Abstract
Polyphenol contents and 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) scavenging activity of cumin (Cuminum cyminum L.) seed extracts were compared depending on their geographical origin (Tunisia and India: TCS and ICS, respectively) and the extraction solvent polarity. The β-carotene bleaching assay, the chelating ability and the reducing power of the most promising solvent extracts were also assessed. In addition, TCS and ICS extracts were acid-hydrolyzed and the phenolics identified by reversed-phase high-performance liquid chromatography (HPLC). Seed phenolic contents and antioxidant activity appeared to be accession and solvent dependent. Extraction with 80% acetone led to the highest polyphenol (18.60 and 16.50 mg gallic acid equivalents (GAE)/g dry weight (DW)), flavonoid (5.91 and 4.99 mg catechin equivalents (CE)/g DW) and tannin (83.23 and 80.23 mg CE/g DW) contents, respectively for TCS and ICS. DPPH scavenging activity, β-carotene bleaching assay, chelating ability and reducing power were maximal in 80% acetone for both TCS and ICS. HPLC analysis revealed several phenolic compounds in C. cyminum seeds, with p-coumaric (4.83 and 2.33 mg/g DW), trans-2-dihydrocinnamic (1.09 and 1.20 mg/g DW) and rosmarinic (0.70 and 1.04 mg/g DW) acids as major phenolics in TCS and ICS, respectively. Thus, phenolic composition of cumin seeds is also origin dependent. Taken together, our findings indicate that cumin might constitute a rich and novel source of natural antioxidants as food additives in food industry and that acetone 80% would be the most appropriate solvent for seed extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants constitute a valuable source of natural antioxidants including vitamins, phenolic compounds and flavonoids (El-Ghorab et al. 2007). Because of their potential carcinogenicity, the utilization of synthetic antioxidants is progressively restricted. This trend is concomitant with an increasing interest for the identification and valorization of natural antioxidants of plant origin. Consumers are also more attentive about healthy and balanced diets, which greatly rely on the utilization of natural preservatives for a healthier lifestyle and natural ways of preserving nutrients (Raghavan 2007). Spices are a potent source of antioxidants, some of them being more efficient and safer than the synthetic antioxidants. According to Pietta (2000), the antioxidant effect of plant products could be mainly ascribed to phenolic compounds, such as flavonoids, phenolic acids, tannins and phenolic diterpenes. Phenolics play an important role in human health owing to their anti-inflammatory, anti-allergic, antimicrobial, anticarcinogenic and antiviral activities (Medina et al. 2007). In plants, these compounds prevent lipid peroxidation and oxidative modification of low-density lipoproteins due to their antioxidant activities (Servili et al. 2004).
Extraction process aims at providing a maximum yield of biosubstances of the highest quality (concentration of target compounds and antioxidant power of the extracts). Yet, literature about the most effective methods and the solvents to extract these compounds is abundant but to some extent contradictory (Contini et al. 2008). Considering the structure of these compounds and their physicochemical properties, it would be impossible to propose a universal extraction protocol. Different solvent systems have been used for the extraction of polyphenols from plant material (Pinelo et al. 2004), but the extraction yield is influenced by the solvent nature and the extraction method (Goli et al. 2004). Phenolic content during the plant growth cycle is also affected by genetic, cultural practices and climatic factors. According to Akowuah et al. (2005), depending on the solvent used for extracting phenolic compounds, extracts obtained from the same plant may vary widely with respect to their antioxidant concentration and activities. In tea, aqueous ethanol seems to be more efficient than methanol and acetone for extracting flavonoids (Wang and Helliwell 2001), whereas water was found to be a better solvent for extracting catechins, as compared to 80% methanol or 70% ethanol (Khokhar and Magnusdotti 2002).
Cumin (Cuminum cyminum L.) of the Apiaceae produces highly nutritional oleaginous seeds which consist in a stripped paired or separate carpel showing nine ridges and oil canals (Pottier-Alapetite 1979). Cumin originates from Egypt, Turkistan and East Mediterranean, but it is cultivated in Iran, China, India, Morocco, south Russia, Japan, Indonesia, Algeria and Turkey (Tuncturk and Tuncturk 2006). In Tunisia, this plant, commonly known as “kammoun”, is a widely used spice in local kitchen. Cumin is an ingredient of most curry powders and many savory spice mixtures and is mainly used to prepare fish dishes in mixture with salt and olive oil. In the city of Sfax (south of Tunisia), it is largely used to prepare many dishes like “Kammounia” and fish plates. It gives bite to plain rice, beans and cakes.
C. cyminum seeds are used for treatment of toothache, dyspepsia, diarrhoea, epilepsy and jaundice (Eikani et al. 1999; Nostro et al. 2005) whereas essential oil of C. cyminum seed exhibits diuretic, carminative, emmenagogic antibacterial, antioxidant, cytotoxic, antidiabetic and antispasmodic properties (Allahghadri et al. 2010). Seeds contain fixed oil (approximately 10%), protein, cellulose, sugar, mineral elements and volatile oil (Li and Jiang 2004). They also contain volatile oil (1–5%) that account for the typical aroma of cumin seeds. Data on C. cyminum phenolics are still scarce and even lacking for Tunisian accessions. In order to assess the effect of solvent system and the geographic origin on phenolic content and antioxidant activities of cumin seeds, we compared the phenolic composition and antioxidant activities of Tunisian and Indian accessions under different extracting solvents. The expected results will be useful for the valorization of this species as a new local source of bioactive compounds for economic and health utilization.
Materials and Methods
Plant Material and Growth Conditions
Two provenances of mature green cumin (C. cyminum L.) seeds were used. The first one was purchased from an herbal market in Menzel Temime, a little town situated in the northeast of Tunisia and called TCS whereas the second one was imported from India (ICS). Seeds of both provenances were cultivated from January 2009 to July 2009 in a greenhouse under controlled conditions (14 h photoperiod; photosynthetic photon flux density 400 mol m−2 s−1; mean temperature and relative humidity 30 ± 5 °C, 55 ± 5% day and 16 ± 2 °C, 90 ± 5% night, respectively). Seeds were sown in 10-L pots filled with a clayey-loamy agricultural soil and were irrigated with tap water. After their harvest, mature seeds were air-dried and stored at 4 °C until used for further analysis.
Preparation of Extracts
Harvested seeds were finely grounded with a type A10 blade-carbide grinding (Ika-Werk, Staufen, Germany), and 2.5 g of the samples was then stirred with 25 mL of solvent for 30 min. Extracts were kept for 24 h at 4 °C, filtered through a Whatman N° 4 filter paper, dried under vacuum and stored at 4 °C until analysis was achieved. Seed extracts were obtained using different pure solvents with increasing polarity: hexane, acetone, acetone 80%, ethanol, methanol, ethanol 80%, methanol 80% and water.
Total Phenolic Content Determination
Total phenolic content was assayed using the Folin–Ciocalteu reagent, following Singleton’s method slightly modified by Dewanto et al. (2002). This method was employed to evaluate the phenolic content of the samples. A calibration curve of gallic acid (ranging from 50 to 400 mg/mL) was prepared and the results, determined by the regression equation of the calibration curve (y = 62.94–0.67, R 2 = 0.99), were expressed as milligram gallic acid equivalents (GAE) per gram dry weight of raw material (DW). In this method, an aliquot (0.125 mL) of a suitable acetone sample was added to 0.5 mL of deionized water and 0.125 mL of Folin–Ciocalteu reagent. The solutions were mixed and incubated at room temperature. After 1 min, 1.5 mL of 7% sodium carbonate (Na2CO3) solution was added. The final mixture was shaken thoroughly and then incubated for 90 min in the dark at room temperature. The absorbance of all samples was measured at 760 nm using a HACH UV–Vis spectrophotometer. Triplicate measurements were made for each sample.
Flavonoid Content Determination
Total flavonoid contents (TFC) were measured according to Dewanto et al. (2002). An aliquot of diluted sample or standard solution of (+)-catechin was added to a 75 μL of NaNO2 solution and mixed for 6 min, before adding 0.15 mL AlCl3 (100 g/L). After 5 min, 0.5 mL of NaOH was added. The final volume was adjusted to 2.5 mL with distilled water and thoroughly mixed. Absorbance of the mixture was determined at 510 nm against the same mixture, without the sample, as a blank. Total flavonoid content was expressed as milligram catechin equivalents (CE) per gram DW, through the calibration curve of (+)-catechin. The calibration curve range was 50–400 μg/mL (R 2 = 0.99). All samples were analysed in three replications.
Assessment of Total Condensed Tannins
Total tannin contents were measured using the modified vanillin assay described by Sun et al. (2002). To 50 μL of properly diluted sample, 3 mL of methanol vanillin solution (4%) and 1.5 mL of H2SO4 were added. The absorption was measured at 500 nm against extract solvent as a blank. The amount of total condensed tannins is expressed as mg (+)-catechin/g DW. The calibration curve range was 50–400 μg/mL (R 2 = 0.99). All samples were analysed in three replications.
Antioxidant Activities
DPPH Assay
The donation capacity of the obtained extracts and essential oils was measured by bleaching of the purple-coloured solution of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) according to the method of Hanato et al. (1998). One millilitre of the extract at different concentrations was added to 0.5 mL of a DPPH·methanolic solution. The mixture was shaken vigorously and left standing at room temperature for 30 min in the dark. The absorbance of the resulting solution was then measured at 517 nm. The antiradical activity was expressed as IC50 (micrograms per millilitre), the antiradical dose required to cause a 50% inhibition. A lower IC50 value corresponds to a higher antioxidant activity of plant extract. The ability to scavenge the DPPH radical was calculated using the following equation:
where A 0 is the absorbance of the control at 30 min and A 1 is the absorbance of the sample at 30 min. BHT was used as a positive control. Samples were analysed in triplicate.
β-Carotene Bleaching Test
A modified method described by Koleva et al. (2002) was used. β-Carotene (0.2 mg), 20 mg of linoleic acid and 200 mg of Tween 40 (polyoxyethylene sorbitan monopalmitate) were mixed with 0.5 mL of chloroform. Chloroform was removed at 45 °C, under vacuum, using a rotary evaporator (Heidolph, Germany). The resulting mixture was immediately diluted with 10 mL of triple-distilled water and was mixed well for 1 to 2 min. The emulsion was further made up to 50 mL with oxygenated distilled water. Aliquots (4 mL) of this emulsion were transferred into different test tubes containing 0.2 mL of test samples. BHA was used for comparative purposes. A control, containing 0.2 mL of corresponding solvent and 4 mL of the above emulsion, was prepared. The tubes were placed, at 50 °C, in a water bath. Absorbances of all the samples at 470 nm were taken at zero time (t = 0), and measurement of absorbance was continued, until the colour of the β-carotene disappeared in the control reaction (t = 180 min), at 15 min intervals. A mixture prepared as above, without β-carotene, served as blank. All determinations were performed in triplicate. The antioxidant activity of the extracts was evaluated in terms of bleaching of the β-carotene using the following formula:
where A t and C t are the absorbance values measured for the test sample and control, respectively, after incubation for 120 min, and C 0 is the absorbance value for the control measured at zero time during the incubation. The results are expressed as IC50 (micrograms per millilitre), the concentration required to cause a 50% β-carotene bleaching inhibition.
Chelating Effect on Ferrous Ions
The ferrous ion chelating activity of different organ extracts was assessed as described by Zhao et al. (2006). Different sample concentrations of the sample were added to 0.05 mL of FeCl2−4H2O solution (2 mM) and incubated at room temperature for 5 min. Then, the reaction was initiated by adding 0.1 mL of ferrozine (5 mM), and the mixture was adjusted to 3 mL with deionized water, shaken vigorously and left standing at room temperature for 10 min. The solution absorbance was then measured spectrophotometrically at 562 nm. The percentage of inhibition of ferrozine–Fe2+ complex formation was calculated using the formula given below:
where A 0 is the absorbance of the ferrozine–Fe2+ complex and A 1 is the absorbance of the test compound. Results were expressed as IC50, efficient concentration corresponding to 50% ferrous iron chelating. EDTA was used as a positive control. Samples were analysed in triplicate.
Reducing Power
The method of Oyaizu (1986) was used to assess the reducing power of different seed extracts. Each extract was mixed with 2.5 mL of sodium phosphate buffer (0.2 mol/L, pH 6.6) and 2.5 mL of potassium ferricyanide (10 g/L), and the mixture was incubated at 50 °C for 20 min; 2.5 mL of trichloroacetic acid (100 g/L) was then added, and the mixture was centrifuged at 650×g for 10 min. The upper layer (2.5 mL) was mixed with 2.5 mL of deionised water and 0.5 mL of ferric chloride (0.01 g/L) and thoroughly mixed. The absorbance was measured at 700 nm against a blank in a spectrophotometer. A higher absorbance indicates a higher reducing power. EC50 value (milligrams per millilitre) is the effective concentration at which the absorbance was 0.5 for reducing power and was obtained from linear regression analysis. Ascorbic acid was used as control.
RP-HPLC Evaluation of Phenolic Compounds
Dried samples from TCS and ICS were hydrolysed according to the method of Proestos et al. (2006) and slightly modified. Twenty millilitres of acetone 80% containing BHT (1 g/l) was added to 0.5 g of a dried sample. Then, 10 mL of 1 M HCl was added. The mixture was stirred carefully and sonicated for 15 min and refluxed in a water bath at 90 °C for 2 h. The obtained mixture was injected to high-performance liquid chromatography (HPLC). The phenolic compound analysis was carried out using an Agilent Technologies 1100 series liquid chromatography (reversed-phase (RP)-HPLC) coupled with an UV–vis multi-wavelength detector. The separation was carried out on a 250 × 4.6-mm, 4-μm Hypersil ODS C18 reversed-phase column at ambient temperature. The mobile phase consisted of acetonitrile (solvent A) and water with 0.2% sulphuric acid (solvent B). The flow rate was kept at 0.5 mL/min. The gradient programme was as follows: 15% A/85% B 0–12 min, 40% A/60% B 12–14 min, 60% A/40% B 14–18 min, 80% A/20% B 18–20 min, 90% A/10% B 20–24 min and 100% A 24–28 min. The injection volume was 20 μl, and peaks were monitored at 280 nm. Samples were filtered through a 0.45-μm membrane filter before injection. Peaks were identified by congruent retention times compared with those of pure standards. Analyses were performed in triplicate.
Statistical Analysis
All extractions and determinations were conducted in triplicates. Data are expressed as mean ± SD. The means were compared by using the one-way and multivariate analysis of variance followed by Duncan’s multiple range tests. The differences between individual means were deemed to be significant at p < 0.05. All analyses were performed by using the “Statistica v 5.1” software.
Results and Discussion
Effect of Provenance and Solvent on Extraction of Phenolics
Total Phenolic Contents
Usually, the solvent used for extraction is chosen according to the purpose of extraction (preparation or analysis), the nature of the assayed components, the physicochemical properties of the matrix, the availability of reagents and equipments, the cost and safety concerns (Yu et al. 2002). Depending on the solvent used, the amount of total phenolic contents (TPC) extracted from cumin seeds ranged from 1.09 to 18.60 mg GAE/g DW, respectively, for water and acetone 80% (Table 1). TPC extracted from TCS were 1.3-fold higher compared to ICS. In both accessions, the highest TPC was obtained with acetone 80%. With respect to TPC extraction yield, solvents used in the present study could be classified in the following decreasing order: acetone 80%, methanol 80%, ethanol 80%, acetone, methanol, ethanol and water. Some exceptions were observed, particularly when water and ethanol were used as extracting solvents. Consistent with previous studies (Turkmen et al. 2006), our findings point out the influence of the extracting solvent and accession on TPC. Zielinski and Kozlowska (2000) showed that 80% acetone is the most efficient solvent mixture for phenol extraction from cereal seeds. Similarly, Zhou and Yu (2004) reported that 50% acetone wheat extracts contained the highest TPC whereas ethanol was the least effective solvent. Eighty percent ethanol and 80% methanol were more efficient than water for extracting total phenolics from peanut skin (Yu et al. 2005).
Recently, El-Ghorab et al. (2010) reported that the highest total phenolic content was found in the cumin methanolic extract (35.5 mg GAE/g DW), whereas the hexane extract showed the lowest one (10.6 mg GAE/g DW). Thippeswamy and Naidu (2005) reported that TPC evaluated in cumin methanolic extract was 9 mg/g DW. In another study, Shan et al. (2005) found a TPC of 2.3 mg/g DW for 80% methanolic extracts of cumin. These changes could be ascribed to the variations in pedoclimatic conditions.
Moreover, the 80% acetone extract obtained from Tunisian provenance showed higher polyphenols content than the Indian one, suggesting that phenolics biosynthesis in C. cyminum is greatly influenced by genetic factors. Previous reports showed that the production of secondary metabolites (such as essential oil) in this species is affected by several factors including genetic ones (Bettaieb et al. 2011).
Total Flavonoids
TFC of cumin seeds varied from 0.52 to 5.91 mg CE/g DW, respectively, for water and 80% acetone. Regarding flavonoid solubility, the solvent classification with respect to their extraction efficiency was similar to that made for polyphenols. Zielinski and Kozlowska (2000) also showed that 80% acetone is an efficient solvent mixture for extracting flavonoids from cereal seeds. Similarly, it was demonstrated that water addition to organic solvents (especially acetone, ethanol or methanol) improved flavonoid extraction yield (Robards and Antolovich 1997). Variance analysis showed that there were significant differences in total flavonoid concentration among the two accessions. TFC extracted from TCS were 11.05% higher than those from ICS.
Condensed Tannins
Cumin is known to contain significant amount of tannins (Uma Pradeep et al. 1993). Our findings confirm this since values of condensed tannin contents (CTC) ranged from 30.92 to 83.23 mg CE/g DW for water and 80% acetone, respectively. As found for phenolics and flavonoids, CTC were found to vary depending on the solvent used. In both accessions, the highest CTC values were recorded when extraction was achieved using 80% acetone (ca. 81 mg EC/g DW in the average), followed by 80% ethanol (ca. 74 mg EC/g DW in the average). Generally, CTC extracted in TCS were 1.3-fold higher than in ICS. Chavan et al. (2001) reported that 70% acetone with or without acid was more efficient than absolute acetone to extract condensed tannins from different peas. Aqueous acetone was chosen as the extraction solvent because it has been reported to be the most effective medium for samples presumably rich in tannin (Naczk and Shahidi 2004). Moreover, the concentrations of total acetone-extractable polyphenols were statistically (P < 0.05) affected by geographical origin. Several studies have highlighted that genetic factors modulated phenolic concentration in different crops; this has been shown for various pecan cultivars grown in Texas State (Villarreal-Lozoya et al. 2007).
Effect of Provenance and Solvent on Cumin Antioxidant Activities
The solvent nature is the most controversial parameter that influences antioxidant capacities (Naczk and Shahidi 2004; Turkmen et al. 2006). The solubility of phenolic compounds is strongly governed by the type of solvent used, the degree of polymerization of phenolics, as well as by the interaction of phenolics with other food constituents and the formation of insoluble complex (Djeridane et al. 2006).
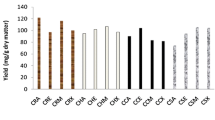
In order to identify the most suitable solvent for assessing antioxidant capacity, the antiradical activity (DPPH test) was evaluated either using the pure solvents or varied mixtures (Fig. 1). Solvent type had significant effect on the DPPH• scavenging activity of C. cyminum extracts. IC50 values ranged from 6.24 μg/mL (80% acetone) to 236 μg/mL (water). Seed extracts with 80% acetone showed the highest ability to reduce DPPH, with IC50 values reaching 6.24 and 15.14 μg/mL for TCS and ICS, respectively. Eighty percent methanol was found also to be the second suitable system (IC50 value of 19.70 and 21.14 μg/mL), followed by ethanol 80% (IC50 value of 22 and 41 μg/mL), respectively, for TCS and ICS. IC50 values using acetone, methanol and ethanol were higher than 100 μg/mL. The lowest antiradical capacity was found in water extracts of C. cyminum seeds (IC50 values were 222 and 236 μg/mL, respectively, for TCS and ICS) (Fig. 1). In addition, ICS exhibited significantly the lowest antiradical activity compared to the TCS irrespective of the solvent used in our study. These results indicate that aqueous solvents are more suitable for extracting DPPH• scavenging agents from C. cyminum seeds. Moreover, the significant variations in antioxidant potential according to the solvents used is essentially due to the difference in polarity, and thus different extractability, of the antioxidative compounds (Djeridane et al. 2006; Maisuthisakul et al. 2007). Several studies showed that solvent polarity leads to significantly different extraction capacities for phenolic compounds in plants (Parida et al. 2004; Galvez et al. 2005).
DPPH scavenging activity (IC50) of different seed extracts (TCS and ICS). Values are means of three replications (N = 3 ± SD). The data marked with the different capital letter, for the solvents, and small letter, for the provenance, in the histograms of each IC50 value share significant differences at P < 0.05 (Duncan test)
In accordance with several studies, adding water at low percentages to the solvent (especially acetone) improved the extracting power and antioxidant activity (Zhao et al. 2006; Zhou and Yu 2004). Zhao et al. (2006) suggested that acetone/water (8:2) is the most recommended solvent system to obtain the highest antioxidant activity in barley. The same correlation is established by Maisuthisakul et al. (2007) by comparing phenolic and DPPH scavenging activities of 26 Thai indigenous plants.
Our findings indicate that aqueous solvents were more effective in terms of extraction power of seed polyphenols and the quenching ability of DPPH than the other solvents. Eighty percent acetone, 80% methanol and 80% ethanol extracts were assessed for complementary antioxidant activities (β-carotene bleaching assay, chelating ability and reducing power). Concerning the β-carotene bleaching assay, the oxidation of linoleic acid generates peroxyl-free radicals due to the abstraction of hydrogen atom from diallylic methylene groups of linoleic acid (Kumaran and Karunakaran 2006). The free radical then will oxidize the highly unsaturated β-carotene. The presence of antioxidants in the extract will minimize the β-carotene oxidation by hydroperoxides. These will be later neutralized by the antioxidants from the extracts. Thus, the degradation rate of β-carotene depends on the antioxidant activity of the extracts. Eighty percent acetone, 80% methanol and 80% ethanol extracts differed in their capacity to inhibit linoleic acid oxidation (Table 2), indicating that the solvent system may significantly influence the antioxidant activity. As observed in the DPPH radical-scavenging capacity measurements, 80% acetone was the most effective means for extracting β-carotene bleaching activities compounds from C. cyminum (followed by 80% methanol and 80% ethanol). Moreover, Tunisian variety exhibited significant higher activity (65.86 μg/mL) than the Indian one (77.05 μg/mL) with acetone 80%. The antioxidative components in the C. cyminum extracts can probably reduce the extent of β-carotene destruction by neutralizing the linoleate-free radical and other free radicals formed in the system.
The effect of solvent and provenance on the antioxidant ability of C. cyminum seeds was also assessed by the estimation of chelating and reducing powers estimation (Table 2). The different extracts showed high antioxidant activities, but 80% acetone led to the highest high chelating power with IC50 values of 23.65 and 19.73 mg/mL, respectively, for TCS and ICS as well as a strong reducing capacity, with IC50 values of 110.34 mg/mL and 93.87 μg/mL, respectively. Comparing TCS and ICS antioxidant capacities showed that unlike to the DPPH and β-carotene bleaching assays, the Indian accession exhibited lower IC50 values and thus higher chelating and reducing powers than the Tunisian one. The reducing capability may serve as a significant indicator of the potential of antioxidant activity (Tanaka et al. 1998). This may be due to a slightly different phenolic composition between the 80% acetone extracts. It has been reported that the solvent polarity and the type of antioxidant substance influence the antioxidant capacity of the extract (Naczk and Shahidi 2004). The reducing property is generally associated with the presence of reductones (Duh 1998), such as ascorbic acid, which have been shown to exert antioxidant action by breaking the free radical chain (Gordon 1990). Reductones are also reported to react directly with peroxides and also with certain precursors of peroxides, thus preventing peroxide formation (Shimada et al. 1992; Xing et al. 2005). On the other hand, ferrous ions are commonly found in foods and are the most effective pro-oxidants (Yamaguchi et al. 1988). Hence, the ferrous ion chelating abilities of the various extracts from C. cyminum seeds would be beneficial.
Identification of Phenolic Compounds by RP-HPLC Assay
Phenolic compounds are naturally present in fruits and vegetables. They are part of everyday diet and are also used as medicines or supplements. Acetone 80% was the most efficient solvent to extract phenolics. For this reason, phenolic composition of TCS and ICS was only evaluated for this extract. No data are available concerning the phenolic composition of C. cyminum seeds. Here, for the first time, polyphenol qualitative and quantitative determination in cumin seeds were performed by RP-HPLC coupled with an UV–visible multi-wavelength detector. Phenolics occur in plants mainly as aglycones, glycosides and esters or are bounded to the cell wall (Soong and Barlow 2006). Therefore, acidic hydrolysis was used to release aglycones. Qualitative and quantitative differences were found between the two accessions (Table 3). TCS and ICS contained comparable phenolic acids (74.53%, 9.49 mg/g DW and 66.24%, 6.41 mg/g DW, respectively). However, TCS contained more flavonoids than ICS (11.43%, 2.88 mg/g DW and 9.68%, 1.77 mg/g DW, respectively).
A total of 19 phenolic compounds were successfully identified in TCS and ICS. The phenolic composition of C. cyminum seeds revealed that p-coumaric acid was more present in TCS (30.49%, 4.83 mg/g DW) compared to ICS (22.91%, 2.33 mg/g DW). Seeds were also characterized by the presence of other phenolic compounds in interesting proportions. TCS showed higher proportions of luteolin (4.65%, 1.29 mg/g DW), syringic acid (9.72%, 0.64 mg/g DW) and even cinnamic acid (6.35%, 0.94 mg/g DW), compared to ICS. However, Indian seeds were richer in trans-2-dihydrocinnamic acid (14.52%, 1.20 mg/g DW) and flavone (5.53%, 0.61 mg/g DW) than TCS.
Variations in phenolic composition could be explained by several factors, whether genetic or related to the geographic origin, plant organ, harvest time, extraction method, type of cultivar and storage conditions (Lawrence 2002). The season and even sunlight duration are also known to influence the plant metabolism since some compounds may be accumulated at a particular period to respond to environmental changes (Koenen 2001).
Ani et al. (2006) studied the phenolic composition of Cuminum nigrum seeds and phenolics including gallic, protocatechuic, caffeic, ellagic, ferulic acids and also flavonols such as quercetin and kaempferol. These authors also found that caffeic and ferulic acids were the predominant compounds. Epidemiological studies have suggested the association between the consumption of phenolic acid rich foods or beverages and the prevention of many diseases (Morton et al. 2000). Recent interest in food phenolics has increased owing to their roles as antioxidants and scavengers of free radicals and their implication in the prevention of many pathologies such as cardiovascular diseases (Ursini et al. 1999) and certain types of cancer (Hudson et al. 2000). Quantitative analysis of total phenolic compounds using HPLC indicates that TCS contained more TPC than ICS (13.51 and 11.76 mg/g, respectively). However, contents obtained by HPLC were significantly lower than those obtained by the spectrophotometrical method. This was predictable due to the low selectivity of Folin–Ciocalteu reagent, as it reacts positively with different phenolic and non-phenolic substances (Que et al. 2006).
Conclusion
The extracting solvent as well as the provenance significantly affected total polyphenol, flavonoids, tannins and antioxidant activities in C. cyminum seeds. In our conditions, the optimal extraction solvent yielding the highest content of phenolic compounds (TPC, TFC and CTC) and their antioxidant capacities (DPPH radical-scavenging capacities, β-carotene bleaching assay, chelating ability and reducing power) was acetone 80%. The major phenolic compound in seeds of the two accessions was p-coumaric acid, which contributes greatly to the high antioxidant activities of this species. The qualitative variability observed in both accessions suggests that phenolic biosynthesis in C. cyminum seeds is greatly influenced by genetic factors. Seeds of both accessions contained high amounts of phenolic compounds and strong antioxidant activities, which is of high economical significance in the perspective of breeding strategies, selecting provenances and solvents with high polyphenols, flavonoids and tannins contents and high antioxidant potential for producing specific health-promoting antioxidants in the food industry.
References
Akowuah, G. A., Ismail, Z., Norhayati, I., & Sadikum, A. (2005). The effect of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chemistry, 93, 311–317.
Allahghadri, T., Rasooli, I., Owlia, P., Nadooshan, M. J., Ghazanfari, T., Taghizadeh, M., et al. (2010). Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. Journal of the Food Sciences, 75, 54–61.
Ani, V., Varadaraj, M. C., & Akhilender Naidu, K. (2006). Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum L.). European Food Research and Technology, 224, 109–115.
Bettaieb, I., Bourgou, S., Sriti, J., Msaada, K., Limam, F., & Marzouk B. (2011). Essential oils and fatty acids composition of Tunisian and Indian cumin (Cuminum cyminum L.) seeds: a comparative study. Journal of the Sciences of Food and Agriculture, doi:10.1002/jsfa.4513.
Chavan, U. D., Shahidi, F., & Naczk, M. (2001). Extraction of condensed tannins from beach pea (Lathyrus maritimus L.) as affected by different solvents. Food Chemistry, 75, 509–512.
Contini, M., Baccelloni, S., Massantini, R., & Anelli, G. (2008). Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chemistry, 110, 659–669.
Dewanto, V., Wu, X., Adom, K., & Liu, R. H. (2002). Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry, 50, 3010–3014.
Djeridane, M. B., Yousfi, D., Nadjemi, P., & Boutassouna, N. (2006). Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chemistry, 97, 654–660.
Duh, P. D. (1998). Antioxidant activity of Budrock (Arctium laooa Linn.) its scavenging effect on free radical and active oxygen. Journal of American Oil Chemist Society, 75, 455–461.
Eikani, M. H., Goodarzni, I., & Mirza, M. (1999). Supercritical carbon dioxide extraction of cumin seeds (Cuminum cyminum L.). Flavour Fragrance Journal, 14, 29–31.
El-Ghorab, A. H., Nauman, M., Anjum, F. M., Hussain, S., & Nadeem, M. A. (2010). Comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). Journal of Agricultural and Food Chemistry, 58, 8231–8237.
El-Ghorab, A. H., Shibamoto, T., & Ozcan, M. (2007). Chemical composition and antioxidant activities of buds and leaves of capers (Capparis ovata Desf. Var. Canesencene) cultivated in Turkey. Journal of Essential Oil Research, 19, 72–77.
Galvez, C. J., Martin-Cordero, P. A., & Houghton, M. (2005). Antioxidant Activity of methanol extracts obtained from Plantago species. Journal of Agricultural and Food Chemistry, 53, 1927–1933.
Goli, A. H., Barzegar, M., & Sahari, M. A. (2004). Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chemistry, 92, 521–525.
Gordon, M. H. (1990). The mechanism of antioxidant action in vitro. In B. J. F. Hudson (Ed.), Food antioxidants (pp. 1–18). London: Elsevier Science.
Hanato, T., Kagawa, H., Yasuhara, T., & Okuda, T. (1998). Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chemical & Pharmaceutical Bulletin, 36, 2090–2097.
Hudson, E. A., Dinh, P. A., Kokubun, T., Simmonds, M. S., & Gescher, A. (2000). Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiology, Biomarkers & Prevention, 9, 1163–1170.
Khokhar, S., & Magnusdotti, S. G. M. (2002). Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. Journal of Agricultural and Food Chemistry, 50, 565–570.
Koenen, E. V. (2001). Medicinal poisonous and edible plants in Namibia. Berlin: Klaus Hess.
Koleva, I. I., Teris, A. B., Jozef, P. H., Linssen, A. G., & Lyuba, N. E. (2002). Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochemical Analysis, 13, 8–17.
Kumaran, A., & Karunakaran, R. J. (2006). Activity guided isolation and identification of free radical scavenging components from an aqueous extract of Coleus aromaticus. Food Chemistry, 100, 356–361.
Lawrence, B. M. (2002). From the sensation to the synthesis. In K. A. D. Swift (Ed.), Advances in flavours and fragrances. Special publication, vol 277 (pp. 57–83). Cambridge: Royal Society of Chemistry.
Li, R., & Jiang, Z. T. (2004). Chemical composition of the essential oil composition (Cuminum cyminum L.) from China. Flavour Fragrance Journal, 19, 311–313.
Maisuthisakul, P., Suttajit, M., & Pongsawatmanit, R. (2007). Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chemistry, 4, 1409–1418.
Medina, I., Gallardo, J. M., Gonzaalez, M. J., Lois, S., & Hedges, N. (2007). Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. Journal of Agricultural and Food Chemistry, 55, 3889–3895.
Morton, L. W., Caccetta, R. A., Ruddey, I. B., & Croft, K. B. (2000). Chemistry and biological effects of dietary phenolic compounds: Relevance to cardiovascular disease. Clinical and Experimental Pharmacology & Physiology, 27, 152–159.
Naczk, M., & Shahidi, F. (2004). Extraction and analysis of phenolics in food. Journal of Chromatography. A, 1054, 95–111.
Nostro, A., Cellini, L., Di Bartolomeo, S., Di Campli, E., Grande, R., & Cannatelli, M. A. (2005). Antibacterial effect of plant extracts against Helicobacter pylori. Phototherapy Research, 19, 198–202.
Oyaizu, M. (1986). Studies on products of the browning reaction prepared from glucose amine. Japanese Journal of Nutrition, 44, 307–315.
Parida, A. K., Das, A. B., Sanada, Y., & Mohanty, P. (2004). Effects of salinity on biochemical components of the mangrove, Aeceras corniculatum. Aquatic Botany, 80, 77–87.
Pietta, P. G. (2000). Flavonoids as antioxidants. Journal of Natural Products, 63, 1035–1042.
Pinelo, M., Rubilar, M., Sineiro, J., & Nunez, M. J. (2004). Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chemistry, 85, 267–273.
Pottier-Alapetite, G. (1979). Flore de la Tunisie. Angiospermes, Dicotylédones Dialypétales (pp. 587–588). Tunis: Imprimerie officielle de la république Tunisienne.
Proestos, C., Boziaris, I. S., Nychas, G. J. E., & Komaitis, M. (2006). Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chemistry, 95, 664–671.
Que, F., Mao, L., & Pan, X. (2006). Antioxidant activities of five Chinese rice wines and the involvement of phenolic compounds. Food Research International, 39, 581–587.
Raghavan, S. (2007). Handbook of spices, seasonings, and flavorings (2nd ed.). Boca Raton: CRC, Taylor & Francis Group.
Robards, K., & Antolovich, M. (1997). Analytical chemistry of frit bioflavonoids-a review. Analyst, 122, 11R–34R.
Servili, M., Selvaggini, R., Esposto, S., Taticchi, A., Montedoro, G., & Morozzi, G. (2004). Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. Journal of Chromatography. A, 1054, 113–127.
Shan, B., Cai, Y. Z., Sun, M., & Corke, H. (2005). Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agricultural and Food Chemistry, 53, 7749–7759.
Shimada, K., Fujikawa, K., & Nakamura, T. (1992). Anti-oxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry, 40, 945–948.
Soong, Y. Y., & Barlow, P. J. (2006). Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chemistry, 97, 524–530.
Sun, J., Chu, Y. F., Wu, X., & Liu, R. H. (2002). Antioxidant and antiproliferative activities of common fruits. Journal of Agricultural and Food Chemistry, 50, 449–454.
Tanaka, M. C., Kuie, W., Nagashima, Y., & Taguchi, T. (1998). Application of antioxidative Maillard reaction products from histidine and glucose to sadine products. Nippon Suisan Gakkaishi, 54, 1409–1410.
Thippeswamy, N. B., & Naidu, K. A. (2005). Antioxidant potency of cumin varieties; cumin, black cumin and bitter cumin on antioxidant systems. European Food Research and Technology, 220, 472–476.
Tuncturk, R., & Tuncturk, M. (2006). Effects of different phosphorus levels on the yield and quality components of cumin (Cuminum cyminum L.). Research Journal of Agriculture and Biological Sciences, 2, 336–340.
Turkmen, N., Sari, F., & Velioglu, Y. S. (2006). Effects of extraction solvents on concentration and antioxidant 1 activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chemistry, 99, 835–841.
Uma Pradeep, K., Geervani, P., & Eggum, B. O. (1993). Common Indian spices: Nutrient composition, consumption and contribution to dietary value. Plant Foods for Human in Nutrition, 44, 137–148.
Ursini, F., Tubaro, F., Rong, J., & Sevanian, A. (1999). Optimization of nutrition: polyphenols and vascular protection. Nutrition Reviews, 57, 241–249.
Villarreal-Lozoya, J. E., Lombardini, L., & Cisneros-Zevallos, L. (2007). Phytochemical constituents and antioxidant capacity of different pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars. Food Chemistry, 102, 1241–1249.
Wang, H., & Helliwell, K. (2001). Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Research International, 34, 223–227.
Xing, R., Liu, S., Guo, Z., Yu, H., & Wang, C. (2005). Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorganic & Medicinal Chemistry, 13, 1573–1577.
Yamaguchi, R., Tatsumi, Y., Asano, M., Kato, K., & Ueno, Y. (1988). Effect of metal salts and fructose on the autoxidation of methyl linoleate in emulsions. Agricultural and Biological Chemistry, 52, 849–850.
Yu, J., Ahmedna, M., & Goktepe, I. (2005). Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chemistry, 90, 199–206.
Yu, L., Haley, S., Perret, J., & Harris, M. (2002). Antioxidant properties of hard winter wheat extracts. Food Chemistry, 78, 457–461.
Zhao, H., Dong, J., Lu, J., Chen, J., Li, Y., Shan, L., et al. (2006). Effect of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.). Journal of Agricultural and Food Chemistry, 54, 7277–7286.
Zhou, K., & Yu, L. (2004). Effects of extraction solvent on wheat bran antioxidant activity estimation. Lebennsmittel-Wissenschaftund-Technologie, 37, 717–721.
Zielinski, H., & Kozlowska, H. (2000). Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. Journal of Agricultural and Food Chemistry, 48, 2008–2016.
Acknowledgements
We wish to express our sincere appreciation to Mr. Abdessattar Rebey for his English assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bettaieb Rebey, I., Bourgou, S., Ben Slimen Debez, I. et al. Effects of Extraction Solvents and Provenances on Phenolic Contents and Antioxidant Activities of Cumin (Cuminum cyminum L.) Seeds. Food Bioprocess Technol 5, 2827–2836 (2012). https://doi.org/10.1007/s11947-011-0625-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0625-4