Abstract
There is growing concern in recent years for consumers about contamination of pesticides in fruits due to increasing use of pesticides in fruits. The objective of this study was to use surface-enhanced Raman spectroscopy (SERS) to detect and characterize pesticides extracted from fruit surfaces. Gold-coated SERS-active nanosubstrates were used for SERS measurement. Three types of pesticides (carbaryl, phosmet, and azinphos-methyl) widely used in apples and tomatoes were selected. Significantly enhanced Raman signals of pesticides were acquired by SERS from the extract of fruit samples and exhibited characteristic patterns of the analytes. Multivariate statistical methods such as partial least squares and principal component analysis were used to develop quantitative and qualitative models. SERS was able to detect all three types of pesticides extracted from fruit samples at the parts per million level. The study of detection limit demonstrated that at 99.86% confidence interval, SERS can detect carbaryl at 4.51 ppm, phosmet at 6.51 ppm, and azinphos-methyl at 6.66 ppm spiked on apples; and carbaryl at 5.35 ppm, phosmet at 2.91 ppm, and azinphos-methyl at 2.94 ppm on tomatoes. Most of these detection limits meet the maximum residue limits established by Food and Agriculture Organization of the United Nations and World Health Organization. Satisfactory recoveries (78–124%) were achieved for samples with concentrations at and larger than the detection limit. These results demonstrate that SERS coupled with novel SERS-active nanosubstrates is a rapid, sensitive, and reliable method for detection and characterization of chemical contaminants in foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organophosphosphate (OP) and carbamate (CB) pesticides have been used for decades to fight against pests to increase the yields of agricultural products. Because of their ability of inactivating acetylcholinesterase, OP and CB are considered to have potent neurotoxicity (Costa 2006; Gupta 2004; He 2000). Several studies suggest that chronic exposure to OP and CB, even at low to mild doses, may lead to long-term adverse neurobehavioral effects (Costa 2006; Wesseling et al. 2002). OP and CB are widely used in fruits and vegetable crops (Kegley and Wise 1998). According to Residue Monitoring Reports published by the US Food and Drugs Administration (US FDA), pesticide residues exist in a large portion of certain types of fruits and vegetables such as apple and tomato (FDA 2006, 2007a). There is growing concern in recent years for consumers about contamination of pesticides in fruits because FDA found that from 2004 to 2007, more domestic fruit samples contain pesticide residues, suggesting that the use of pesticides in fruits is increasing (FDA 2007b). Therefore, reliable and consistent detection methods for OP and CB in fruits and vegetables are crucially needed.
Current methods adopted by AOAC for detection of OP and CB in fruits and vegetables are various thin-layer chromatography and gas chromatography setups (AOAC 2005; Ortelli et al. 2005; Rawn et al. 2006). In recent years, novel analytical methods for detection of OP and CB pesticides in fruits and vegetables have been developed, including liquid chromatography–mass spectrometry (Grimalt et al. 2007; Liu et al. 2005; Ortelli et al. 2005), fluorescence polarization immunoassay (Kolosova et al. 2003), multienzyme inhibition assay (Walz and Schwack 2007), and biosensors (Valdés-Ramírez et al. 2008; Zhang et al. 2005). Although these methods can be used to detect trace amount of pesticide residues, they are time-consuming, labor-intensive, and often require complicated procedures of sample preparation. In addition, a narrow range of application is another major hindrance to applying these techniques for food analysis.
Vibrational spectroscopic methods such as Raman spectroscopy have long been considered a useful analytical technique to evaluate food safety and quality (Lu et al. 2011). Although Raman spectrum can provide “fingerprint-like” information about various chemical and biochemical components in a complex system with little or no sample preparation, traditional Raman spectroscopy is only suitable for measuring compounds present in high concentration because only one out of one million photons undergoes Raman scattering, thus generating only weak signals. Surface-enhanced Raman spectroscopy (SERS), discovered in the 1970s (Albrecht and Creighton 1977; Fleischmann et al. 1974; Jeanmaire and Van Duyne 1977), significantly enhances the sensitivity of traditional Raman spectroscopy. Over the last 10 years, with rapid development of nanotechnology and nanomaterials, the interest in SERS was revived. SERS is a technique in which probed molecules are absorbed onto the roughened surface of transition metals, resulting in significant enhancement of the Raman signals by many orders of magnitude in highly localized optical fields of nanostructures. Coupled with metal nanosubstrates, SERS can increase the intensity of Raman signals by more than one million times due to the effects of electromagnetic field enhancement and chemical enhancement (Haynes et al. 2005; Kneipp et al. 2002). Many applications are being explored using novel nanosized or nanostructured substrates for SERS measurements. For example, nanosized particles can be used as SERS-active substrates because they have a very high surface/volume aspect ratio, which is an important parameter to ensure that a great number of probe molecules are captured in the close vicinity of the metal surface. To date, gold and silver are two most frequently used materials for fabrication of SERS-active nanosubstrates.
SERS has been used in rapid detection and characterization of various food contaminants and adulterants, including banned seafood drugs such as crystal violet and malachite green (He et al. 2008a; Lai et al. 2011), melamine and its derivatives (He et al. 2008b; Liu et al. 2010), and banned food dye Sudan-1 (Shadi et al. 2010). The objective of this study was to use SERS coupled with novel nanosubstrates for rapid detection of OP and CB pesticides extracted from fruit surfaces. Two types of fruits (i.e., apple and tomato) were selected in this study because they are major targets of pesticide residue monitoring and have been found to be heavily contaminated with various pesticides (FDA 2006, 2007a). Multivariate statistical methods were used to analyze SERS spectral data and develop quantitative and qualitative models for data analysis.
Materials and Methods
Materials
Two OP pesticides (azinphos-methyl and phosmet) and one CB pesticide (carbaryl) were purchased from Fisher Scientific Inc. (Pittsburgh, PA, USA). Organic apples (Granny Smith) and tomatoes were purchased from a local supermarket. Organic fruits were selected and cleaned to ensure that no pesticide residues existed on the samples.
Sample Preparation
Pure pesticide solutions: 100 ppm (w/v) of azinphos-methyl, phosmet, and carbaryl stock solutions were prepared using a mixed solvent system (acetonitrile/H2O = 1:1, v/v). Solutions of 50, 10, 5, 1, 0.5, and 0.1 ppm pesticides were prepared by serial dilutions from the 100 ppm solution. The solvent without pesticides was used as the control.
Apples and tomatoes were weighed, and their diameters were measured. Assuming that an apple or a tomato is of spherical shape, the surface area of the fruit was calculated. Using these data, given designated pesticide concentration on a fruit (parts per million, or equivalently, micrograms per gram), the mass of pesticide (microgram) that should be spiked on 1 cm2 of fruit skin was obtained. Then, certain amounts of pesticide solutions were dropped with a pipetter onto a piece of ~4 cm2 fruit skin freshly peeled from the fruit. Extra care was taken to ensure even distribution of the solution on the fruit skin. The skin of the samples were then blown dry, cut into small pieces, and placed in conical tubes containing 4 mL of mixed solvent (acetonitrile/H2O = 1:1, v/v). After vigorous vortexing for 1 min, the mixture was sonicated using an ultrasonic processor equipped with a 6.5-mm tapered microtip (Sonics & Materials, Inc. Newtown, CT, USA) for 5 min with 30-s working and 10-s interval at an amplitude of 30%. Finally, the supernatant was filtered with a 0.22-μm syringe filter, and the filtrate was used for SERS measurement.
SERS Substrates
The SERS substrates, Q-SERS™ G1, were obtained from Nanova Inc. (Columbia, MO, USA). Q-SERS™ substrates are gold-based nanostructures fabricated on a silicon wafer. A volume of ~0.3–0.5 μL of the filtrate from previous step was dropped on the surface of a substrate using a micropipette. The substrate, which was fixed on a glass slide, was then placed on a hot plate and heated at 40°C until the solvent completely evaporated.
SERS Measurement
A Renishaw RM1000 Raman Spectrometer System (Gloucestershire, UK) equipped with a Leica DMLB microscope (Wetzlar, Germany) was used in this study. This system is equipped with a 785-nm near-infrared diode laser source. During the measurement, light from the high power (maximum at 300 mW) diode laser was directed and focused onto the sample at a microscope stage through a ×50 objective. Raman scattering signals were detected by a 578 × 385 pixels charge-coupled device array detector. The size of each pixel was 22 × 22 μm. Spectral data were collected by WiRE 3.2 software (Gloucestershire, UK). In this study, spectra of samples were collected using a ×50 objective with 10-s exposure time, 0% focus, and ~20 mW laser power in the extended mode. Detection ranges for each pesticide were 550–1,650 cm−1 for azinphos-methyl, 550–1,800 cm−1 for phosmet, and 600–1,700 cm−1 for carbaryl. The detection range was determined in a way that the range was as narrow as possible, but no obvious signals were missed.
Data Analysis
The software Delight version 3.2.1 (D-Squared Development Inc., LaGrande, OR, USA) was used in data analysis. SERS spectral data were analyzed following previously developed methods with slight modification (He et al. 2008a; Liu et al. 2010). Briefly, data pre-processing algorithms including polynomial subtract and Gaussian smoothing were employed to subtract the baseline shift and eliminate high frequency noises from the instrument. The partial least squares (PLS) model, a multivariate statistical regression model, was constructed to predict analyte concentrations in tested samples. The PLS model was validated by leave-one-out cross validation, which uses all but one sample to build a calibration curve and repeats for each sample in the data set. The number of PLS latent variables was optimized based on the lowest root mean square error of prediction (RMSEP) values to avoid overfitting of spectral data.
In this equation, n is the number of samples, ĉ i is the predicted pesticide concentration (ppm), and c i is the actual pesticide concentration (ppm). The correlation coefficient (R) and RMSEP were used to evaluate the model. The higher the R value or the lower the RMSEP value is, the better predictability the model has.
The detection limit (DL) with 99.86% confidence interval can be calculated from the PLS calibration curve based on characteristic peaks in SERS spectra using the following formula (Strickland and Batt 2009):
in which σ is the standard error of predicted concentration, and m is the slope of the calibration curve. In a PLS model, σ equals to RMSEP.
Principal component analysis (PCA) is a statistical technique used to reduce a multidimensional data set to its most dominant features, to remove random variations (noise), and to retain the principal components (PCs) that explain most variations between sample treatments (Goodacre et al. 1998). In this study, spectral data were smoothed with a Gaussian function at 4 cm−1 followed by a second derivative transformation with a 12 cm−1 gap before PCA was conducted.
Concentrations of pesticide solutions extracted from fruit samples were determined via PLS using the calibration curve. The recovery percentage was calculated with quantified pesticide concentrations divided by spiked pesticide concentrations.
Results and Discussion
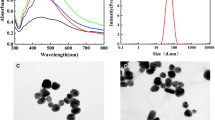
Figure 1 shows an SEM image of the surface of a gold-coated Q-SERS™ G1 substrate, which exhibits two layers of nanostructures. The larger nanoparticles have sizes of 20-60 nm and their surfaces are covered by smaller nanoparticles with sizes of 4-6 nm. Most of those bigger nanoparticles with sizes of 40-50 nm are connected, but some of them have a gap of about 20 nm between each other. The structures of nanosubstrates are not highly uniform when observing at nanoscale. Thus, the SERS enhancement factors at different locations are expected to be different depending on the size and spacing of nanoparticles. However, if one spot on the substrate is compared to another at microscale, the structures of substrates are very uniform. During SERS measurement, a laser beam with a diameter of 5 μm was typically applied. If a spot with a diameter of 5 μm is compared with another spot of identical size on a substrate, the numbers of active hotspots should be at the same level, and the enhancement factors of these two spots are almost identical. The surface coverage of the nanoparticles by a thin layer of gold is uniform and complete, which is confirmed by the fact that no silicon peak was detected in the SERS spectra.
Figure 2 shows molecular structures of three types of pesticides (azinphos-methyl, carbaryl, and phosmet). To confirm that no interfering signals were introduced by solvent or other factors, SERS spectra of the three pesticides in both solution and solid forms were obtained and compared, as shown in Fig. 3. For all three pesticides, signals of solution and solid forms agree well with each other, indicating that the solvent used did not produce interfering signals in measurement. The intensity of some peaks in spectra obtained from the solution is apparently different from their counterparts in spectra obtained from the solid (e.g., a peak at 730 cm−1 in carbaryl spectra and peaks in 580–740 cm−1 region in phosmet spectra). This may be due to the interaction between solute and solvent molecules. These peaks were not used for quantification analysis.
Average SERS spectra (n = 8) of pesticide solutions and solid (a1 azinphos-methyl solution, a2 azinphos-methyl solid, b1 carbaryl solution, b2 carbaryl solid, c1 phosmet solution, c2 phosmet solid). The concentration of all solutions is 50 ppm. Measurements were conducted with a 10-s exposure time and ~20 mW laser power. Spectra were presented with smoothing at 4 cm−1 and baseline adjustment by subtracting a second order polynomial function
SERS was used to measure solutions containing different concentrations of three pesticides. Average SERS spectra (n = 8) of carbaryl are shown in Fig. 4, while similar results were obtained for the other two pesticides (data not shown). The carbaryl spectra are highly consistent with a previous report using Ag nanoparticle-coated Si nanowire as SERS substrate, in which the intensity of 1,380 and 1,440 cm−1 peaks decreased as concentration of carbaryl decreased (Wang et al. 2010). Band assignments were summarized in Table 1 based on other published data (Boese and Martin 2004; Fischer et al. 1997; Socrates 2004; Trotter 1977). For example, a strong peak at 1,380 cm−1 may be due to the symmetric vibration of the naphthalene ring. The naphthalene ring of carbaryl is mono-substituted, and the 1,440 cm−1 peak may be from unspecified vibrations of this ring. Another strong peak at 1,565 cm−1 can be attributed to the stretching of C=C double bonds in the naphthalene ring. In general, our results agreed well with previous reports.
Second derivative transformation is a powerful tool in analysis of spectral data. It can separate overlapping peaks, eliminate baseline effects, and enhance spectral resolution. Spectra of carbaryl solutions were preprocessed using second derivative transformation. Figure 5 shows the most prominent feature at 1,380 cm−1 in the spectra. This figure demonstrates that SERS is able to differentiate spectral patterns between different concentrations of carbaryl in a mixed solvent. A drawback of this approach is that it may not provide a direct discrimination between samples containing relatively high concentrations of pesticides (larger than 10 ppm). As shown in Fig. 5, the height of the peak at 1,380 cm−1 does not differ much between the spectral samples of 10 and 50 ppm, which is also the case for the other two pesticides.
PLS analysis was applied on spectra of all three pesticide samples. RMSEP values obtained from the PLS models of carbaryl with different latent variables are shown in Fig. 6. The spectral data were preprocessed with smoothing at 4 cm−1 in the whole spectral region. The lowest RMSEP value was achieved when five latent variables were used, suggesting that the optimal number of latent variables to construct a PLS model is 5. The optimal number for building PLS models is 5 for azinphos-methyl and 6 for phosmet. PLS prediction results for carbaryl (n = 54) are shown in Fig. 7 by plotting predicted pesticide concentrations against actual pesticide concentrations (R = 0.84; RMSEP = 1.954 × 10−5). For azinphos-methyl and phosmet, R values are 0.98 and 0.85, and RMSEP values are 7.269 × 10−6 and 1.823 × 10−5. With R values all larger than 0.83, results of PLS analyses suggest that PLS can serve as a reliable method to quantify pesticides.
PCA was conducted based on the spectra acquired from all three pesticides to investigate if SERS can differentiate different pesticides. The results of using the first two PCs to classify samples are shown in Fig. 8, indicating that PCA with the first two PCs could be used to discriminate the three pesticide samples quite well. Although Fig. 8 shows clear segregation between the three pesticide samples, a small portion of samples overlapped with each other. Because a SERS spectrum reflects the characteristics of chemical structure of the analyte, it is possible that one of the PCs can be assigned to common chemical groups. Both azinphos-methyl and phosmet are organophosphorus compounds and possess a functional group [S = P(OCH3)2], so when using the first two PCs to classify them, overlapping of data clusters may occur. In contrast, carbaryl does not contain such phosphorus group, but it has a naphthalene structure, which is similar to aromatic rings that the other two pesticides have. This would explain why some of carbaryl samples overlapped with those of phosmet and azinphos-methyl. SERS spectra acquired from higher concentrations of samples exhibited more significant differences from each other because richer information of chemical structures of the analytes was reflected in SERS spectra.
SERS was used to detect carbaryl, phosmet, and azinphos-methyl extracted from real food samples. Detection limits for the pesticides were calculated by Eq. 2. As an example, calibration curve of carbaryl extracted from apple skin is shown in Fig. 9. Results of detection limit for three pesticides are summarized in Table 2, indicating that satisfactory prediction results could be obtained by SERS. Maximum residue limits (MRLs) of the three pesticides tested in this study have been established for apples and tomatoes by Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO), as shown in Table 3 (FAO/WHO 1967, 1984, 2009). Comparing the MRLs with the results in Table 2, it can be concluded that the SERS method meets the requirement of carbaryl and phosmet. For azinphos-methyl, more efficient extraction procedures and better performing substrates is needed to improve the result.
It was argued that a thicker waxy outer layer of tomato skin favors penetration of pesticides because the layer contributes to the stability of pesticides in it (Gunther and Blinn 1955). This claim was supported by the fact that after being applied to whole tomatoes, most of dimethoate, profenofos, and pirimiphos-methyl, all of which are organophosphorus pesticides, were found to be retained in the skin of tomatoes (Abou-Arab 1999). In addition, peeling reduces over 83% of the three organophosphorus pesticides in tomatoes (Abou-Arab 1999). For apples, it was shown that 24 h after application, over 85% of azinphos-methyl and phosmet residues locate in the skin and the outside 2 mm of the apple flesh (Wise et al. 2009). Therefore, our method of peeling the fruit and applying pesticide solutions onto the skin did not cause significant sampling errors compared to common approaches such as extracting pesticides from whole fruit homogenates.
To calculate the recovery of pesticides, we took an approach different from common chromatographic methods in which the calibration curves obtained from pure pesticide solutions were used to quantify the analyte extracted from food samples. For quantification analysis based on PLS, some studies suggest that the standards and unknown samples should be of the same matrix composition (Sentellas et al. 2001). Therefore, we used the calibration curve obtained from pesticides extracted from real foods to predict the concentrations of the samples, and calculated the recovery percentage accordingly (Table 4). The recoveries are satisfactory for samples with concentrations around or larger than their corresponding detection limits (77.7 to 124.3%). For comparison, several previous studies using different methods to detect these pesticides in fruits and vegetables show recoveries ranging from 70 to 110% (Grimalt et al. 2007; Kolosova et al. 2003; Liu et al. 2005; Walz and Schwack 2007; Zhang et al. 2005). While these methods do show better detection limits (ranging from 15 ppb to 3 ppm for different pesticides in various food matrices), it must be noted that complex and time-consuming processes should not be incorporated as part of sample preparation procedure of SERS as the rapidness is the main advantage of SERS methods.
We propose that when SERS methods are applied to test for chemical samples in food, the standards should be the chemical extracted from the foods that have been spiked with known concentrations of the analyte, in contrast to chromatographic methods in which standards are pure chemical solutions. The steps of extraction should be the same for standards and samples. Today’s food safety situation calls for development of rapid, sensitive, and reliable methods that are suitable for on-site detection. Compared with other methods, SERS has the following advantages to meet such requirements: simplicity of sample preparation, acceptable accuracy and reliability, and wide applications. In addition, more effective yet simple extraction techniques and better performing substrates will greatly improve the capability of SERS methods.
Conclusions
Our study shows that using SERS coupled with novel gold-coated nanosubstrates, three types of pesticides (azinphos-methyl, phosmet, and carbaryl) can be quantitatively measured and qualitatively distinguished and characterized. With a few steps of simple sample preparation, trace amount of pesticides in apples and tomatoes can be rapidly extracted and detected by SERS, and the detection limits meet the MRLs set by FAO/WHO. Future research is needed to test for more types of pesticides on vegetables and fruits, study the interferences from other compounds, and compare the SERS method with other alternative techniques such as HPLC and GC-MS.
References
Abou-Arab, A. A. K. (1999). Behavior of pesticides in tomatoes during commercial and home preparation. Food Chemistry, 65(4), 509–514.
Albrecht, M. G., & Creighton, J. A. (1977). Anomalously intense Raman spectra of pyridine at a silver electrode. Journal of the American Chemical Society, 99(15), 5215–5217.
AOAC. (2005). Official methods of analysis of aoac international. Arlington: AOAC International.
Boese, A. D., & Martin, J. M. L. (2004). Vibrational spectra of the azabenzenes revisited: Anharmonic force fields. Journal of Physical Chemistry A, 108(15), 3085–3096.
Costa, L. G. (2006). Current issues in organophosphate toxicology. Clinica Chimica Acta, 366(1–2), 1–13.
FAO/WHO. (1967). Carbaryl (pesticide residues in food: 1967 evaluations). Available at: http://www.inchem.org/documents/jmpr/jmpmono/v067pr03.htm. Accessed 1 October 2010.
FAO/WHO. (1984). Phosmet (pesticide residues in food: 1984 evaluations). Available at: http://www.inchem.org/documents/jmpr/jmpmono/v84pr37.htm. Accessed 1 October 2010.
FAO/WHO. (2009). Pesticide residues in food (MRLS/EMRLS). Available at: http://www.codexalimentarius.net/mrls/pestdes/jsp/pest_q-e.jsp. Accessed 1 October 2010.
FDA. (2006). Pesticide residue monitoring program results and discussion fy 2006. Available at: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/Pesticides/ResidueMonitoringReports/ucm125187.htm. Accessed 22 September 2010.
FDA. (2007a). Pesticide monitoring program fy 2007. Available at: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/Pesticides/ResidueMonitoringReports/ucm169577.htm#summary. Accessed 22 September 2010.
FDA. (2007b). Pesticide monitoring program fy 2007. Available at: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/Pesticides/ResidueMonitoringReports/default.htm. Accessed 30 April 2011.
Fischer, G., Smith, D. M., & Nwankwoala, A. U. (1997). The electronic spectroscopy of 1,2,3-triazine. Chemical Physics, 221(1–2), 11–21.
Fleischmann, M., Hendra, P. J., & McQuillan, A. J. (1974). Raman spectra of pyridine adsorbed at a silver electrode. Chemical Physics Letters, 26(2), 163–166.
Goodacre, R., Timmins, E. M., Burton, R., Kaderbhai, N., Woodward, A. M., Kell, D. B., et al. (1998). Rapid identification of urinary tract infection bacteria using hyperspectral whole-organism fingerprinting and artificial neural networks. Microbiology, 144(5), 1157–1170.
Grimalt, S., Pozo, Ó. J., Sancho, J. V., & Hernández, F. (2007). Use of liquid chromatography coupled to quadrupole time-of-flight mass spectrometry to investigate pesticide residues in fruits. Analytical Chemistry, 79(7), 2833–2843.
Gunther, F. A., & Blinn, R. C. (1955). Analysis of insecticides and acaricides: A treatise on sampling, isolation and determination, including residue methods. New York: Interscience.
Gupta, R. C. (2004). Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicology Mechanisms and Methods, 14(3), 103–143.
Haynes, C. L., McFarland, A. D., & Van Duyne, R. P. (2005). Surface-enhanced Raman spectroscopy. Analytical Chemistry, 77(17), 338a–346a.
He, F. (2000). Neurotoxic effects of insecticides—Current and future research: A review. Neurotoxicology, 21(5), 829–835.
He, L., Kim, N.-J., Li, H., Hu, Z., & Lin, M. (2008a). Use of a fractal-like gold nanostructure in surface enhanced Raman spectroscopy for detection of selected food contaminants. Journal of Agricultural and Food Chemistry, 56(21), 9843–9847.
He, L., Liu, Y., Lin, M., Awika, J., Ledoux, D. R., Li, H., et al. (2008b). A new approach to measure melamine, cyanuric acid, and melamine cyanurate using surface enhanced Raman spectroscopy coupled with gold nanosubstrates. Sensing and Instrumentation for Food Quality and Safety, 2(1), 66–71.
Jeanmaire, D. L., & Van Duyne, R. P. (1977). Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. Journal of Electroanalytical Chemistry, 84(1), 1–20.
Kegley, S. E., & Wise, L. J. (1998). Pesticides in fruits and vegetables. Mill Valley: University Science Books.
Kneipp, K., Kneipp, H., Itzkan, I., Dasari, R. R., & Feld, M. S. (2002). Surface-enhanced Raman scattering and biophysics. Journal of Physics: Condensed Matter, 14(18), R597–R624.
Kolosova, A. Y., Park, J.-H., Eremin, S. A., Kang, S.-J., & Chung, D.-H. (2003). Fluorescence polarization immunoassay based on a monoclonal antibody for the detection of the organophosphorus pesticide parathion-methyl. Journal of Agricultural and Food Chemistry, 51(5), 1107–1114.
Lai, K., Zhang, Y., Du, R., Zhai, F., Rasco, B., & Huang, Y. (2011). Determination of chloramphenicol and crystal violet with surface enhanced Raman spectroscopy. Sensing and Instrumentation for Food Quality and Safety, 5(1), 19–24.
Liu, M., Hashi, Y., Song, Y., & Lin, J.-M. (2005). Simultaneous determination of carbamate and organophosphorus pesticides in fruits and vegetables by liquid chromatography–mass spectrometry. Journal of Chromatography A, 1097(1–2), 183–187.
Liu, B., Lin, M., & Li, H. (2010). Potential of sers for rapid detection of melamine and cyanuric acid extracted from milk. Sensing and Instrumentation for Food Quality and Safety, 4(1), 13–19.
Lu, X., Al-Qadiri, H., Lin, M., & Rasco, B. (2011). Application of mid-infrared and Raman spectroscopy to the study of bacteria. Food and Bioprocess Technology, 4(6), 919–935.
Ortelli, D., Edder, P., & Corvi, C. (2005). Pesticide residues survey in citrus fruits. Food Additives and Contaminants, 22(5), 423–428.
Rawn, D. F. K., Quade, S. C., Shields, J. B., Conca, G., Sun, W.-F., Lacroix, G. M. A., et al. (2006). Organophosphate levels in apple composites and individual apples from a treated canadian orchard. Journal of Agricultural and Food Chemistry, 54(5), 1943–1948.
Sentellas, S., Saurina, J., Hernández-Cassou, S., Galceran, M. T., & Puignou, L. S. (2001). Multivariate calibration methods for quantification in strongly overlapping capillary electrophoretic peaks. Journal of Chromatography A, 909(2), 259–269.
Shadi, I. T., Xu, Y., & Goodacre, R. (2010). Quantitative analysis of the banned food dye sudan-1 using surface enhanced Raman scattering with multivariate chemometrics. Journal of Physical Chemistry C, 114(16), 7285–7290.
Socrates, G. (2004). Infrared and Raman characteristic group frequencies. New York: Wiley.
Strickland, A. D., & Batt, C. A. (2009). Detection of carbendazim by surface-enhanced Raman scattering using cyclodextrin inclusion complexes on gold nanorods. Analytical Chemistry, 81(8), 2895–2903.
Trotter, P. J. (1977). Azo dye tautomeric structures determined by laser-Raman spectroscopy. Applied Spectroscopy, 31(1), 30–35.
Valdés-Ramírez, G., Fournier, D., Ramírez-Silva, M. T., & Marty, J. L. (2008). Sensitive amperometric biosensor for dichlorovos quantification: Application to detection of residues on apple skin. Talanta, 74(4), 741–746.
Walz, I., & Schwack, W. (2007). Multienzyme inhibition assay for residue analysis of insecticidal organophosphates and carbamates. Journal of Agricultural and Food Chemistry, 55(26), 10563–10571.
Wang, X. T., Shi, W. S., She, G. W., Mu, L. X., & Lee, S. T. (2010). High-performance surface-enhanced Raman scattering sensors based on ag nanoparticles-coated si nanowire arrays for quantitative detection of pesticides. Applied Physics Letters, 96, 053104–053104.
Wesseling, C., Keifer, M., Ahlbom, A., McConnell, R., Moon, J.-D., Rosenstock, L., et al. (2002). Long-term neurobehavioral effects of mild poisonings with organophosphate and n-methyl carbamate pesticides among banana workers. International Journal of Occupational and Environmental Health, 8(1), 27–34.
Wise, J. C., Vanderpoppen, R., & Vandervoort, C. (2009). Curative activity of insecticides on rhagoletis pomonella (Diptera: Tephritidae) in apples. Journal of Economic Entomology, 102(5), 1884–1890.
Zhang, Y., Muench, S. B., Schulze, H., Perz, R., Yang, B., Schmid, R. D., et al. (2005). Disposable biosensor test for organophosphate and carbamate insecticides in milk. Journal of Agricultural and Food Chemistry, 53(13), 5110–5115.
Acknowledgements
This research was supported by the Open Project Program (No. SKLF-KF-201108) of State Key Laboratory of Food Science and Technology, Jiangnan University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, B., Zhou, P., Liu, X. et al. Detection of Pesticides in Fruits by Surface-Enhanced Raman Spectroscopy Coupled with Gold Nanostructures. Food Bioprocess Technol 6, 710–718 (2013). https://doi.org/10.1007/s11947-011-0774-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0774-5