Engineering (pp. 39-1–39-35). Boca Raton: CRC Press.
Kaku, M., Kamada, H., Kawata, T., Koseki, H., Abedini, S., Kojima, S., Motokawa, M., Fujita, T., Ohtani, J., Tsuka, N., Matsuda, Y., Sunagawa, H., Hernandes, R. A. M., Ohwada, N., & Tanne, K. (2010). Cryopreservation of periodontal ligament cells with magnetic field for tooth banking. Cryobiology, 61, 73–78.
Kaku, M., Kawata, T., Abedini, S., Koseki, H., Kojima, S., Sumi, H., Shikata, H., Motokawa, M., Fujita, T., Ohtani, J., Ohwada, N., Kurita, M., & Tanne, K. (2012). Electric and magnetic fields in cryopreservation: a response. Cryobiology, 64, 304–305.
Kim, Y. B., Jeong, J. Y., Ku, S. K., Kim, E. M., Park, K. J., & Jang, A. (2013a). Effects of various thawing methods on the quality characteristics of frozen beef. Korean Journal for Food Science of Animal Resources, 33(6), 723–729.
Kim, Y. B., Woo, S. M., Jeong, J. Y., Ku, S. K., Jeong, J. W., Kum, J. S., & Kim, E. M. (2013b). Temperature changes during freezing and effect of physicochemical properties after thawing on meat by air blast and magnetic resonance quick freezing. Korean Journal for Food Science of Animal Resources, 33(6), 763–771.
Ku, S. K., Jeong, J. Y., Park, J. D., Jeon, K. H., Kim, E. M., & Kim, Y. B. (2014). Quality evaluation of pork with various freezing and thawing methods. Korean Journal for Food Science of Animal Resources, 34(5), 597–603.
Mok, J. H., Choi, W., Park, S. H., Lee, S. H., & Jun, S. (2015). Emerging pulsed electric field (PEF) and static magnetic field (SMF) combination technology for food freezing. International Journal of Refrigeration, 50, 137–145.
Naito, M., Hirai, S., Mihara, M., Terayama, H., Hatayama, N., Hayashi, S., Matsushita, M., & Itoh, M. (2012). Effect of a magnetic field on drosophila under supercooled conditions. PloS One, 7.
Nakagawa, T., Mihara, M., Noguchi, S., Fujii, K., Ohwada, T., Niino, T., Sato, I., Yamashita, H., Masamune, K., & Dohi, T. (2012). Development of pathology specimen preparation method by supercooling cryopreservation under magnetic field. Academic Collaborations for Sick Children, 5, 21–27.
Ngapo, T. M., Babare, I. H., Reynolds, J., & Mawson, R. F. (1999). Freezing and thawing rate effects on drip loss from samples of pork. Meat Science, 53(3), 149–158.
Otero, L., Rodríguez, A. C., Pérez-Mateos, M., & Sanz, P. D. (2016). Effects of magnetic fields on freezing: application to biological products. Comprehensive Reviews in Food Science and Food Safety, 15(3), 646–667.
Otero, L., Pérez-Mateos, M., Rodríguez, A. C., & Sanz, P. D. (2017). Electromagnetic freezing: effects of weak oscillating magnetic fields on crab sticks. Journal of Food Engineering, 200, 87–94.
Owada, N. (2007). Highly-efficient freezing apparatus and highly-efficient freezing method. United States Patent US 7,237,400B2.
Owada, N., & Kurita, S. (2001). Super-quick freezing method and apparatus therefor. United States Patent US 2001/6250087B1.
Pérez Chabela, M. L., & Mateo-Oyague, J. (2006). Frozen meat: Quality and shelf life. In Y. H. Hui (Ed.), Handbook of food science, technology, and Engineering (pp. 115-1–115-9). Boca Raton: CRC Press.
Suzuki, T., Takeuchi, Y., Masuda, K., Watanabe, M., Shirakashi, R., Fukuda, Y., Tsuruta, T., Yamamoto, K., Koga, N., Hiruma, N., Ichioka, J., & Takai, K. (2009). Experimental investigation of effectiveness of magnetic field on food freezing process. Transactions of the Japan Society of Refrigerating and Air Conditioning Engineers, 26, 371–386.
Watanabe, M., Kanesaka, N., Masuda, K., & Suzuki, T. (2011). Effect of oscillating magnetic field on supercooling in food freezing. Proceedings of the 23 rd IIR International Congress of Refrigeration; refrigeration for sustainable development, August 21–26, Prague, Czech Republic. 1, 2892–2899.
Woo, M. W., & Mujumdar, A. S. (2010). Effects of electric and magnetic field on freezing and possible relevance in freeze drying. Drying Technology, 28, 433–443.
Wowk, B. (2012). Electric and magnetic fields in cryopreservation. Cryobiology, 64, 301–303.
Yamamoto, N., Tamura, S., Matsushita, J., & Ishimura, K. (2005). Fracture properties and microstructure of chicken breasts frozen by electromagnetic freezing. Journal of Home Economics of Japan, 56(3), 141–151.
Acknowledgments
This work has been supported by the Spanish MINECO through the Project AGL2012-39756-C02-01. A. C. Rodríguez has been supported by a grant from Spanish MINECO for the carrying out of a brief stay in a R & D centre, as an additional action to the pre-doctoral contract BES-2013-065942 from MINECO, jointly financed by the European Social Fund, in the framework of the National Program for the Promotion of Talent and its Employability (National Sub-Program for Doctors Training). We would also like to acknowledge Air Products for supporting the supervisory costs of C. James and S. J. James at FRPERC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez, A.C., James, C. & James, S.J. Effects of Weak Oscillating Magnetic Fields on the Freezing of Pork Loin. Food Bioprocess Technol 10, 1615–1621 (2017). https://doi.org/10.1007/s11947-017-1931-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1931-2