Abstract
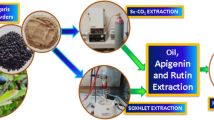
Seabuckthorn (Hippophae rhamnoides L.) berry oil having high nutraceutical, cosmeceutical, and therapeutic activity has been extracted from dried seabuckthorn (SBT) whole berry powder using supercritical carbon dioxide (SC-CO2), a green process for extraction of bioactives. The SC-CO2 process was optimized using Box–Behnken design. Three SC-CO2 parameters namely extracting pressure, extracting temperature, and time of extraction were examined. The optimal SC-CO2 conditions were determined, and the quadratic response surfaces were drawn from the mathematical models. A maximum recovery of 85.12% tocopherol, 71.73% carotene, and an EC50 of 29.02 mg/ml (from DPPH assay) was obtained after SC-CO2 extraction at 44 °C, 345 bar, and run time of 80 min. Use of methanol as an entrainer at 30% v/w of SBT berry at optimized conditions further increased the extraction efficiency and the potency. This extract can be used for varied nutraceutical, cosmeceutical, and pharmaceutical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seabuckthorn (SBT) is a branched and thorny nitrogen-fixing deciduous shrub of Elaeagnaceae family, native to Eurasia, and has been domesticated in several countries including India, China, Nepal, Pakistan, Myanmar, Russia, Britain, Germany, Finland, Romania, and France at a high altitude of 2,500–4,300 m. SBT is a protective natural agent to human health since all parts of the plant are considered to be a good source of a large number of bioactive substances (Arimboor et al. 2006). Cossuta et al. (2007) described this significant antioxidant activity to be related majorly to the contents of carotenoids and tocopherols. Various parts of SBT, especially berries, have been used as raw materials for nutraceuticals and therapeutic properties. Traditionally, SBT is used as herbal medicine for burns and wound healing, relieving cough, and aiding digestion for centuries in China and Russia. Recently, the nutritional importance of the berries in the form of health drinks has been increasing in North America, Europe, and India (Kaljurand et al. 2007).
Over 200 bioactive components have been found in SBT, of which many are investigated in SBT berries. SBT contains a series of chemical compounds including carotenoids, tocopherols, sterols, flavonoids, phenolics, lipids, and ascorbic acid. These compounds are of interest due to their biological and therapeutic activities including those as an antioxidant (Chauhan et al. 2007; Yang et al. 2011a), antitumoral (Padmavathi et al. 2005), antiproliferative (Grey et al. 2010), hepatoprotective (Geetha et al. 2008), antimicrobial (Nemtanu et al. 2009), in dry eye syndrome (Larmo et al. 2010), and immunomodulation (Dorhoi et al. 2006). SBT berries are also rich in vitamins like B1, B2, K, P, and phytosterols. Despite its highly acidic nature and exotic flavor, SBT berries have a good potential for producing various processed products like ready-to-serve beverage, squash, syrup, jam, and jellies. Products on the market from SBT range from oil, juice, and food additives to candies, jellies, cosmetics, and shampoos (Bal et al. 2011). SBT berry has 20–24% oil which has a high percentage of essential unsaturated fatty acids like oleic acid, palmitoleic acid, palmitic acid, and linoleic acid, of which palmitoleic acid is a principal constituent of skin fat. SBT extract is also recommended for skin softening and anti-wrinkle products. This fatty acid can also nourish skin if taken in adequate quantities, and is useful for treating systemic skin disorders such as atopic dermatitis (Yang et al. 2000). SBT berry oil contains bioactives such as tocopherols, carotenoids, and phytosterols which are valuable and protects against variety of illness. The carotenoids protect the skin against sun damage. Tocopherols and tocotrienols are powerful antioxidants that protect against sun damage as well. Phytosterols replace cholesterol in the skin, and by doing so, they improve the reduced barrier functions of the skin; besides, it also soothens irritation (Guliyev et al. 2004). The composition and contents of SBT actives vary greatly with variation in latitude and altitude (Zheng et al. 2010). Yang et al. (2011b) found content of inositols to be inversely related to air temperature and directly related to annual precipitation. Yang (2009) found methyl inositol and β-d-glucopyranose to be useful marker in determining harvesting time and different species of SBT berry. Apart from berries, SBT seeds (Sharma et al. 2008) and leaves (Zu et al. 2006) also contain important bioactives which possess potential applications.
The global climate change and massive wide scale use of organic solvents by a diverse range of global industries represents a serious threat to the environment. In response, the Montreal Protocol, introduced in 1987, is dynamic and evolving and has restricted the manufacture and use of other solvents in addition to CFCs. Consequently, worldwide, there is pressure for industry to adopt new sustainable processes that minimizes or eliminates the need of environmentally damaging organic solvents. This has manifested in significant efforts by scientists in designing environment-friendly research protocols. Extraction and isolation of natural products from various sources conventionally generates large amount of waste organic solvents. Pereira and Vicente (2010) reported environmental benefits arising from energy efficiency or reduced usage of non-renewable resources by emerging thermal and non-thermal technologies. These thermal methods such as solvent-free microwave-assisted extraction have been applied by researchers for extraction of phenolics from SBT pomace (Périno-Issartier et al. 2010) and SBT berries (Michel et al. 2011). They found the extracts to possess potent antioxidant activity in short extraction time. An eco-friendly alternative to the use of organic solvents in natural product extraction is the application of supercritical fluid extraction (SFE) protocol. The emerging stricter environmental regulations concerning the use of common industrial solvents, most of which are hazardous to human health, have led to the increasing popularity and growth of the SFE technologies, especially those employing supercritical carbon dioxide (SC-CO2).
The extraction of nutraceuticals using SFE technique covers principles of green technology as it is solvent-free and safe (Shi and Le Maguer 2000). Supercritical fluids are advantageous due to simplicity and less degradation of thermolabile compounds (Bruno et al. 1993). As reported by Van der Velde et al. (1992), SFE competes with conventional organic solvent extraction. SFE requires less time than conventional extraction due to higher mass transfer rates in supercritical fluids than in liquid solvents. Sajfrtová et al. (2010) used SFE for extraction of β-sitosterol from SBT seeds and found the yield to be up to five times higher using SFE technique.
To the best of our knowledge, no reports which correlate bioactives in SC-CO2 extraction of SBT berries and its antioxidant activity, is available. The present work reports on the optimization of SFE of SBT bioactives and its correlation to free radical scavenging activity (DPPH∙). Furthermore, the work also suggests the use of suitable entrainer to enhance the extraction efficiency and potency of extract.
Materials and Methods
Materials
The fruit of SBT (Hippophae spp.), harvested on September 24, 2009, were procured from Sindhu Fruit Processing Industry, Leh, India. The fruits were orange-colored and ripe as estimated by their carotene content (Andersson et al. 2009). The fruits were sundried by the processors to a moisture content of 0.5%. The dried fruit was then ground to a particle size of 340 μm. All the chemicals and solvents used in the present study were of AR grade and purchased from Sigma-Aldrich and S.D. Fine-Chem. Ltd, Mumbai, India. CO2 cylinders were supplied by Bombay Carbon Dioxide Gas Company, Mumbai, India.
SC-CO2 Extraction of SBT Berry Powder
For each experiment, 25 g of dried SBT berry powder was subjected to SC-CO2 extraction. Pure CO2 of >99% purity was used for the extraction. For extractions by SC-CO2 with an entrainer, the required quantity of entrainer was uniformly mixed with the SBT berry powder prior to extraction and immediately filled into the extraction vessel. The flow rate of the CO2 was kept constant at 10 g/min. The time of extraction was kept constant as 60 min, unless otherwise specified.
Laboratory scale supercritical equipment (Thar SFE-500) of Thar Technologies, Inc. PA, USA was used. A plug of cotton was pushed to the closed end of a SS 316 insulated high-pressure extraction vessel fitted with a filter-containing metal frit and tamped tightly in place. SPEED matrix (a hydromatrix and dispersing agent) was added to eliminate the dead volume of the extraction vessel, and a second plug of cotton was placed. A known amount of sample (25 g) was placed in the vessel and a third plug of cotton was placed above the sample matrix. The vessel was packed firmly to ensure that CO2 diffused uniformly through the sample matrix. The vessel was kept in place and a thermocouple was connected to the vessel body. A pressure-tight cyclone collector made of SS 316 was used to collect the extract from CO2.
Conventional Organic Solvent Extraction
Dried SBT berry powder was extracted in a Soxhlet extractor for 2 h with petroleum ether at 70 °C. The extract was used for determination of total tocopherols, total carotenes, total phenolics, total flavonoids, and free radical scavenging activity. The extraction yields of Soxhlet extract was taken as control (100%).
Optimization of the Selected Process Variables Using Box–Behnken Design
Statistical approach was used for optimizing the extraction of SBT actives from dried berry powder by varying the operating parameters (Granato et al. 2011). Box–Behnken design was used which places points on the midpoints of the edges of the (hyper-) cubical design region and also at the center. A Box–Behnken experimental design of three independent variables, varied at three levels, was used to obtain the combination of values that optimizes the response within the region of the three-dimensional observation space. The experiments were designed using Design Expert version 8.0.1 trial version (Stat-Ease, Minneapolis, MN, USA). Regression analysis was performed on the data obtained from the design experiments. Coding of the variables was done according to the Eq. 1:
where x i is the dimensionless value of an independent variable, X i is the real value of an independent variable, X cp is the real value of an independent variable at the center point, and ΔX i is the step change of real value of the variable i corresponding to a variation of a unit for the dimensionless value of the variable i. The experiments were carried out in triplicate to estimate the variability and reproducibility of measurements. The relationship of the independent variables and the response was calculated by the following second-order polynomial equation.
Y is the predicted response, β 0 is a constant, β i is the linear coefficient, β ii the squared coefficient, and β ij is the cross-product coefficient, k is the number of factors. The second-order polynomial coefficients and the response surface plots were obtained using Design Expert version 8.0.1, and the model was validated for the process conditions used in this study. Xu et al. (2008) optimized the SC-CO2 of SBT berry using response surface methodology and monitored the yields of tocopherols, carotenes, and oil, but no correlation of actives with the antioxidant activity was shown. Furthermore, the effect of entrainers was not investigated.
The extract obtained after SC-CO2 extraction was estimated for total tocopherols, total carotenes, and free radical scavenging activity (DPPH).
Effect of Entrainer
Supercritical CO2 is highly nonpolar in nature. Thus, when a polar solvent (entrainer) is mixed with SC-CO2, the polarity of the supercritical CO2 is altered which, in turn, influence the extraction yield. Here, the SBT berry powder was moistened with entrainers such as methanol, ethanol, and 2-propanol at 10%, 20%, and 30% v/w of SBT berry powder and extracted using optimized conditions (temperature, pressure, and extraction time of 44 °C, 345 bar, and 80 min, respectively).
Analytical Methods
Proximate Composition of SBT Berry Powder
The proximate constituents of the raw material affect the process of extraction. Hence, the samples were analyzed for their proximate compositions. Moisture content (MC) was determined by drying the samples at 105 ± 1 °C until constant weight (AACC 1976). Ash content was determined by heating the dried samples in a muffle furnace at 550 °C for 6 h (AOAC 1975). Protein content was calculated by estimating nitrogen by Kjeldahl method (N × 6.25) (AOAC 1975) using semi-automatic Kel Plus-ELITE EX equipment. Fat content was determined by using petroleum ether (60–80° fraction) as solvent (AOAC 1975) using semi-automatic SOCS PLUS SCS 4 fat extraction system (Pelican equipments, Chennai, India). Carbohydrate content was calculated by difference.
Determination of Total Tocopherols
The extract obtained after extraction was evaporated under vacuum in a rotary evaporator (Buchi rotavapor R-124, Switzerland) and redissolved in ethanol. The amount of tocopherols extracted from SBT berry powder was analyzed by HPLC using ZORBAX Eclipse XDB C18 Analytical Column (4.6 × 250 mm, 5 μm) and eluted with methanol at 1 ml/min. The tocopherol content was monitored at 292 nm with a diode array detector. The peak identity and λ max values of the compound were confirmed by the retention time and characteristic UV spectra of standard α-tocopherol. Extracted tocopherols were quantified and expressed as α-tocopherol.
Determination of Total Carotene
Carotenoids were assayed following the method described by Ranjith et al. (2006). Briefly, 1 ml aliquot of oil (extracted from berry) in hexane (0.1 g/ml) was added to 0.5 ml of 5 g/l NaCl, vortexed for 30 s, and centrifuged at 1,500g for 10 min. The supernatant was diluted, and the absorbance at 460 nm was measured. The amount of carotenoids was calculated by plotting a calibration curve with authentic standards of β-carotene (1–10 μg/ml) and expressed as β-carotene. The mean ± standard error of three replications was recorded.
Determination of Flavonoids
The AlCl3 method (Lamaison and Carnet 1990) was used for the quantification of the total flavonoid content of SBT berry extract. A total of 1.0 ml of 2% AlCl3.6H2O was added to equal volumes of the extract. The mixture was shaken and the absorbance was read at 440 nm. Flavonoid contents were expressed in milligrams of quercetin equivalent/100 g dry weight of SBT berry powder.
Free Radical Scavenging Activity (DPPH·)
The relative stability of the 2,2-diphenyl-1-picrylhydrazyl (DPPH·) radical has been widely used to evaluate the antioxidant activity of various plant extracts and pure compounds. DPPH radical-scavenging activity by SBT berry extract was measured according to the method of Brand-Williams et al. (1995) with some modifications. One milliliter of extract was mixed with 1 ml of a 100-μM solution of DPPH in methanol. The mixture was shaken vigorously and kept in the dark at room temperature for 20 min. Absorbance was measured at 517 nm using methanol as the blank. The DPPH solution was prepared daily, stored in flasks, covered, and kept in the dark at 4 °C until the measurements were taken. The scavenging activity of the extracts was expressed as EC50 (DPPH) which represents the concentration of extract that gives rise to a 50% reduction in DPPH absorbance and was determined by linear regression analysis.
The percentage of inhibition of the DPPH radical is defined as
where A control is the absorbance of the control (containing all reagents except the sample), A sample is the absorbance of the sample, both measured at 517 nm in a steady state.
EC50 is based on the reduction of an alcoholic solution of DPPH owing to the donation of hydrogen by an antioxidant compound. As the bioactives from SBT (tocopherols, carotenes) are potential antioxidants, the higher the total bioactives, the higher will be the radical scavenging activity and, thus, the lower will be EC50. In other words, the extract is more potent when the EC50 value is lower.
Fatty Acid Profiling of SBT Berry Extract Using GC-MS
The oil extracted from SBT berry using SC-CO2 at optimal condition (44 °C, 350 bar, and 80 min with 30% v/w of methanol as entrainer) was analyzed by GC-MS. The fatty acid methyl esters (FAME) were prepared by acid catalyzed transesterification of the extracted oils. Briefly, to 200 mg of the extracted oil, 10 ml of 3 M methanolic HCl containing 5 g/L cupric acetate was added and the mixture was kept for 8 h with constant stirring. After the reaction is completed, FAME were extracted with hexane and were used for GC-MS analysis. GC-MS using Varian 220-MS ion trap mass spectrophotometer (Varian Inc, Walnut Creek, CA, USA) connected to Varian 450-GC equipped with CP-SIL 88 capillary column (25 m × 0.25 mm i.d. × 0.39 mm o.d., Varian). The oven temperature was programmed to increase from 120 to 230 °C at a rate of 2 °C/min, with an initial hold of 5 min, and a final hold of 20 min. The injector was programmed and maintained at 250 °C. The flow rate of the carrier gas (helium) was adjusted to 1.0 ml/min. The samples were injected using the split sampling technique with a ratio of 1:20. GC-MS was operated at an ionization voltage of 70 eV and a trap temperature at 220 °C with a mass range of 40–350 amu. The percent composition of oil was determined using GC-MS peak areas without any correction factors. The identification of compounds was carried out according to the databank (NIST MS Search 2.0) of GC-MS used here.
Statistical Analysis
All results presented in the paper are mean ± SD of at least three determinations. Statistical analysis was carried out using Microsoft Office Excel 2007. Student's t test was performed to determine the statistical significance of the results. Correlations reaching a minimal confidence level of 95% were considered as being statistically significant.
Results and Discussion
Proximate Analysis of SBT Berries
The dried SBT berry, harvest of September 2009 season, had moisture content of 5.54% ± 0.03%. The protein, fat, ash, and total carbohydrate content of dried SBT berries were found to be 7.19% ± 0.25%, 23.92% ± 1.26%, 2.40% ± 0.04%, and 66.49% ± 1.55%, respectively. The results are expressed on moisture-free basis.
Conventional Organic Solvent Extraction
The Soxhlet extraction of powdered SBT berries with petroleum ether resulted in 21.10 and 44.68 mg of α-tocopherol and β-carotene/100 g dry berries powder, respectively. The free radical scavenging activity of the Soxhlet extract, expressed as EC50, was found to be 16.87 mg/ml. Soxhlet extraction resulted in extraction of 23.92 g of oil from 100 g powdered SBT berries. The yields obtained from Soxhlet extraction are taken as control (100%).
Optimization of Extraction Conditions by Using Box–Behnken Design
The combined effect of three independent variables A, temperature (°C); B, pressure (bar); and C, time (minutes) for the extraction of SBT berries actives was examined using Box–Behnken design. The experimental results for the Box–Behnken design are shown in Table 1. The analysis of variance (ANOVA) for DPPH, tocopherols, and carotenes extraction are presented in Table 2.
The ANOVA of the quadratic model indicated that the model is significant for tocopherols, carotenes, and free radical scavenging activity (DPPH assay) with the model F values of 27.07, 18.08, and 122.17, respectively, and is calculated as ratio of mean square (MS) regression and mean square residual. Model P values (Prob > F) were very low 0.001, 0.0005, and <0.0001 for tocopherols, carotenes, and free radical scavenging activity (DPPH assay), respectively, again signifying the model to be significant
where SS stands for sum of squares. The subscript T refers to group/model (between group variation), while subscript E refers to error/residual (within group variation).
As the aim for the extraction of SBT berries was to get the extract with maximum antioxidant activity, free radical scavenging activity (DPPH assay) was used as an indicator for determining optimum extraction conditions. The P values were used as a tool to check the significance of each of the coefficients, which, in turn are necessary to understand the pattern of the mutual interactions between the test variables. The smaller the magnitude of the P, the more significant is the corresponding coefficient. P values less than 0.05 indicates the model terms to be significant. The coefficient estimates and the corresponding P values suggests that among the test variables used in the study pressure, time, temperature2, pressure2, time2, and mutual interaction between pressure × time were significant model terms, whereas temperature, temperature × pressure, and temperature × time were not found to be significant. The second-order response model found after analysis for the regression was
The R 2 value of 0.9937 indicated that the model, as fitted, explained 99.37% of the variability in SFE of SBT berries. A very high degree of precision and a good deal of reliability of the experimental values were indicated by a very low value of the coefficient of variation. A validation run resulted in EC50, tocopherol and carotene content of 29.02 mg/ml, 85.12% and 71.73% as compared with the model predicted yield of 28.22 mg/ml, 85.07% and 70.72%, respectively. Figure 1 shows the significant interactions between the pressure and time for radical scavenging and carotene recovery when temperature is held at zero level (45 °C). The optimized condition found for SFE of SBT berry powder is temperature 44 °C, pressure 345 bar, and 80 min of extraction time.
Effect of Entrainer
The extraction efficiency of SBT actives from powdered SBT berries and thus antioxidant potency can further be enhanced by modifying the polarity of SC-CO2 by use of entrainers. Hence, attempts were made to extract SBT actives using different entrainers such as methanol, ethanol, and 2-propanol with SC-CO2. The experiments were performed with the Box–Behnken optimized extraction conditions.
The amount of entrainer solvent used varied from 10% to 30% (v/w) of powdered SBT berries. The extraction yields for methanol was better as compared to ethanol and 2-propanol. The recovery yields for tocopherol and carotenes were 90.82% and 67.12%, respectively when the concentration of entrainer, methanol, was 30% (v/w). Also, the extract had a greater antioxidant activity as seen by the decrease in EC50 from 29.02 to 18.85 mg/ml with 30% (v/w) of methanol as an entrainer. This increase in antioxidant potency could be due to the increased solubility of flavonoids in presence of more polar entrainers and thereby helping in its co-extraction (Table 3).
Fatty Acid Profiling of SBT Berry Oil
The main components in the oil were palmitoleic acid, oleic acid, linolenic acid, and palmitic acid which made up 98.23% of total oils. Table 4 enlist the composition of fatty acid of SBT berry obtained using SC-CO2 extraction. The result showed that SBT berry contained 74.68% unsaturated fatty acid (UFA) leading to high medicinal and nutritional value of berry oil, since supplementation of UFA is associated with many health benefits including cardiovascular, benign prostate hypertrophy, postprandial lipidemia. The major component of SBT berry oil is an omega-7 fatty acid, palmitoleic acid, constituting 38.07% of total oil. This is one of the only two sources of omega-7 from plant source available today. It is a naturally occurring component of healthy skin and is a highly effective antioxidant providing strong anti-aging support due to its ability to control free radicals. It is also a strong emollient, thereby soothes and moisturizes the skin and helps in regeneration of tissues.
Conclusions
SBT berries are a valuable asset of bioactives such as tocopherols, carotenoids, and flavonoids which could be concentrated by SC-CO2. Box–Behnken design was found to be valuable tool to estimate the effect of temperature, pressure, and time of extraction and their interactions for optimizing the extraction of tocopherols, carotenes, and radical scavenging activity of SBT berry extract. SC-CO2 extraction of dehydrated SBT berry powder with methanol as an entrainer at 30% v/w of dried berry powder resulted in further increase in percentage recovery of SBT actives with an increase in potency of the extract measured by EC50 value. Thus, SC-CO2 extraction of SBT berry powder is an efficient alternative to conventional solvent extraction for its application in foods/feeds, pharmaceuticals, and cosmetics.
References
AACC. (1976). Approved methods of American Association of Cereal Chemists, method 44–15 A, St. Paul- MN.
AOAC. (1975). Official method of analysis. Association of Analytical Chemists, Washington, D.C., 12th Ed.
Andersson, S. C., Olsson, M. E., Johansson, E., & Rumpunen, K. (2009). Carotenoids in sea buckthorn (Hippophae rhamnoides L.) berries during ripening and use of pheophytin a as a maturity marker. Journal of Agricultural and Food Chemistry, 57, 250–258.
Arimboor, R., Venugopalan, W., Arumughan, C., & Sawhney, R. (2006). Integrated processing of fresh Indian sea buckthorn (Hippophae rhamnoides) berries and chemical evaluation of products. Journal of the Science of Food and Agriculture, 86(14), 2345–2353.
Bal L. M., Meda V., Naik S. N., & Satya, S. (2011). Sea buckthorn berries: A potential source of valuable nutrients for nutraceuticals and cosmoceuticals. Food Research International, doi:http://10.1016/j.foodres.2011.03.002
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of free radical method to evaluate antioxidant activity. LWT Food Science and Technology, 28(1), 25–30.
Bruno, T., Castro, C. A. N., Hamel, J. F. P., & Palavra, A. M. F. (1993). Supercritical fluid extraction of biological products. In J. F. Kennedy & J. M. S. Cabral (Eds.), Recovery processes for biological materials (pp. 303–354). Chichester: Wiley.
Chauhan, A. S., Negi, P. S., & Ramteke, R. S. (2007). Antioxidant and antibacterial activities of aqueous extract of Seabuckthorn (Hippophae rhamnoides) seeds. Fitoterapia, 78, 590–592.
Cossuta, D., Simandi, B., Hohmann, J., Doleschall, F., & Keve, T. (2007). Supercritical carbon dioxide extraction of sea buckthorn (Hippophae rhamnoides L.) pomace. Journal of the Science of Food and Agriculture, 87(13), 2472–2481.
Dorhoi, A., Dobrean, V., Zǎhan, M., & Virag, P. (2006). Modulatory effects of several herbal extracts on avian peripheral blood cell immune responses. Phytotherapy Research, 20, 352–358.
Geetha, S., Jayamurthy, P., Pal, K., Pandey, S., Kumar, R., & Sawhney, R. C. (2008). Hepatoprotective effects of sea buckthorn (Hippophae rhamnoides L.) against carbon tetrachloride induced liver injury in rats. Journal of the Science of Food and Agriculture, 88, 1592–1597.
Granato, D., Branco, G. F., & Calado, V. M. (2011). Experimental design and application of response surface methodology for process modelling and optimization: A review. Food Research International. doi:10.1016/j.foodres.2010.12.008.
Grey, C., Widén, C., Adlercreutz, P., Rumpunen, K., & Duan, R. (2010). Antiproliferative effects of sea buckthorn (Hippophae rhamnoides L.) extracts on human colon and liver cancer cell lines. Food Chemistry, 120, 1004–1010.
Guliyev, V., Gulb, M., & Yildirim, A. (2004). Hippophae rhamnoides L.: Chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. Journal of Chromatography B, 812, 291–307.
Kaljurand, M., Gorbatsova, J., Lougas, T., & Vokk, R. (2007). Comparison of the contents of various antioxidants of sea buckthorn berries using CE. Electrophoresis, 28, 4136–4142.
Lamaison, J. L. C., & Carnet, A. (1990). Teneurs en principaux flavonoides des fleurs de Crataegus monogyna Jacq et de Crataegus laevigata (Poiret D. C) en fonction de la vegetation. Pharmaceut Acta Helve, 65, 315–320.
Larmo, P. S., Jarvinen, R. L., Setala, N. L., Yang, B., Viitanen, M. H., Engblom, J. R. K., et al. (2010). Oral sea buckthorn oil attenuates tear film osmolarity and symptoms in individuals with dry eye. The Journal of Nutrition, 140, 1462–1468.
Michel, T., Destandau, E., & Elfakir, C. (2011). Evaluation of a simple and promising method for extraction of antioxidants from sea buckthorn (Hippophae rhamnoides L.) berries: Pressurised solvent-free microwave assisted extraction. Food Chemistry, 126, 1380–1386.
Nemtanu, M. R., Mineal, R., Mazliu, E., Setnic, S., Mitru, E., Balotescu, C., et al. (2009). Effects of ionizing radiation on the antioxidant and antimicrobial activities of sea buckthorn oil. Acta Horticulture, 826, 255–260.
Padmavathi, B., Upreti, M., Singh, V., Rao, A. R., Singh, R. P., & Rath, P. C. (2005). Chemoprevention by Hippophae rhamnoides: Effects on tumorigenesis, phase II and antioxidant enzymes, and IRF-1 transcription factor. Nutrition and Cancer, 51, 59–67.
Pereira, R. N., & Vicente, E. E. (2010). Environmental impact of novel thermal and non-thermal technologies in food processing. Food Research International, 43, 1936–1943.
Périno-Issartier, S., Zill-e-Huma, Abert-Vian, M., & Chemat, F. (2010). Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food and Bioprocess Technology, doi:10.1007/s11947-010-0438-x
Ranjith, A., Sarin Kumar, K., Venugopalan, V. V., Arumugan, C., Sawhney, R. C., & Singh, V. (2006). Fatty acids, tocols and carotenoids in pulp oil of three sea buckthorn species (Hippophae rhamnoids, H. salicifolia, and H. tibetana) grown in the Indian Himalayas. Journal of the American Oil Chemists' Society, 83, 359–364.
Sajfrtová, M., Ličková, I., Wimmerová, M., Sovová, H., & Wimmer, Z. (2010). β-Sitosterol: Supercritical carbon dioxide extraction from sea buckthorn (Hippophae rhamnoides L.) seeds. International Journal of Molecular Sciences, 11, 1842–1850.
Sharma, U. K., Sharma, K., Sharma, N., Sharma, A., Singh, H. P., & Sinha, A. K. (2008). Microwave-assisted efficient extraction of different parts of Hippophae rhamnoides for the comparative evaluation of antioxidant activity and quantification of its phenolic constituents by reverse-phase high-performance liquid chromatography (RP-HPLC). Journal of Agricultural and Food Chemistry, 56, 374–379.
Shi, J., & Le Maguer, M. (2000). Lycopene in tomatoes: Chemical and physical properties affected by food processing. Critical Reviews in Food Science and Nutrition, 40, 1–42.
Van der Velde, E. G., de Haan, W., & Liem, A. K. (1992). Supercritical fluid extraction of polychlorinated biphenyls and pesticides from soil. Comparison with other extraction methods. Journal of Chromatography A, 626, 135–143.
Xu, X., Gao, Y., Liu, G., Wang, Q., & Zhao, J. (2008). Optimization of supercritical carbon dioxide extraction of sea buckthorn (Hippophae rhamnoides L.) oil using response surface methodology. LWT–Food Science and Technology, 41, 1223–1231.
Yang, B. (2009). Sugars, acids, ethyl β-d-glucopyranose and a methyl inositol in sea buckthorn (Hippophae rhamnoides) berries. Food Chemistry, 112, 89–97.
Yang, B., Kalimo, K. O., Tahvonen, R. L., Mattila, L. M., Katajisto, J. K., & Kallio, H. P. (2000). Effect of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on the fatty acid composition of skin glycerophospholipids of patients with atopic dermatitis. The Journal of Nutritional Biochemistry, 11, 338–340.
Yang, B., Ahotupa, M., Määttä P., & Kallio, H. (2011a). Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Research International, doi:http://10.1016/j.foodres.2011.02.025
Yang, B., Zheng, J., & Kallio, H. (2011). Influence of origin, harvesting time and weather conditions on content of inositols and methylinositols in sea buckthorn (Hippophae rhamnoides) berries. Food Chemistry, 125, 388–396.
Zheng, J., Kallio, H., Linderborg, K., & Yang, B. (2010). Sugars, sugar alcohols, fruit acids, and ascorbic acid in wild Chinese sea buckthorn (Hippophae rhamnoides ssp. sinensis) with special reference to influence of latitude and altitude. Food Research International, doi:http://10.1016/j.foodres.2010.10.007
Zu, Y., Li, C., Fu, Y., & Zhao, C. (2006). Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. Journal of Pharmaceutical and Biomedical Analysis, 41, 714–719.
Acknowledgments
Financial support of this work by Department of Biotechnology, Government of India, New Delhi, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kagliwal, L.D., Pol, A.S., Patil, S.C. et al. Antioxidant-Rich Extract from Dehydrated Seabuckthorn Berries by Supercritical Carbon Dioxide Extraction. Food Bioprocess Technol 5, 2768–2776 (2012). https://doi.org/10.1007/s11947-011-0613-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0613-8