Abstract
The purpose of this work was to investigate the rheological behavior of Spanish honeys under different conditions (at different temperatures and concentrations). All the samples were characterized to determine their physicochemical (moisture, 0Brix, pH, ash, conductivity, color, total acidity, diastase activity, 5-hydroxymethylfurfural content, sugar content) and thermal (glass transition temperature) profiles. The honeys samples (80.4–82 0Brix) behaved as Newtonian fluid; as expected, their viscosity increased with the solid content and decreased with the temperature. Two experimental viscosity models (Arrhenius and Vogel–Taumman–Fulcher) were checked using the experimental data to correlate the influence of temperature on honey viscosity. A simplified model was proposed to describe the combined effect of the temperatures and concentrations (0Brix) on the viscosity of Spanish honeys. The dynamic viscosity and complex viscosity had the same magnitude at 40 °C, 45 °C, and 50 °C for of all the samples and the Cox Merz rule could be applied at these temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Honey is a biological product whose characteristics changes with time. Its chemical composition varies between certain limits, depending on its floral origin, on the soil and climate, on the material released, as well as on the procedure in the extraction and marketing of honey (Saénz-Laín and Gómez-Ferreras 2000). Honey is a semiliquid product (water, 15–20% approximately) which contains a complex mixture of carbohydrates, mainly glucose and fructose; other sugars are present as traces, depending on the floral origin. Moreover, organic acids, lactones, amino acids, minerals, vitamins, enzymes, pollen, wax, and pigments are present (Fallico et al. 2004). The viscosity of honey is influenced by temperature and moisture, as well as the presence of crystals and colloids. Water content is an essential qualitative factor that affects not only the viscosity of honey, but also its rheological properties (Sopade et al. 2004; Lazaridou et al. 2004; Juszczak and Fortuna 2006; Yanniotis et al. 2006; Kang and Yoo 2008). The moisture content can vary from year-to-year, depending on the environmental conditions and the beekeepers’ handling during the harvest.
Recently, the rheological properties of honey from different countries have been analyzed: Argentina (Recondo et al. 2006), Australia (Bhandari et al. 1999; Mossel et al. 2000; Sopade et al. 2002, 2004), China (Junzheng and Changying 1998; Chen et al. 2009), Korea (Yoo 2004; Kang and Yoo 2008), Germany (Samanalieva and Senge 2009), Greece (Lazaridou et al. 2004, Yanniotis et al. 2006), India (Kumar and Mandal 2009), Israel (Cohen and Weihs 2010), Jordan (Abu-Jdayil et al. 2002), Poland (Juszczak and Fortuna 2006; Witczak et al. 2011), and Spain (Gómez Diaz et al. 2009). Knowledge of the flow behavior of concentrated food stuff is useful in quality control, calculating energy requirements, process control, and selection of proper process equipment (Kaya et al. 2008).
The parameters obtained from oscillatory tests are very sensitive to chemical and physical changes; therefore, they are useful for rheological evaluation in dairy systems (Guinee et al. 2000 Kahyaoglu and Kaya 2003; Villegas and Costell 2007), honey (Yoo 2004), biopolymers (Chronakis et al. 2000; Salinas et al. 2011; Steffolani et al. 2011), etc. The correlation between steady shear and dynamic shear parameters could be observed for food systems. The Cox–Merz rule is used to predict steady shear viscosity from complex shear viscosity and vice versa (Steffe 1996).
where, η* is the complex viscosity (Pa·s), η is the steady viscosity (Pa·s), w is the angular velocity (rad · s−1) and γ is the shear rate (s−1).
The purpose of this study was: to analyze the physicochemical, thermal, and rheological properties of Spanish honeys; to observe the temperature and concentration dependence on honey viscosity; to obtain a model using the two parameters (temperature and soluble solid content); and to evaluate the application of the Cox–Merz rule in the case of Spanish honeys and the influence of the temperature on this rule.
Materials and Methods
Materials
The honey varieties used: four monofloral (eucalyptus, orange, rosemary, and summer savory), one polyfloral, and one nectar (honeydew) purchased from Spanish market. All the honey samples were in fluid state.
Physical–Chemical Analysis
Moisture content of honey samples were obtained by measuring the refractive index at 20 °C using a digital refractometer (3 T Atago Abbe refractometer, Atago Co., Tokyo, Japan). The water content and 0Brix concentration was determined based on a Chataway table (Bogdanov 2002). 5-Hydroxymethylfurfural content (HMF), diastase activity, pH, ash, total acidity, and electrical conductivity were analyzed in accordance with the harmonized methods of the European Honey Commission (Bogdanov 2002). Color was measured by reflectance spectroscopy using a spectrocolorimeter Minolta CM-3600d (Osaka, Japan), the samples were placed in 20 mm thick holders and measured against a black and white background. Translucency was determined by applying the Kubelka–Munk theory for multiple scattering to the reflection spectra (Hutchings 1999). Color coordinates CIEL* a* b* were obtained from R ∞ between 400 and 700 nm for D65 illuminant and from two observer (Talens et al. 2001). All the tests were performed in duplicate.
Glucose, fructose, and sucrose in honey samples was made by a HPLC 10ADVP–SHIMADZU, with RI detector, according to a method described by Bogdanov (2002). The linear regression factor of the calibration curves was higher than 0.9982 for all sugars. Sugars were quantified by comparison of the peak area obtained with those of standard sugars. The results for each sugar were expressed as gram per 100 g honey. Values of parameters were expressed as the mean ± standard deviation to a confidence interval for mean of 95%.
Thermal Properties
Glass transition temperature was measured by a differential scanning calorimeter (Mettler Toledo DSC-1, Mettler Toledo, USA). The instrument was calibrated with indium. For the differential scanning calorimeter studies, the honey samples (10–25 mg in hermetically sealed 40 μl aluminum pans) were rapidly cooled with liquid nitrogen to −130 °C, held 10 min before heating to 50 °C at 10 °C/min. The software, STARe Excellence Software, was used in obtaining the glass transition temperature, which was defined as the point of inflection of the heat capacity change.
Steady State Rheology
Viscosity measurements were carried out on the honey samples at different temperatures (25 °C, 30 °C, 35 °C, 40 °C, 45 °C, and 50 °C), with Haake RheoStress 1 rheometer, equipped with control thermo bath and a coaxial cylinder system. The rheological properties of honey can be influenced by the presence of crystals and air bubbles (Abu-Jdayil et al. 2002; Bhandari et al. 1999, Mossel et al. 2000). Before being used, they were warmed up to 55 °C to dissolve any crystals, and kept in flasks at 30 °C to remove air bubbles that could interfere in rheological studies.
The shear rate vs. viscosity curve was obtained for shear rates from 0 to 100 s−1. The honey sample was allowed to reach the desired temperature 20 min. Each measurement was taken in duplicate. Values of parameters were expressed as the mean ± standard deviation to a confidence interval for mean of 95%.
Dynamic State Rheology
The dynamic rheological properties of honey samples were obtained with a RheoStress 1 rheometer (Thermo Haake, Germany) at different temperatures (25 °C, 30 °C, 40 °C, 45 °C, and 50 °C), using a parallel plate system (Ø 60 mm) at a gap of 500 μm. A batch of each composition was prepared and at least two measurements were performed on each batch, using a fresh sample for each measurement. After loading the sample, a waiting period of 5 min was used to allow the sample to recover itself and to reach the desired temperature. In order to determine the linear viscoelastic region, stress sweeps were run at 1 Hz first. Then, the frequency sweeps were performed over the range w = 0.628–62.8 rad/s at 1 Pa stress. The 1 Pa stress was in the linear viscoelastic region. A Rheowin Job software (v. 2.93, Haake) was used to obtain the experimental data and to calculate the η *. Each measurement was taken in duplicate. Values of parameters were expressed as the mean ± standard deviation to a confidence interval for mean of 95%.
Statistical Analysis
In order to establish statistical differences between the means of the physicochemical, thermal, and rheological parameters, one-factor analysis of variance, with least significant difference at significance level 0.05 calculated by Fisher’s test was applied using the Statgraphics Plus 5.1. The variables were weighted with the inverse of the standard deviation of all objects in order to compensate for the different scales of the variables.
Results and Discussion
Table 1 shows the result of the characterization of honeys according to their physicochemical composition. The refractive index, resulting from the digital refractometer, was obtained, and then using the Chataway table the value of moisture and 0Brix concentration was calculated (Bogdanov 2002). All honeys tested met the threshold requirement of moisture content required by the Codex Alimentarius (max. 20%) (Codex Alimentarius 2001), with mean values ranging between 16.24% and 17.96%. As expected, the lowest water content was registered by the honeydew sample (Gómez Díaz et al. 2005).
The highest value of pH was observed for honeydew and the lowest for eucalyptus honey. The acidic nature of honey is attributed to the presence of organic acids and the variation in acidity among different honey types may be a result of the variation in these constituents (Nanda et al. 2003). The pH values of Spanish honeys were in the same range as those reported in the case of Algerian honeys (Ouchemoukh et al. 2007), Indian honeys (Ahmed et al. 2007), and Turkish honeys (Kaya et al. 2008).
The lowest total acidity and diastase activity levels were registered in rosemary and orange honeys, considerably lower than those obtained in honeydew, polyfloral, eucalyptus, and summer savory. Previous studies show that orange honey has the lowest acidity and conductivity levels among floral honeys (Corbella and Cozzolino 2006; Terrab et al. 2003a; Serrano et al. 2004). The results for the polyfloral honeys were in the intermediate range, which is logical, given that they derive from a variety of different nectars (Corbella and Cozzolino 2006). In this study, the conductivity ranged between 183.2 and 1,378 μS·cm−1; the highest value being registered for the honeydew sample, as in other papers (Escriche et al. 2009).
The wide variability of honey composition is also reflected by the ash content. This parameter which is generally used to classify honey (floral, mixed, or honeydew honey), is mainly determined by soil and climatic characteristics, with values between 0.13% and 0.92%. The honeydew sample had higher ash content than the unifloral and polyfloral samples (Codex Alimentarius 1993).
In this study, HMF content, which is widely recognized as an indicator of freshness, was always lower than 14.85 mg/kg. This parameter in honey is related to its quality and heating processing but has not been related to the origin of sample (Anklam 1998). These low values demonstrate that honey was quite fresh and had not been subjected too much heat treatment (Bogdanov 2002. This finding is corroborated with the fact that diastase activity was high in all the honeys analyzed, especially in polyfloral and honeydew ones. The diastase activity results were well over eight, the minimum level established by the European Commission Directive relating to honey (2001).
To characterize the honey samples in terms of color, they were plotted in their corresponding positions on the a*-b* and a*-L* color spaces (Fig. 1a–b). On the a*-b* color space, the nearer a honey is to the origin, the less purity of color it has, and the further away it from the origin it is, the greater its purity is. The honeys with the greatest purity of color were the orange, rosemary, and summer savory ones; the honeydew honey, followed by the polyfloral honey and eucalyptus, exhibiting the least purity of color. Rosemary, orange, and summer savory honeys in particular, had the greatest yellow component (highest b* values), and polyfloral honey had the greatest red component (the highest a* value). Figure 1b shows that rosemary, summer savory and orange honeys were clearer (higher L* value) than the other varieties. Honeydew did not show only the less purity of color of all the honeys analyzed but it also showed to be the darkest one (lowest L* value). The color values obtained were within the expected ranges for each of the honeys studied. The most color values reported in the literature generally correspond to measurements made by the Pfund scale (mm; Corbella and Cozzolino 2006; Persano-Oddo et al. 1995). Although only a few studies had used CIELAB (L*, a*, b*) to measure color in nectar and honeydew honey (Terrab et al. 2003a, b; Lazaridou et al. 2004; Soria et al. 2004; Escriche et al. 2009), their results were similar to this study.
Sugars represent the main components of any type of honey. Reducing sugars (invert sugar), mainly fructose and glucose, are the major constituents of honey (Kucuk et al. 2007). The actual proportion of fructose to glucose, in any particular honey, depends largely on the source of the nectar (Anklam 1998).The total content of glucose and fructose was over 60 g/100 g of honey in accordance with the 2001/110/CE Directive. The highest concentration was registered in the case of rosemary, followed by the honeydew sample. The fructose/glucose ratio was calculated for all samples. This ratio gives information about the crystallization state of honey, when fructose is higher than glucose honey is fluid. In all the cases, this ratio was higher than 1 (Table 1), which was confirmed by the crystallization state of the mentioned honey sample; all the honeys were in liquid state. Regarding the sugars profile, invert sugar (fructose and glucose) represented the largest portion of honey composition but small quantities of sucrose was also present; the highest content of sucrose was registered in the case of the eucalyptus sample. The values of invert sugar showed normal levels similar to those reported in the case of Algerian honeys (Ouchemoukh et al. 2007), Argentinian honeys (Baroni et al. 2009), Moroccan honeys (Terrab et al. 2003b), and Romanian honeys (Mărghiţaş et al. 2009).
The glass transition temperature (T g ) is shown in Table 1; it is commonly related to a relaxation effect whose amplitude depends on the thermal history of the samples. In the case of Spanish honeys, the glass transition temperature ranged from −47.78 °C to −40.52 °C.
A significantly higher glass transition temperature (−40.52 °C) was exhibited by the honey sample obtained from nectar source (honeydew) which is attributed to a lower moisture content. Generally, honeys with high moisture content exhibited low T g , in line with the plasticization effect of water, which is related to the ability of the water molecules to weaken hydrogen bonds, dipole–dipole, and intra- and inter-macromolecular interactions (Mossel et al. 2000). The T g values generally increase with increasing molecular weight. The major sugars present in honey are fructose and glucose (Ouchemoukh et al. 2007) which contribute to the glass transition of honey samples. The sugar ratio depends on the floral source and environmental conditions therefore influence honey T g . In addition, T g varies widely for a specific food as it depends on many factors such as sample preparation and size, heating/cooling rate, sample holding time, moisture content, etc.
The values of glass transition temperature were in the same range with the ones reported in the case of Australian honeys (Sopade et al. 2002), Argentinean honeys (Recondo et al. 2006), and Indian honeys (Ahmed et al. 2007.
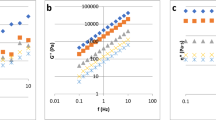
In Figs. 2 and 3, the steady and dynamic state rheology behavior of Spanish honey is shown. The η* is independent on frequency while the η is independent on shear rate, both exhibiting a Newtonian plateau. The Newtonian behavior of honey has been observed by many authors during in recent years (Yoo 2004; Juszczak and Fortuna 2006; Yanniotis et al. 2006; Gómez Diaz et al. 2009; Kumar and Mandal 2009; Cohen and Weihs 2010). In Table 2, the steady state viscosity of Spanish honeys as a function of moisture content and temperature is shown. The relationship between the apparent viscosity and the shear rate and complex viscosity and frequency, respectively, were generally constant functions. The viscosities did not change much (the coefficient of variation <10%) with increasing shear rate (Fig. 2) but they substantially decreased as the temperature increased (Table 2). This was expected as honey is Newtonian liquid and highly sensitive to temperatures (Assil et al. 1991; Bhandari et al. 1999; Junzheng and Changying 1998; Mossel et al. 2000; White 1975, 1978). Honey with high moisture content is generally less viscous, which complies with the studies published on the ability of water to dilute food systems (Steffe 1996). As temperature increases, the average speed of the molecules in honey increases and the amount of the time they spend “in contact” with their nearest neighbors decreases; thus, as temperature increases, the average intermolecular forces decrease and consequently the viscosity decreases (Patil and Muskan 2009).
In Table 3, the influence of temperature and moisture content on the viscosity of honey from Australia (Sopade et al. 2002), China (Junzheng and Changying 1998), Greece (Yanniotis et al. 2006), Israel (Cohen and Weihs 2010), and Poland (Juszczak and Fortuna 2006) is presented. The combined influence of temperature and moisture content on viscosity was the same as that reported in the presented study for Spanish honeys, the viscosity decreases with increasing moisture content and temperature.
Effect of Temperature
The flow behavior of the Spanish honeys was further described by the Arrhenius, and Vogel–Taumman–Fulcher (VTF) models:
-
Arrhenius model (Eq. 2)
where, η0 is a constant, R is the gas constant [kJ·mol−1·K−1], and E a activation energy (is an energy barrier to flowing) [kJ·mol−1], and T absolute temperature [K].
-
VTF model (Eq. 3)
where, η∞ is the viscosity at T = ∞ and T 0 is the absolute temperature at which the relaxation time relevant to molecular displacements becomes infinite. The value T 0 was fixed at 184 K, which was estimated from data reported by Parker and Ring (1995) for aqueous sugar systems of similar concentration, and B was calculated using nonlinear regression.
The activation energies (E a ) values (Table 4), calculated by the Arrhenius model ranged between 84.07 and 91.35 kJ/mol, and the higher they were, the more viscous the honey was. Similar values were reported for Argentinean honeys (Recondo et al. 2006), Greek honeys (Yanniotis et al. 2006), Jordanian honeys (Zaitoun, et al. 2001), and Polish honeys (Juszczak and Fortuna 2006). These values were considerably lower than those established for Australian honeys (Bhandari et al. 1999, Sopade et al. 2002). The latter result was probably due to the fact that the authors cited performed measurements at lower temperatures (2 °C, 4 °C, and 7 °C). As suggested by Mossel et al. (2000), in the Arrhenius equation, lower temperature measurement results in higher deviations from the experimental data. The activation energy respected the moisture content percentage, so the highest one was achieved by honeydew and the smallest one by rosemary. The material constant (pre-exponential factor in the Arrhenius equation), according to Al-Malah et al. (2001) and Zaitoun et al. (2001), represents viscosity at a temperature approaching infinity. The higher the values of these constants were, the higher the viscosity of the samples was; these values were in the same range with the ones reported in the case of Israeli honeys (Cohen and Weihs 2010
The viscosity vs. temperature relation was also described using the Vogel–Taumman–Fulcher model. The parameters of that model, determined by nonlinear regression, are shown in Table 4. The value of constant B was in the same order (1,600–1,954 K) as reported for viscosity at different temperature of Argentinean honeys (Recondo et al. 2006), sucrose (Parker and Ring 1995), fructose and glucose (Ollet and Parker 1995, and crystallization kinetics of lactose and lactose–trehalose systems (Mazzobre et al 2001).
Effect of Concentration
The highest values of viscosity were registered by the honeydew sample, lower for eucalyptus and summer savory, and the lowest for the rosemary honey. Table 2 shows the values of the viscosity at the temperatures studied, which were calculated using the Newtonian fluid formula. In all the cases, the coefficient of regression (R 2) exceeded 0.99. Honeydew exhibited the highest viscosity whereas rosemary the lowest one. The results confirm the previous research data concerning the dependence of honey viscosity on water content (Al-Malah et al. 2001; Mossel et al. 2000, Sopade et al. 2002; Zaitoun et al. 2001). The increase in viscosity of honey with soluble solid content (C, 0Brix) can be described by power law and exponential models (Rao et al. 1984; Ibarz et al. 1987).
where, η1, η2, b 1, and b 2 are constants, and C is the concentration in 0Brix.
In order to calculate the model constants, the viscosity data were fitted to Eqs.4 and 5 by nonlinear regression. The resulting values of the constants are presented in Table 5. The R 2 are fairly similar, so the two models are suitable for describing the effect of the soluble solids of the Spanish honeys.
At a given temperature, the activation energy for flow depends on the soluble solid content which can be described by several models (Giner et al. 1996). We used two models, similar to Eqs.6 and 7.
where A 1, A 2, B 1, and B 2 are constants. The E a values and their respective concentrations were fitted to Eqs. 4 and 5 by nonlinear regression to determine the model parameters. The calculated parameters for these models are given in Table 6. Based on R 2, we observed that the exponential model was marginally better than the power law model in describing the dependency of E a on soluble solid content.
Combined Effect of Temperature and Concentration
For practical applications, it is useful to obtain an equation describing the combined effect of temperature and concentration on honey viscosity (Ibarz et al. 1992). The following models were investigated (Eqs. 8 and 9):
The viscosity, concentration, and temperature data were fitted to these models by the nonlinear regression and the values of the model constants were determined. The values of these constants are summarized in Table 7. It can be observed that both models showed similar activation energy as in the case of clarified cherry juice and grape pekmez, both displaying Newtonian behavior (Ibarz et al. 1992, Kaya et al. 2008.
Taking into account the statistical R 2, shown in Table 7, it could be concluded that the best fit corresponds to Eq. 8. Therefore, for the interval of concentrations (C, 0Brix) and temperatures (T) studied, the following equation (Eq. 10) was proposed to evaluate the η of Spanish honeys:
Cox–Merz Rule
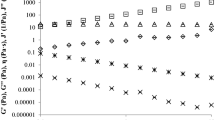
Cox–Merz rule (1958) states that the complex shear viscosity becomes nearly equal to the steady shear viscosity when angular velocity is equal to shear rate (Eq. 1). This rule was studied for many polymers, solutions, and complex food systems (Da Silva and Rao1992; Tiziani and Vodovotz 2005; Ahmed, Ramaswamy and Sashidhar 2007; Yaşar et al. 2009; Fissore et al. 2009; Augusto et al. 2011), but not for Newtonian honey. The relationship between dynamic η* and the η data at frequency range of 0.62–62.83 rad/s and shear rate of 0.62–62.83 s−1 were studied for the Spanish honeys (Fig. 4). The comparison was carried out at 25 °C, 30 °C, 40 °C, 45 °C, and 50 °C. The results of the application of Cox–Merz rule for honey samples (orange, rosemary, summer savory, eucalyptus, polyfloral, and honeydew) are shown in Fig. 3a–f. None of the honeys samples obeyed the Cox–Merz rule at 25 °C and 30 °C; at this temperature, it was observed that the magnitude of the η* values were lower than those of the η values at angular velocities or shear rates. In the case of non-Newtonian fluid when the data set of η* vs. η are parallel the deviation is probably adjusted using shift factor (α; Tiziani and Vodovotz 2005; Eq. 11):
In the Newtonian fluid case, the data set of η* vs. η are parallel and parallel with the frequency and shear rate and the data set could be adjusted with the vertical α (Eq. 12):
The shift factor (Table 8) decreased with the increasing in temperature in all the honeys samples. Considering the magnitude of α shift factor and moisture content correlation between these two parameters could not be made.
At 40 °C, 45 °C, and 50 °C, the Cox Merz rule is obeyed by all the honey samples (orange, rosemary, eucalyptus, polyfloral, honeydew, and summer savory), α shift factor is closed to 1 (1.001–1.036). The gap between the magnitudes of η* and η values in the case of the honey samples at 25 °C and 30 °C could be explained by the structure decay due to effect of stress deformation applied to the system by dynamic or steady shear (Chamberlain and Rao 1999).
Another relationship (Eq. 13) which is able to effectively relate steady shear and dynamic rheological properties was proposed by Bistanzy and Kokini (1983)):
where, κ and \( \beta \) are empirical constants determined from experimental data. In Table 9, the κ and β constants for Spanish honeys are presented; their magnitude is not correlated with the moisture content. The values of the two parameters are closed to the ones reported in the case of stick margarine, peanut butter, and marshmallow fluff (Kokini 1992).
Conclusion
The types of honey analyzed in this paper comply with the Codex Alimentarius Standards and CE directives with respect to their physicochemical composition. The honeydew sample exhibited a maximum soluble solid content, higher pH, conductivity, diastase activity, and total acidity, lower glass transition temperature, the least purity of color and was the darkest one. Spanish honeys are Newtonian, and the honey viscosity was affected by the soluble solid content and temperature complying with the literature. The effects of soluble solid content and temperature on viscosity were described by power law and exponential models. A model was developed to describe the temperature and soluble solid content on the Spanish honeys viscosity, irrespective of their origin, which is a suitable tool for predicting the rheological behavior of honeys according to its temperature and concentration for the industrial process. Steady shear viscosity and complex viscosity of the Spanish honeys fits a modified Cox Merz rule for the Newtonian fluid with the introduction of a vertical shift factor. The modified Cox Merz rule could be applied for all the honeys at 40 °C, 45 °C, and 50 °C; the steady shear viscosity and complex viscosity could be predicted from each other.
References
Abu-Jdayil, B., Al-Majeed Ghzawi, A., Al-Malah, K. I. M., & Zaitoun, S. J. (2002). Heat effect on rheology of light- and darkcolored honey. Journal of Food Engineering, 51(1), 33–38.
Ahmed, J., Ramaswamy, H. S., & Sashidhar, K. C. (2007). Rheological characteristics of tamarind (Tamarindus indica L.) juice concentrates, LWT. Food Science and Technology, 40(2), 225–231.
Ahmed, J., Prabhu, S. T., Raghavan, G. S. V., & Ngadi, M. (2007). Physico-chemical, rheological, calorimetric and dielectric behaviour of selected Indian honey. Journal of Food Engineering, 79, 1207–1213.
Al-Malah, K. I. M., Abu-Jdayil, B., Zaitoun, S., & Ghzawi, A. A. M. (2001). Application of WLF and Arrhenius kinetics to rheology of selected dark-colored honey. Journal of Food Process Engineering, 24, 341–357.
Anklam, E. (1998). A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chemistry, 63, 549–562.
Assil, H. I., Sterling, R., & Sporns, P. (1991). Crystal control in processed liquid honey. Journal of Food Science, 56(4), 1034–1037. 1041.
Augusto, P. E. D., Falguera, V., Cristianini, M., & Ibarz, A. (2011). Viscoelastic properties of tomato juice: applicability of the Cox–Merz rule. Food and Bioprocess Technology. doi:10.1007/s11947-011-0655-y.
Baroni, M. V., Arrua, C., Nores, M. L., Fayé, P., Díaz, M., Chiabrando, G. A., & Wunderlin, D. A. (2009). Composition of honey from Córdoba (Argentina): assessment of north/south provenance by chemometrics. Food Chemistry, 114, 727–733.
Bhandari, B., D’Arcy, B., & Chow, S. (1999). Rheology of selected Australian honeys. Journal of Food Engineering, 41(1), 65–68.
Bistanzy, K. L., & Kokini, J. L. (1983). Comparison of steady shear rheological properties and small amplitude dynamic viscoelastic properties of fluid food materials. Journal of Texture Studies, 14, 113–124.
Bogdanov S., (2002) Harmonised methods of the international honey commission. Swiss Bee Research Centre, FAM, Liebefeld, CH-3003 Bern, Switzerland.
Chamberlain, E. K., & Rao, M. A. (1999). Rheological properties of acid converted waxy maize starches in water and 90% DMSO/10% water. Carbohydrate Polymers, 40, 251–260.
Chen, Y. W., Lin, C. H., Wu, F. Y., & Chen, H. H. (2009). Rheological properties of crystallized honey prepared by new type of nuclei. Journal of Food Process Engineering, 32, 512–527.
Chronakis, I. S., Doublier, J. L., & Piculell, L. (2000). Viscoelastic properties for kappa- and iota-carrageenan in aqueous NaI from the liquid-like to the solid-like behaviour. International Journal of Biological Macromolecules, 28(1), 1–14.
Codex Alimentarius. (1993). Standard for honey, ref. no. CL 1993/14, SH. Rome: Codex Alimentarius Commission FAO/WHO.
Codex Standard (Codex Alimentarius) 12–1981, Rev. 2 (2001) Revised codex standard for honey
Cohen, I., & Weihs, D. (2010). Rheology and microrheology of natural and reduced-calorie Israeli honeys as a model for high-viscosity Newtonian liquids. Journal of Food Engineering, 100(2), 366–371.
Corbella, E., & Cozzolino, D. (2006). Classification of the floral origin of Uruguayan honeys by chemical and physical characteristics combined with chemometics. LWT- Food Science and Technology, 39, 534–539.
Cox, W. P., & Merz, E. H. (1958). Correlation of dynamic and steady flow viscosities. Journal of Polymer Science, 28, 619–622.
Da Silva, J. A. L., & Rao, M. A. (1992). Viscoelastic properties of food hydrocolloid dispersions. In M. A. Rao & J. F. Steffe (Eds.), Viscoelastic properties of foods. London, UK: Elseiver.
Escriche, I., Visquert, M., Juan-Borras, M., & Fito, P. (2009). Influence of simulated industrial thermal treatments on the volatile fractionsof different varieties of honey. Food Chemistry, 112, 329–338.
European Commission Directive relating to honey (2001). 2001/110/CE of 02/12/2001.
Fallico, B., Zappalà, M., Arena, E., & Verzera, A. (2004). Effects of heating process on chemical composition and HMF levels in Sicilian monofloral honeys. Food Chemistry, 85(2), 305–313.
Fissore, E. N., Matkovic, L., Wider, E., Rojas, A. M., & Gerschenson, L. N. (2009). Rheological properties of pectin-enriched products isolated from butternut (Cucurbita moschata Duch ex Poiret). LWT- Food Science and Technology, 42(8), 1413–1421.
Giner, J., Ibarz, A., Garza, S., & Xhian-Quan, S. (1996). Rheology of clarified cherry juices. Journal of Food Enginnering, 30, 147–154.
Gómez Diaz, D., Navaza, J. M., & Quintans, L. C. (2009). Effect of temperature on the viscosity of honey. International Journal of Food Properties, 12(2), 396–404.
Gómez Díaz, D., Navaza, J. M., & Quintans, L. C. (2005). Rheological behaviour of Galician honeys. European Food Research and Technology, 222, 439–442.
Guinee, T. P., Auty, M. A. E., & Fenelon, M. A. (2000). The effect of fat content on the rheology, microstructure and heat-induced functional characteristics of Cheddar cheese. International Dairy Journal, 10, 277–288.
Hutchings, J. B. (1999). Food color and appearance (2nd ed.). Gaithersburg, Md: Aspen Publishers.
Ibarz, A., Vicente, M., & Graell, J. (1987). Rheological behavior of apple juice and pear juice and their concentrates. Journal of Food Engineering, 6, 257–267. doi:10.1016/0260-8774(87)90013-6.
Ibarz, A., Pagán, J., & Miguelsanz, R. (1992). Rheology of clarified fruit juices. II. Blackcurrant juices. Journal of Food Engineering, 15, 63–67. doi:10.1016/0260-8774(92)90040-D.
Junzheng, P., & Changying, J. (1998). General rheological model for natural honeys in China. Journal of Food Engineering, 36(2), 165–168.
Juszczak, L., & Fortuna, T. (2006). Rheology of selected Polish honeys. Journal of Food Engineering, 73(1), 43–49.
Kahyaoglu, T., & Kaya, S. (2003). Effect of heat treatment and fat reduction on the rheological and functional properties of Gaziantep cheeese. International Dairy Journal, 13, 867–875.
Kang, K. M., & Yoo, B. (2008). Dynamic rheological properties of honeys at low temperatures as affected by moisture content and temperature. Food Science and Biotechnology, 17(1), 90–94.
Kaya, A., Ko, S., & Gunasekaran, S. (2008). Viscosity and color change during in situ solidification of grape pekmez. Food and Bioprocess Technology, 4(2), 241–246.
Kokini, J. L. (1992). Rheological properties of food. In D. R. Heldman & D. B. Lund (Eds.), Handbook of food engineering (pp. 1–38). New York: Marcel Dekker.
Kucuk, M., Kolayh, S., Karaoglu, S., Ulusoy, E., Baltaci, C., & Candan, F. (2007). Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chemistry, 100, 526–534.
Kumar, J. S., & Mandal, M. (2009). Rheology and thermal properties of marketed Indian honey. Nutrition and Food Science, 39(2), 111–117.
Lazaridou, A., Biliaderis, C. G., Bacandritsos, N., & Sabatini, A. G. (2004). Composition, thermal and rheological behaviour of selected Greek honeys. Journal of Food Engineering, 64(1), 9–21.
Mărghiţaş, L., Dezmirean, D., Moise, A., Bobis, O., Laslo, L., & Bogdanov, S. (2009). Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chemistry, 112, 863–867.
Mazzobre, M. F., Soto, G., Aguilera, J. M., & Buera, P. (2001). Crystallization kinetics of lactose in systems co-lyophilized with trehalose. Analysis by differential scanning calorimetry. Food Research International, 34(10), 903–911.
Mossel, B., Bhandari, B., D’Arcy, B., & Caffin, N. (2000). Use of Arrhenius model to predict rheological behaviour in some Australian honeys. Lebensmittel-Wissenschaft und Technologie, 33, 545–552.
Nanda, V., Sarkar, B. C., Sharma, H. K., & Bawa, A. S. (2003). Physico–chemical properties and estimation of mineral content in honey produced from different plants in Northern India. Journal of Food Compost. Anal, 16, 613–619.
Ollet, A. L., & Parker, R. (1995). The viscosity of supercooled fructose and its glass transition temperature. Journal of Texture Studies, 21, 355–362.
Ouchemoukh, S., Louaileche, H., & Schweitzer, P. (2007). Physicochemical characteristics and pollen spectrum of some Algerian honeys. Food Control, 18, 52–58.
Parker, R., & Ring, S. G. (1995). A theoretical analysis of diffusion controlled reactions in frozen solutions. CryoLetters, 16, 197–208.
Patil, U., & Muskan, K. (2009). Essentials of biotechnology. New Delhi, India: International Publishing House.
Persano-Oddo, L., Gioia-Piazza, M., & Zellini, G. (1995). Caratteristiche cromatiche dei mieli uniflorali. Apicoltura, 10, 109–120.
Rao, M. A., Cooley, H. J., & Vizali, A. A. (1984). Flow properties of concentrated juices at low temperatures. Food Technology, 38, 113–119.
Recondo, M. P., Elizalde, B. E., & Buera, M. P. (2006). Modelling temperature dependence of honey viscosity and of related supersaturated model carbohydrate systems. Journal of Food Engineering, 77(1), 126–134.
Saénz-Laín, C., & Gómez-Ferreras, C. (2000). Mieles españolas: características e identificación mediante el análisis del polen. Madrid, Spain: Mundi-Prensa.
Salinas, M. V., Zuleta, A., Ronayne, P., & Puppo, M. C. (2011). Wheat flour enriched with calcium and inulin: a study of hydration and rheological properties of dough. Food and Bioprocess Technologies. doi:10.1007/s11947-011-0691-7.
Samanalieva, J., & Senge, B. (2009). Analytical and rheological investigations into selected unifloral German honey. European Food Research and Technology, 229, 107–113.
Serrano, S., Villarejo, M., Espejo, R., & Jodral, M. (2004). Chemical and physical parameters of Andalusian honey: classification of citrus and eucalyptus honeys by discriminant analysis. Food Chemistry, 87, 619–625.
Sopade, P. A., Halley, P., Bhandari, B., D’Arcy, B., Doebler, C., & Caffin, N. (2002). Application of the Williams–Landel–Ferry model to the viscosity–temperature relationship of Australian honeys. Journal of Food Engineering, 56(1), 67–75.
Sopade, P. A., Halley, P. J., D’Arcy, B. R., Bhandari, B., & Caffin, N. (2004). Dynamic and steady-state rheology of Australian honeys at subzero temperatures. Journal of Food Process Engineering, 27(4), 284–309.
Soria, A. C., Gonzalez, M., de Lorenzo, C., Martínez-Castro, I., & Sanz, J. (2004). Characterization of artesanal honeys from Madrid (Central Spain) on the basis of their melissopalynological, physicochemical and volatile composition data. Food Chemistry, 85, 121–130.
Steffe, J. (1996). Rheological methods in food process engineering—second edition. USA: Freeman Press.
Steffolani, M. E., Ribotta, P. D., Perez, G. T., Puppo, M. C., & León, A. E. (2011). Use of enzymes to minimize dough freezing damage. Food and Bioprocess Technologies. doi:10.1007/s11947-011-0538-2.
Talens, P., Martinez-Navarrete, N., Fito, P., & Chiralt, A. (2001). Changes in optical and mechanical properties during osmodehydrofreezing of kiwi fruit. Innovative Food Science and Emerging Technologies, 3, 191–199.
Terrab, A., Gonzalez, G. A., Diez, M. J., & Heredia, F. J. (2003a). Mineral content and electrical conductivity of honeysproduced in Northewet Morocco and their contribution to the characterisation of unifloral honeys. Journal of the Science of Food and Agriculture, 83, 637–643.
Terrab, A., Gonzalez, G. A., Diez, M. J., & Heredia, F. J. (2003b). Characterisation of Moroccan unifloral honeys using multivariate analysis. European Food Research and Technology, 218, 88–95.
Tiziani, S., & Vodovotz, Y. (2005). Rheological effects of soy protein addition to tomato juice. Food Hydrocolloids, 19(1), 45–52.
Villegas, B., & Costell, E. (2007). Flow behaviour of inulin–milk beverages. Influence of inulin average chain length and milk fat content. International Dairy Journal, 17, 776–781.
White, J. W. (1975). Physical characteristics of honey. In E. Crane (Ed.), Honey: a comprehensive survey (pp. 207–239). London: Morrison and Gibs.
White, J. W., Jr. (1978). Honey. Advances in Food Research, 24, 287–274.
Witczak, M., Juszcak, L., & Galkowska, D. (2011). Non-Newtonian behaviour of heather honey. Journal of Food Engineering, 104(1), 532–537.
Yanniotis, S., Skaltsi, S., & Karaburnioti, S. (2006). Effect of moisture content on the viscosity of honey at different temperatures. Journal of Food Engineering, 72(4), 372–377.
Yaşar, K., Kahyaoglu, T., & Şahan, N. (2009). Dynamic rheological characterization of salep glucomannan/galactomannanbased milk beverages. Food Hydrocolloids, 23(5), 1305–1311.
Yoo, B. (2004). Effect of temperature on dynamic rheology of Korean honeys. Journal of Food Engineering, 65, 459–463.
Zaitoun, S., Ghzawi, A., Al-Malah, K. I. M., & Abu-Jdayil, B. (2001). Rheological properties of selected light colored Jordanian honey. International Journal of Food Properties, 4, 139–148.
Acknowledgments
This paper was supported by the project "Knowledge provocation and development through doctoral research PRO-DOCT contract no. POSDRU/88/1.5/S/52946 ", project co-funded by the European Social Fund through Sectoral Operational Program Human Resources 2007–2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oroian, M., Amariei, S., Escriche, I. et al. Rheological Aspects of Spanish Honeys. Food Bioprocess Technol 6, 228–241 (2013). https://doi.org/10.1007/s11947-011-0730-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0730-4