Abstract
This paper investigated the feasibility for pasteurizing raw (100 %) pomegranate juice in a commercial scale pulsed electric field (PEF) processing system. The juice was processed at 35 and 38 kV/cm for 281 μs at 55 °C with a flow rate of 100 L/h. Effect of PEF processing on microbial stability, color, °Brix, pH, sediment, antioxidant activity, total phenolic content, anthocyanin, and sensory properties after the treatments and during storage at 4 °C for 12 weeks were studied and compared to those of thermally processed juice. PEF treatments significantly (p < 0.05) inhibited the growth of total aerobic bacteria, which remained at <2.5 log colony-forming units (CFU)/ml during the 12-week storage. No yeast and mold were detected (<0.69 log CFU/ml) in the PEF-treated juices during storage up to weeks 10 and 12, which is similar to the thermally processed juice. There were no significant differences in pH and °Brix values between the PEF processed juice and unprocessed juice. PEF processing did not alter the contents of total phenolics and anthocyanin as compared to unprocessed juice. PEF processing had significantly (p < 0.05) less impact on the color of pomegranate juice than thermal processing. PEF-treated juice had the same consumer satisfaction scores as the unprocessed juice, which were significantly (p < 0.05) higher than thermally processed juice samples. There was no significant difference between the two PEF treatments in all results. This study demonstrated that PEF technology extended microbial shelf-life and preserved the major quality and nutritional characteristics of pomegranate juice, and hence, is technically feasible for commercialization in the juice industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pomegranate juice is an important source of anthocyanins, such as 3-glucosides and 3, 5-diglucosides of delphinidin, cyanidin, and pelargonidin (Du et al. 1975). Anthocyanins are responsible for the bright red color of pomegranate juice, and the red color is one of its major quality parameters of pomegranate juice that affects consumers sensory acceptance (Alighourchi and Barzegar 2009; Gil et al. 2000; Hamutal et al. 2009; Patras et al. 2010). Health-promoting effects of pomegranate juice are attributable to its remarkably high antioxidant capacity (Aviram et al. 2000; Hamutal et al. 2009). The antioxidant activity of the pomegranate juice can be correlated to the phenolic composition. Anthocyanins and other phenolic compounds of pomegranate juice have preventive and therapeutic effects on cardiovascular disease, inflammation, and different types of cancers (Aviram et al. 2000; Lansky and Newman 2007; Malik et al. 2005), in particular, it has been demonstrated that the consumption of this juice decreases the susceptibility of low-density lipoprotein to aggregation and retention.
Unfortunately, the bioactive compounds are quickly affected by exogenic factors such as oxygen, light, and especially pH and temperature. Therefore, there is a real need to minimize the degradation of functional molecules during pasteurization process and storage time of the pomegranate juice, in order to ensure an optimal sensorial and nutraceutical quality. The challenge of preserving the sensorial and nutraceutical properties while extending the microbiological shelf-life suggests the need of non-thermal technologies for pasteurization of fresh pomegranate juice.
Pulsed electric field (PEF) is a non-thermal processing method used to maximize quality, enhance shelf stability, and maintain food safety by inactivating spoilage and pathogenic microorganisms. PEF has been demonstrated to be effective against various pathogenic and spoilage microorganisms and retain fresh quality attributes of food without appreciable loss of flavor, color, and bioactive compounds (Charles-Rodriguez et al. 2007; Cserhalmi et al. 2006; Elez-Martı´nez et al. 2006; Hodgins et al. 2002; Jin and Zhang 1999; Min et al. 2007; Yeom et al. 2000). Fresh orange juice, tomato juice, and applesauce processed in a commercial scale PEF system achieved extended shelf-life at 4 °C (Jin et al. 2009; Min et al. 2003a, b). Cost analyses of a commercial scale PEF system using orange juice as a model have been reported by Jin and Zhang (2002) and Sampedro et al. (2013). However, there are no publications reporting the use of a commercial scale PEF processing system for pomegranate juice and the effects of PEF on the physicochemical properties, bioactive compounds, and shelf-life of pomegranate juice during storage at refrigerated temperature. The objectives of this research were (1) to investigate the effects of commercial scale PEF processing on the microbiological shelf-life of pomegranate juice, and (2) to compare the quality, nutritional, and sensory values of PEF processed pomegranate juice with those of thermally processed juice immediately after processing and during storage at 4 °C for 12 weeks.
Materials and Methods
Juice Preparation
Pomegranate juices were from AMC Incorporation, Spain, including unpasteurized (UP), pasteurized (one Ohmic treatment at 105 °C for 5 s, PT), and double heat processed (first Ohmic treatment at 105 °C for 5 s and second pasteurization at 88 °C for 30 s, DT). UP and PT juices were bulk-packaged (20 L/bag), and DT juice was packaged in 750-ml PET bottles. PT and DT juices were processed in Spain and used for comparison studies. All juices (UP, PT, and DT) were frozen before shipping to the USDA research facility. The frozen juices were shipped at refrigerated temperature and received within 2 days then stored at −20 °C. UP and PT juices were thawed at 4 °C for 3 days prior to PEF processing. Half of UP juice was used for PEF processing. Another half of UP juice, PT juice, and PEF processed juices (PEF1 and PEF2) were packaged in 750-ml PET bottles inside a Class II Type 2A biosafety cabinet. All 750-ml PET bottles were provided by AMC, Spain.
Pulsed Electric Field Processing System
A commercial scale pulsed electric field (PEF) continuous processing system, operating at a flow rate of 100 L/h, was used for this pilot scale study. The high-voltage pulse generator (OSU-6 Model, Diversified Technologies, Inc, Bedford, MA), originally designed for processing up to 2,000 L/h of orange juice, provides biopolar square waveform pulses with a maximum peak voltage of ± 60 kV. The high-voltage pulse generator operates at a maximum repetition rate of 2,000 pulses per second (pps) and pulse width of 1 ∼ 10 μs. Pulses were monitored with a high-voltage probe (VD-60; Northstar, Albuquerque, NM, USA), current monitors (Model 110; Pearson, Palo Alto, CA, USA), and oscilloscopes (TDS-210; Tektronix, Beaverton, OR, USA). Figure 1 shows the flow chart of this system.
Pulsed Electric Field Treatment Conditions
The treatment conditions for the pilot scale experiments were selected based on our preliminary studies on the reduction of Escherichia coli O 157:H7 and nonpathogenic E. coli ATCC 35218 in inoculated juice as determined using a lab scale PEF unit and validated with E. coli ATCC 35218 in peptone water in the commercial scale system. Each PEF treatment chamber, through which the juice passed during processing, consisted of two boron carbide electrodes and a ceramic insulator. The inner diameter of the chambers was 0.807 cm, and the gap distance between the electrodes was 1.27 cm. Six chambers were connected in series (electrically in parallel), thus enabling the products to flow sequentially through all six treatment zones. Counter flow heat exchangers, controlled by independent PID controllers, maintained the outlet temperature of each chamber at 55 °C. Two field strengths (35 and 38 kV/cm) were used in this study. The processing conditions are listed in Table 1, and were monitored and recorded by a USDA-developed data acquisition system using National Instruments LabVIEW (Austin, TX) software. All temperature probes were calibrated as system prior to the start of the study.
Juice Packaging and Storage
Thermally pasteurized (PT), PEF-processed (PEF1, PEF2), and unpasteurized (UP) pomegranate juices were packaged into 750-ml PET bottles as described in the previous section. Double ohmic-processed (DT) juice was kept in the original 750-ml PET bottles. All juice samples were stored at 4 °C. Three bottles of juice samples from each treatment were randomly selected for microbial analysis, and another three bottles were used for quality analysis at each sampling time.
Microbiological Analysis
Total aerobic plate count (TPC) and total yeast and molds (YMC) were determined on plate count agar (PCA, BBL/Difco Laboratories, Sparks, MD, USA) and dichlorane rose bengal chloranphenicol agar (DRBC, Merck, Germany), respectively, using a100-μL surface plating method. PCA plates were incubated at 37 °C for 24 h, and DRBC plates at 25 °C for 5 days. Results were expressed as colony-forming units (CFU) per milliliter.
Antioxidant Activity Analysis
Antioxidant activity of pomegranate juice was determined using the automated oxygen radical absorbance capacity (ORAC) method according to Ronald et al. (2005) and Wang et al. (1996). Juice samples were centrifuged at 8,000 rpm for 10 min, and supernatants were used for ORAC assay. The automated ORAC assay was carried out on a BioTek Synergy HT Multi-mode Microplate Reader (Winooski, VT, USA) with a fluorescence attachment. Fluorescent filters were set to pass light with an excitation wavelength of 485 nm and an emission wavelength of 528 nm. In a final assay, 150 μl 0.08 μM sodium fluorescein (fluorescent probe) was used as a target of free radical damage, 25 μl 2,2′-azobis(2-amidino-propane) dihydrochloride (AAPH; 4 × 10−3 M) as a peroxyl radical generator, 25-μl diluted supernatants as sample, and Trolox as a control standard. The analyzer was programmed to record the fluorescence each minute after AAPH addition. Final results were calculated using the differences of areas under the curves between a blank [25 μl 75 mM phosphate buffer (pH 7.4)] and a sample and are expressed as sample, fresh weight basis (Ronald et al. 2005; Wang et al. 1996). There were four measurements for each sample from one replicate, and there were a total of 12 measurements for each treatment.
Total Phenolic Content Analysis
Total phenolic content was measured using the Folin-Ciocalteu colorimetric method (Fan 2005). Juice sample (100 μl) used for the antioxidant activity assay was mixed with 200 μl of Folin-Ciocalteu reagent (Sigma Chemical Co, St. Louis, MO, USA), and incubated for 5 min at 25 °C. Then, 3 ml of 50 g/kg sodium carbonate was added. Absorbances at 760 nm were recorded for the mixtures after 2-h incubation at 25 °C. Total phenolic content was expressed as gallic acid equivalents in grams per milliliter of fresh samples. There were four measurements for each sample from one replicate, and there were a total of 12 measurements for each treatment.
Anthocyanin Content Analysis
The total anthocyanin content was estimated by the pH-differential method (Lako et al. 2007) using two buffer systems: potassium chloride buffer, pH 1.0 (0.025 M) and sodium acetate buffer, pH 4.5 (0.4 M) on a UV–vis spectrophotometer (UV-2401 PC, Shimadzu corporation, Kyoto, Japan). Samples were diluted in pH 1.0 and pH 4.5 buffers and then measured at 520 and 700 nm. The absorbance was calculated as A = (A 520nm − A 700nm) pH1.0 − (A 520nm − A 700nm) pH4.5.
The monomeric anthocyanin pigment concentration was calculated as cyanidin-3-glucoside. The monomerix anthocyanin pigment (milligrams per liter) = A × MW × DF × 1,000/(ε × 1), where A = absorbance, MW = molecular weight (449.2), DF = dilution factor, and ε = molecular absorptivity (26,900). All measurements were done in triplicate, and averages were reported.
pH, Total Soluble Solids, and Sediment Measurements
The pH and total soluble solid (TSS) content of the juice were measured using a pH meter (Thermo Electron Corp., Beverly, MA) and a digital refractometer (Reichert, Inc., Depew, NY, USA), respectively. The refractometer was calibrated using distilled water, and the measurement was done using the temperature compensated mode. The soluble solid content was expressed as ˚Brix. The sediment was determined by measuring the height of sediment at the bottom of the bottle bottom. Three samples were used, and all measurements were made in triplicate and average results reported.
Color
The color of juice samples were measured in International Commission on Illumination L*, a*, b* three-dimensional color space using ColorQuest XE spectrophotometer (Hunter Associates Lab, Reston, VA) where L designates lightness and measured the relative lightness and darkness of juice with L = 0 corresponding to black and L = 100 corresponding to white. The a value measured green to red with positive values indicating more red, and negative values indicating more green. The b value measured blue to yellow with positive value signaling more yellow and negative value signaling more blue. Three measurements were performed, and results were averaged. In addition, total color difference (∆E) was calculated using the following equations:
where L 0*, a 0*, and b 0* are the control values for unprocessed pomegranate juice at day 0, and L*, a*, and b* are the values for the treated samples at each storage time.
Sensory Evaluation
The sensory evaluation of four different treatments of the pomegranate juice was conducted at Auburn University. The juice samples at day 0 were immediately frozen and kept at −20 °C before the sensory evaluation. A total of 30 consumers were recruited. Consumers were asked about their degree of satisfaction of four pomegranate juice samples based on the overall appearance, overall flavor, pomegranate flavor, and acceptability using nine-point scales (1 = dislike extremely to 9 = like extremely). Approximately 20 ml of each sample was served. Unsalted crackers and water were provided for palate cleansing between samples.
Statistical Analysis
Data were analyzed using analysis of variance with SAS version 9.1 software (SAS Institute, Cary, NC). Duncan’s multiple range test was used to determine the significant differences of mean values. Significance was defined at p < 0.05.
Results and Discussion
Effect of PEF Processing on the Survival of TPC and YMC
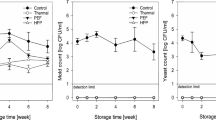
The effects of PEF treatments on the survival of total aerobic bacteria (TPC) and yeasts and molds (YMC) are shown in Fig. 2. Natural bacteria grew well in untreated pomegranate juice, from ca. 3 log CFU/ml at 0 day to 7 log CFU/ml after 4 weeks at 4 °C. PEF treatments reduced the bacteria to 2.0–2.3 log CFU/ml and maintained these levels through the 12-week storage at 4 °C (Fig. 2a). There was no significant difference in TPC between the two PEF treatments. For comparison, thermally pasteurized (PT) and double thermally pasteurized (DT) juice samples had no TPC detected (<0.69 log CFU/ml) during the entire storage period. Molds and yeasts also grew well in untreated juice samples, reaching 7 log CFU/ml after 4 weeks at 4 °C (Fig. 2b). PEF treatments significantly (p < 0.05) reduced YMC from ca. 2.6 log CFU/ml to undetectable levels (<0.69 log CFU/ml) after the treatments and kept these levels until 10 weeks for PEF treatment 1 (35 kV/cm) and 12 weeks for PEF treatment 2 (38 kV/cm), where approximately 1.5 and 2.1 log CFU/ml of YMC were observed, respectively. Similar to TPC, there was no YMC detected in DT juice samples. However, molds and yeasts grew over the storage time in PT juice samples, reaching 4.4 log CFU/ml by the end of 12 weeks.
Survival of TPC and YMC in pomegranate juice during storage at 4 °C as affected by PEF and thermal treatments. a TPC, b YMC. UP unpasteurized, PEF1 35 kV/cm, PEF2 38 kV/cm, PT single thermal treatment, DT double thermal treatment. Error bars represent the standard deviation of the mean. Asterisk under detection limit
PEF process in laboratory scale PEF systems has been reported to extend the microbiological shelf-life of fruit juices, such as orange (Min et al. 2006), tomato (Aguilo-Aguayo et al. 2008), and apple and cranberry (Jin and Zhang, 1999; Raso et al. 1998). In this study, PEF processing extended the microbial shelf-life of pomegranate juice to 12 weeks at 4 °C, while both TPC and YMC were under 2.5 log CFU/ml, which indicates effective inactivation of spoilage microorganisms by PEF. Using the same commercial scale PEF processing system, other studies demonstrated that PEF extended the shelf-life of tomato juice, orange juice, applesauce, and yogurt (Jin et al. 2009; Min et al. 2003a, b; Yeom et al. 2004).
PEF processing was more effective against yeasts and molds than vegetative bacteria, as the two PEF treatments achieved similar reduction (<0.69 log CFU/ml) of YMC compared to the double thermal treatment (DT) until weeks 10 and 12 (Fig. 2b). Yeasts are more sensitive than vegetative bacteria to PEF treatment due to their larger cell size (Jeyamkondan et al. 1999). For cranberry juice, the PEF-treated sample had less growth of molds and yeasts than that of aerobic bacteria after 7- and 14-day storage (Jin and Zhang 1999). The YMC for PEF-processed apple cider throughout the 4-week storage period at 4 °C were <1 log CFU/ml, while TPC of PEF-processed cider increased to 2.4 log CFU/ml by the end of the storage (Azhu Valappil et al. 2009).
Effect of PEF Processing on Total Phenolics, Anthocyanin and Antioxidant Activity (ORAC)
The effects of PEF processing and thermal processing on the concentrations of total phenolics, total anthocyanin, and antioxidant in pomegranate juice during storage at 4 °C for 12 weeks are shown in Fig. 3. Two groups of samples were observed based on total phenolic concentrations: thermally processed samples (PT and DT) were in one group, and PEF processed samples were grouped with unprocessed samples (Fig. 3a), indicating that PEF processing didn’t alter the total phenolic contents as compared to unprocessed juice. Similar to total phenolics, the PEF processed and unprocessed juice samples had similar concentrations of total anthocyanin (Fig. 3b). Storage time didn’t affect the changes of total phenolics, but did slightly decrease the total anthocyanin concentrations for all samples stored at 4 °C for 12 weeks.
Effects of PEF and thermal treatments on the retention of total phenolics, anthocyanin, and antioxidant activity (ORAC) of pomegranate juice during storage at 4 °C for 12 weeks. a Total phenolics, b anthocyanin, c antioxidant activity. UP unpasteurized, PEF1 35 kV/cm, PEF2 38 kV/cm, PT single thermal treatment, DT double thermal treatment. Error bars represent the standard deviation of the mean
The variation in the antioxidant activity of PEF-processed pomegranate juice during storage is shown in Fig. 3c. The antioxidant activity of PEF-processed pomegranate juice was similar to that in unpasteurized juice during the first 4 weeks of storage. The additional storage time didn’t significantly (p > 0.05) affect the antioxidant activity of PEF-processed pomegranate juices. There was no significant difference in antioxidant activity between the two PEF-treated pomegranate juices during the storage at 4 °C for 12 weeks.
In this study, PEF processing didn’t significantly (p > 0.05) impact the nutritional values of pomegranate juice as the PEF-processed juice samples had similar total phenolic, anthocyanin, and antioxidant compounds to those in unpasteurized juice samples (Fig. 3). Although anthocyanin compounds in all samples decreased during the storage, the smallest decrease of the compound was observed in PEF juices.
With respect to the influence of thermal processing on total phenolic compounds, significant increases were observed in the thermally treated samples (Fig. 3a), which is not correlated to anthocyanin (Fig. 3b). Augments in the phenolic content of juices after thermal processing have been widely reported and connected to the breakdown of polymeric phenolics (Yildiz et al. 2009; Hager et al. 2010). It may be due to increase of ellagitannins after the thermal treatments. Ellagitannins have been regarded as the main non-colored phenolics of pomegranate juices. Among them, punicalagin isomers accounted for the high antioxidant capacity of some pomegranate juices (Gil et al. 2000). Punicalagins were present in low concentrations at fresh juices before pasteurization; however, they significantly (p < 0.05) increased for all juices after thermal treatment.
Effect of PEF Processing on Color
Changes were observed when the color characteristics were analyzed by colorimetric meter after processing and during the storage period (Fig. 4). PEF-treated juice samples become less bright (lower L* values), and more red (higher a* values), and had similar yellowness (b* value) as compared to unpasteurized juice samples. In contrast, the thermally processed juice samples had significantly (p < 0.05) lower L* and b* values than those for PEF-treated and unpasteurized juice samples.
Effects of PEF and thermal treatments on the stability of color of pomegranate juice during storage at 4 °C for 12 weeks. a L* value, b a* value, c b* value, and d ΔE value. UP unpasteurized, PEF1 35 kV/cm, PEF2 38 kV/cm, PT single thermal treatment, DT double thermal treatment. Error bars represent the standard deviation of the mean
Fresh-like color is an important quality parameter for consumer acceptance of juice. A decrease in L* and b* values for thermally pasteurized juices indicated that the juice color became darker and less yellow than unpasteurized and PEF-processed juices. Interestingly, noticeable decreases in a* values for double thermally treated samples (DT) were observed, indicating a loss in redness, while thermally pasteurized samples (PT) had similar a* values as unpasteurized and PEF-processed samples. The a* values for treated samples were highly correlated with the anthocyanin pigment contents in Fig. 3b, as the Pearson correlation coefficients for PEF1, PEF2, PT, and DT were 0.81, 0.75, 0.85, and 0.78, respectively.
When the color (L*, a* and b*) of unpasteurized juice sample at 0 day was used as a base point, total color difference (∆E) between unpasteurized and PEF or thermally treated juice samples clearly showed that PEF processing had significantly (p < 0.05) less impact on the color of pomegranate juice than thermal processing (PT and DT; Fig. 4), while thermally treated samples had significantly (p < 0.05) higher ΔE (P < 0.05) compared to both PEF samples at week 0 and throughout the 12 weeks. PEF-processed juice showed maximum color stability during 12 weeks of storage. The less color changes caused by PEF were further proved by visual observation (Fig. 5). Figure 5 shows photos of the juice samples stored at 4 °C for 1 and 12 weeks. Thermal treatments are known to have pronounced effects on the color of juice due to degradation of color pigments, while better color preservation in PEF-treated juice compared to heat-pasteurized juice has been documented (Jin and Zhang 1999; Lee and Coates 1999; Min et al. 2003a, b; Azhu Valappil et al. 2009; Yeom et al. 2000). The current study demonstrates that PEF processing had the least impact on the color changes compared to thermally processed juices immediately after processing and during storage at 4 °C for 12 weeks.
Effect of PEF Processing on °Brix, pH, and Sediment
Effects of thermal processing and PEF processing on the change of TSS and pH of pomegranate juice during storage at 4 °C for 12 weeks are shown in Fig. 6. The °Brix values of all juice samples except DT samples stayed at 16.5 during storage at 4 °C for 12 weeks (Fig. 6a). The DT samples showed slightly lower °Brix values. All juice samples had similar pH value around 3.85 and did not significantly (p > 0.05) change during the whole storage period (Fig. 6a).
Effects of PEF treatments on the stability of Brix, pH, and sediment of pomegranate juice during storage at 4 °C for 12 weeks. a Total soluble solid content (solid line) and pH (dashed line). b Sediment. UP unpasteurized, PEF1 35 kV/cm, PEF2 38 kV/cm, PT single thermal treatment, DT double thermal treatment. Error bars represent the standard deviation of the mean
Figure 6b shows juice sediments as affected by processes and storage time. After a 3-week storage, all samples had stabilized with regard to the sediments. Based on the amounts of sediment, the juice samples could be divided into three groups, unpasteurized (UP), PEF processed (PEF1 and PEF2), and thermally processed (PT and DT). Unpasteurized juice samples had the most sediment and thermally processed juice samples had the least sediments. The difference in juice sediments between each sample can also be observed in Fig. 5. PEF processing had less impact on the sediments of the juices than the thermal processing.
Effect of PEF Processing on Consumer Acceptance
The overall satisfaction scores of consumer tests are presented in Table 2. Consumers preferred unpasteurized (UP) and PEF-processed (PEF) juice samples to those thermally processed (DT and PT) (p < 0.05). Although there were no statistical differences in all scores between PEF and UP juices, UP juice had the highest score of appearance (6.9 vs. 6.7), while PEF juice got the highest scores of overall flavor and overall acceptability (6.3 and 6.4, respectively). In contrast, both thermally treated juice samples had the lowest scores of pomegranate flavor, appearance, overall flavor, and overall acceptability, which is significantly (p < 0.05) different from UP and PEF juices. Similar results were also observed in other PEF-processed foods (Azhu Valappil et al. 2009; Evrendilek et al. 2004; Jin et al. 2009; Yeom et al. 2004).
Conclusion
This study demonstrated that PEF-processed pomegranate juice in a commercial scale system had a similar microbiological shelf-life as thermally processed juices while having similar quality, nutritional, and sensory values as the unpasteurized juices, which further confirmed that PEF was effective against spoilage microorganisms and to retain fresh quality attributes of food without appreciable loss of flavor, color, and bioactive compounds as investigated by other researchers. Therefore, we conclude that PEF processing technology is technically feasible for commercial application for juice products.
References
Aguilo-Aguayo, I., Odriozola-Serrano, I., Quintao-Teixeira, L. J., & Martin-Belloso, O. (2008). Inactivation of tomato juice peroxidase by high-intensity pulsed electric fields as affected by process conditions. Food Chemistry, 107(2), 949–955.
Alighourchi, H., & Barzegar, M. (2009). Some physicochemical characteristics and degradation kinetic of anthocyanin of reconstituted pomegranate juice during storage. Journal of Food Engineering, 90, 179–185.
Aviram, M., Dornfeld, L., Rosenblat, M., Volkova, N., Kaplan, M., Coleman, R., et al. (2000). Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. American Journal of Clinical Nutrition, 71, 1062–1076.
Azhu Valappil, Z., Fan, X., Zhang, H., & Rouseff, R. (2009). Impact of thermal and nonthermal processing technologies on unfermented apple cider aroma volatiles. Journal of Agricultural and Food Chemistry, 57, 924–929.
Charles-Rodriguez, A. V., Nevarez-Moorillon, G. V., Zhang, Q. H., & Ortega-Rivas, E. (2007). Comparison of thermal processing and pulsed electric fields treatment in pasteurization of apple juice. Food and Bioproducts Processing, 85(2), 93–97.
Cserhalmi, Z., Sass-Kiss, A., To´th-Markus, M., & Lechner, N. (2006). Study of pulsed electric field treated citrus juices. Innovative Food Science & Emerging Technologies, 7, 49–54.
Du, C., Wang, P., & Francis, F. (1975). Anthocyanins of pomegranate, Punica granatum. Journal of Food Science, 40, 417–418.
Elez-Martı´nez, P., Soliva-Fortuny, R., & Martı´n-Belloso, O. (2006). Comparative study on shelf life of orange juice processed by high intensity pulsed electric fields or heat treatment. European Food Research and Technology, 222, 321–329.
Evrendilek, G. A., Yeom, H. W., Jin, Z. T., & Zhang, Q. H. (2004). Safety and quality evaluation of a yogurt-based drink processed by a pilot plant PEF system. Journal of Food Processing Engineering, 27, 197–212.
Fan, X. (2005). Antioxidant capacity of fresh-cut vegetables exposed to ionizing radiation. Journal of the Science of Food and Agriculture, 85, 995–1000.
Gil, M. I., Tomás-Barberán, F. A., Hess-Pierce, B., Holcroft, D. M., & Kader, A. A. (2000). Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. Journal of Agricultural and Food Chemistry, 48, 4581–4589.
Hager, T.-J., Howard, L.-R., & Prior, R.-L. (2010). Processing and storage effects on the ellagitannin composition of processed blackberry products. Journal of Agricultural and Food Chemistry, 58(22), 11749–11754.
Hamutal, B. N., Sylvie, J., Effi, T., Moti, H., Amnon, G., Ilan, S., et al. (2009). Seasonal and cultivar variations in antioxidant and sensory quality of pomegranate (Punica granatum L.) fruit. Journal of Food Composition and Analysis, 22, 189–195.
Hodgins, A. M., Mittal, G. S., & Griffiths, M. W. (2002). Pasteurization of fresh orange juice using low-energy pulsed electrical field. Journal of Food Science, 67, 2294–2299.
Jeyamkondan, S., Jayas, D. S., & Holley, R. A. (1999). Pulsed electric field processing of foods: a review. Journal of Food Protection, 62, 1088–1096.
Jin, T., Zhang, H. Q., Li, S. Q., Kim, M., Dunne, C. P., Yang, T., et al. (2009). Quality of applesauces processed by pulsed electric fields and HTST pasteurization. International Journal of Food Science and Technology, 44, 829–839.
Jin, T., & Zhang, H. (1999). Pulsed electric fields inactivate microorganisms and preserve quality of cranberry juice. Journal of Food Processing and Preservative, 23(6), 481–497.
Jin, T., & Zhang, Q. H. (2002). Cost evaluation of a commercial scale PEF system. 2002 IFT Annual Meeting, technical program 91E-21, Anaheim, CA, USA.
Lako, J., Trenerry, V. C., Wahlqvist, M., Wattanapenpaiboon, N., Sotheeswaran, S., & Premier, R. (2007). Phytochemical flavonols carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chemistry, 101, 1727–1741.
Lansky, E. P., & Newman, R. A. (2007). Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. Journal of Ethnopharmacology, 109, 177–206.
Lee, H. S., & Coates, G. A. (1999). Thermal pasteurization effects on color of red grapefruit juices. Journal of Food Science, 64, 663–666.
Malik, A., Afaq, F., Sarfaraz, S., Adhami, V. M., Syed, D. N., & Mukhtar, H. (2005). Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proceedings of the National Academy of Sciences of the United States of America, 102, 14813–14818.
Min, S., Jin, Z., Min, S., Yeom, H., & Zhang, Q. (2003a). Commercial-scale pulsed electric field processing of orange juice. Journal of Food Science, 68, 1265–1271.
Min, S., Jin, Z. T., & Zhang, Q. H. (2003b). Commercial scale pulsed electric field processing of tomato juice. Journal of Agricultural and Food Chemistry, 51, 3338–3344.
Min, S., Jin, Z. T., Min, S. K., Yeom, H., & Zhang, Q. H. (2006). Commercial-scale pulsed electric field processing of orange juice. Journal of Food Science, 64(4), 1265–1271.
Min, S., Evrendilek, G. A., & Zhang, H. Q. (2007). Pulsed electric fields: processing system, microbial and enzyme inhibition, and shelf life extension of foods. IEEE Transactions on Plasma Science, 35(1), 59–73.
Patras, A., Brunton, N. P., O'Donnell, C., & Tiwari, B. (2010). Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends in Food Science & Technology, 21, 3–11.
Raso, J., Calderon, M. L., Gongora, M., Barbosa-Canovas, C., & Swanson, B. G. (1998). Inactivation of mold ascospores and conidiospores suspended in fruit juices by pulsed electric fields. Journal of Food Science, 31(7–8), 668–672.
Ronald, L., Wu, X., & Schaich, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry, 53, 4290–4302.
Sampedro, F., McAloon, A., Yee, W., Fan, X., Zhang, H. Q., & Geveke, D. J. (2013). Cost analysis of commercial pasteurization of orange juice by pulsed electric fields. Innovative Food Science and Emerging Technologies, 17, 72–78.
Wang, H., Cao, G., & Ronald, L. (1996). Total antioxidant capacity of fruits. Journal of Agricultural and Food Chemistry, 44, 701–705.
Yeom, H. W., Streaker, C. B., Zhang, Q. H., & Min, D. B. (2000). Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. Journal of Agricultural and Food Chemistry, 48, 4597–4605.
Yeom, H. W., Evrendilek, G. A., Jin, Z. T., & Zhang, Q. H. (2004). Processing of yogurt-based products with pulsed electric fields: microbial, sensory and physical evaluation. Journal of Food Processing and Preservative, 28(3), 161–178.
Yildiz, H., Bozkurt, H., & Icier, F. (2009). Ohmic and conventional heating of pomegranate juice: effects on rheology, color, and total phenolics. Food Science and Technology International, 15(5), 503–512.
Acknowledgments
This project was in collaboration with AMC Group, Spain under the Cooperative Research and Development Agreement (#58-3K95-2-1543). The authors thank Anita Parameswaran and Kim Sokorai for excellent technical laboratory assistance, Andy Bigley for outstanding PEF pilot system operation, and Xiaoying Zhang (Auburn University) for superb sensory evaluation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity employer.
Rights and permissions
About this article
Cite this article
Guo, M., Jin, T.Z., Geveke, D.J. et al. Evaluation of Microbial Stability, Bioactive Compounds, Physicochemical Properties, and Consumer Acceptance of Pomegranate Juice Processed in a Commercial Scale Pulsed Electric Field System. Food Bioprocess Technol 7, 2112–2120 (2014). https://doi.org/10.1007/s11947-013-1185-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1185-6