Abstract
In this study, we describe the extraction of different valuable compounds from winter melon seeds using supercritical carbon dioxide extraction combined with pressure swing technique (SCE-PST). The effects of the extraction variables, namely pressure, holding time (HT), and continuous extraction time (CT), were optimized by response surface methodology (RSM) to maximize the crude extraction yield (CEY). The optimal conditions were at pressure of 181.35 bar, HT of 9.93 min, and CT of 50.14 min. Under these conditions, the experimental CEY was 235.70 ± 0.11 mg g−1 with a relatively strong antioxidant activity (64.42 ± 0.21 % inhibition of DPPH· radicals, 67.36 ± 0.34 % inhibition of ABTS·+ radicals) and considerable amount of phenolic compounds (42.77 ± 0.40 mg gallic acid equivalent/g extract). The high-performance liquid chromatography (HPLC) analysis revealed that the bioactive phenolic compounds increased significantly using PST (p < 0.05), where gallic acid had the highest concentration (0.688 ± 0.34 mg g−1). The extract obtained using optimal SCE-PST conditions contained more than 83.65 % total unsaturated fatty acids (UFAs) and linoleic acid accounted for 67.33 ± 0.22 % in the total extract. From the results, the SCE efficiency in terms of extract quantity and quality has been enhanced significantly applying PST. Finally, the results were compared with previous published findings using supercritical carbon dioxide, ultrasound-assisted, and Soxhlet extraction. It was found that higher CEY could be achieved using Soxhlet extraction even through the quality of SCE-PST extracts in terms of antioxidant activity and phenolic compounds was better.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different conventional extraction methods have been used to isolate natural compounds from seeds. Conventional extraction methods are usually characterized by poor quality of extracts due to application of high temperatures resulting in degradation of heat-labile bioactive compounds. In recent years, increasing demand for the highest quality natural products as well as attention for environment pollution has inducted strict regulations during commercial production. Environmental-friendly techniques are becoming more interesting in order to develop the “Green Chemistry” concept (Kamran Khan et al. 2010). Supercritical fluid extraction (SFE) is a popular technique for extraction due to its high efficiency, short extraction time, and absence of chemical residues. Since the 1980s, various potential applications of SFE have been documented (Valcárcel and Tena 1997; Cao and Ito 2003; Lee et al. 2000). Carbon dioxide (CO2) is the most popular supercritical fluid solvent owing to its low cost, non-flammability, easy availability, non-toxicity, and low critical temperature (31.1 °C) and pressure (73.8 bar). The SFE technique makes high reliability and consistency in safety and quality of valuable thermo-sensitive compounds, which are degraded during conventional extraction methods, and also meet the consumer demand for natural products (Wang and Weller 2006; Bimakr et al. 2012). Therefore, SFE using CO2 was introduced as an alternative to the conventional extraction methods of valuable compounds from different natural sources. Pressure swing technique (PST) has been performed to improve the SFE process (Salto 1995). Supercritical carbon dioxide extraction (SCE) combined with PST (SCE-PST) consists on pressurization and depressurization steps has been applied for separating cashew nut shell liquid and palm kernel oil (Smith Jr. et al. 2003; Zaidul et al. 2007). To date, there is lack of knowledge about the effect of applying SCE-PST for improving extraction efficiency in terms of quantity and quality of extracts.
Optimization of the experimental condition is a critical and inevitable step in developing a successful extraction process due to the effect of various variables on the process efficiency. Response surface methodology (RSM) is a statistical approach used to optimize experimental conditions through generation of a mathematical model (Triveni et al. 2001; Bas and Boyaci 2007; Wang et al. 2008). RSM is a faster and more economical method than classic one-variable-at-a-time or full factorial experimentation (Liu et al. 2009). Optimization of SCE of grape seed, walnut, rosemary, cottonseed, rosehip seed, and extraction of phenolic and astaxanthin has been successfully performed using RSM (Lee et al. 2000; Oliveira et al. 2002; Rezzoug et al. 2005; Bhattacharjee et al. 2007; Machmudah et al. 2007; Stévigny et al. 2007; Thana et al. 2008).
Benicasa hispida (Thunb.) Cogn. (Syn. Benincasa cerifera, Cucurbitaceae family), commonly known as winter melon, is originated from southeast Asia and has been cultivated for at least 2000 years. This fruit is large and seedy with white color and spongy flesh. Storage of ripe winter melon without injury for long periods of time, even for a year, can be achieved in dry and cool atmosphere (Zaini et al. 2011). Index of Nutritional Quality (INQ) data showed that B. hispida has been valued as a high quality vegetable (Mingyu et al. 1995; Zaini et al. 2011). In our preliminary experiments, the proximate analysis of B. hispida seeds was compared with those presented in literature for groundnut and sunflower (Ensminger et al. 1990), and two Cucurbitaceae species including Cucumis melo var. flexuosus and Citrullus lanatus (Mariod et al. 2009). Significant differences were observed among the seeds in their content of moisture, crude protein, fats/oils, crude fiber, ash, and carbohydrate contents. The protein content of B. hispida seeds (18.45 ± 0.8 g/100 g) was higher than C. melo var. flexuosus (15.70 ± 0.25 g/100 g) but still lower than the other seeds. The oil content of B. hispida (31.26 ± 0.6 %) seed was higher than the two Cucurbitacea species seeds. The high percentages of oil make these seeds suitable for the oil industry (Al-Khalifa 1996; Nyam et al. 2009). These findings showed that B. hispida seeds may have the potential to be used as high protein and oil sources in some food formulations, although it should be considered that the composition and nutritional values vary with the place of origin, stage of maturity, and subsequent storage (Al-Naqeeb et al. 2009).
In the current study, for the first time, the use of SCE-PST as a new technique for extraction of valuable bioactive compounds from B. hispida seeds was investigated. The effect of some process variables, namely pressure, holding time (HT), and continuous extraction time (CT), was investigated and then optimized by RSM to maximize crude extraction yield (CEY). The major bioactive flavonoids were analyzed using high-performance liquid chromatography (HPLC) to investigate the effect of PST on their extraction. The extract obtained under optimal extraction conditions was also analyzed using gas chromatography (GC) to determine the fatty acid composition. Furthermore, the antioxidant activity in terms of 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS˙+) and 1, 1-diphenyl-2-picrylhydrazyl (DPPH˙) radical scavenging activity (RSA) and total phenolic content (TPC) of extracts were determined. The results obtained in the current study were compared with those reported previously on the application of SCE (Bimakr et al. 2013), ultrasound-assisted extraction (UAE) (Bimakr et al. 2012), and conventional Soxhlet extraction (Mandana et al. 2012) to find out the most efficient and preferable extraction technique to obtain valuable compounds from B. hispida seeds.
Materials and Methods
Materials
Whole winter melon fruits were purchased from a local market in Serdang, Selangor, Malaysia. The fruits were cut, seeds separated manually, and washed under tap water. Ventilated oven (Heraeus Vacutherm VT6025, Germany) was used to dry seeds (40 °C for 24 h). The samples were ground in a grinder mill (MX-335, Panasonic, Malaysia) for 10 s to produce a powder with an approximate size of 1.5–2.5 mm. Carbon dioxide (CO2, SFE grade) contained in a diptube cylinder was purchased from MOX Company in Malaysia. Ethanol (EtOH, 99.5 %, analytical grade) and n-hexane (analytical grade) were obtained from Scharlau Chemical, European Union. Fatty acid methyl ester (FAME) standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium methoxide, potassium persulphate, 2-2′azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS˙+), 1,1-diphenyl-2-picrylhydrazyl (DPPH˙), and Folin-Ciocalteu reagent (FCR) were purchased from Fisher (Pittsburgh, PA, USA). Phenolic compound standards including gallic acid (GA), catechin (CA), naringenin (NA), myricetin (MY), and quercetin (QU) were purchased from Sigma-Aldrich Chemie Gmbh Munich, Germany.
Supercritical Carbon Dioxide Extraction Combined with Pressure Swing Technique
The extraction process setup is depicted in Fig. 1. All SCE-PST extractions were carried out in a supercritical fluid apparatus (ABRP200, Pittsburgh, PA, USA). The CO2 pressure to the extractor was controlled with a back pressure regulator. The extraction vessel was loaded with 40 g of B. hispida ground seeds mixed with glass beads (120 g with 2.00 mm in diameter) brought to extraction temperature (46 °C). The temperature was chosen based on preliminary experiments. For un-interrupted SCE process (SCE without using PST), CO2 was compressed to the desired pressure and allowed to flow through the extractor over selected continuous extraction time (Fig. 2a).
For interrupted SCE process (SCE combined with PST), at given pressure (125–200 bar) and temperature (46 °C), the extraction vessel containing the sample was pressurized with CO2 which held for different holding time (HT, 6–18 min) and then the vessel was depressurized (Fig. 2b). The swing was applied three times based on preliminary experiments and then continuous time (CT, 30–60 min) was performed. After the extraction was completed, the CO2 feed supply was closed off and depressurization was carried out. The rate of depressurization is an important step that should be considered during the process. A rapid depressurization produces liquid CO2 as well as dry ice. As a consequence, the trapped CO2 inside the solid matrix expands which results in breakage of the cells (Martinez 2008). In the current study, depressurization step generally required about 3 min for reducing pressure from 125 bar and 4 to 5 min from pressure 200 or 275 bar, respectively. The CO2 and EtOH flow rates were 10 and 1 g/min, respectively. EtOH, which is allowed to use in the food and pharmaceutical industries, was applied as co-solvent due to its good miscibility with CO2 and non-toxicity (Sánchez-Vicente et al. 2009). After extraction, the co-solvent was removed from the extract using vacuum rotary evaporator (Eyela, A-1000S, Japan) at 40 °C.
Determination of Crude Extraction Yield
The crude extracts were weighed gravimetrically using an analytical Mettler Toledo balance (±0.0001 g) (Mettler Toledo GmbH, Greinfensee, Switzerland), and the CEY was calculated according to the following Equation:
Where m e is the crude extract mass (g) and m s is the extracted sample mass (g). The results of CEY (n = 3) were expressed as mg (± standard deviation) per g of sample.
Antioxidant Assays
The extract obtained under optimum conditions was subjected to different radical scavenging assays: (1) 1, 1-diphenyl-2-picrylhydrazyl (DPPH˙) and (2) 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS˙+).
DPPH˙ Radical Scavenging Activity
Reaction with the DPPH˙ radical was performed as previously described (Bimakr et al. 2013). Briefly, an aliquot of test sample (0.1 mg/mL) (dissolved in EtOH) was added into 3 mL of an ethanolic solution of DPPH˙ (60 μM) and the absorbance was measured at 515 nm for 60 min with 10 min intervals using UV–Vis spectrophotometer (Thermo 4001/4 UV–Vis Spectrophotometer, Thermo Fisher Scientific). Ethanolic DPPH˙ solution was used as a blank sample. The inhibition percent of scavenged DPPH˙ (%DPPHsc) was calculated as 100 × (Ab − As) / Ab, where Ab was the absorbance of the blank and As was the absorbance of the sample.
ABTS˙+ Radical Scavenging Activity
ABTS˙+ (2,2´-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt) radical scavenging activity was assessed as previously described (Bimakr et al. 2013). The ABTS˙+ solution was prepared by mixing 7 mM ABTS and 2.45 mM potassium persulphate and then incubated in the dark at room temperature for 16 h. The ABTS˙+ solution was diluted with 80 % (v/v) ethanol to obtain an absorbance of 0.700 ± 0.005 at 734 nm. The ABTS·+ solution (3.9 mL) was added to 0.1 mg/mL test sample. Absorbance of the mixture was recorded at 734 nm for 10 min with 2 min intervals using UV–Vis spectrophotometer (Thermo 4001/4 UV–Vis Spectrophotometer, Thermo Fisher Scientific). The blank test was conducted with ethanol instead of crude extract. The inhibition percent of scavenged ABTS˙+ (%ABTSsc) was calculated as 100 × (Ab − As) / Ab, where Ab was the absorbance of the blank and As was the absorbance of the sample.
Determination of Total Phenolic Content
The TPC of the extract obtained under optimum conditions was determined using Folin-Ciocalteu reagent (FCR) according to the procedure described by Singleton et al. (1999). Deionized water (1 mL) was mixed with 10 mg of test sample. Then, 1 mL of FCR (freshly diluted 10 times with distilled water) was added. After 5 min, 7.5 mL of aqueous carbonate sodium (Na2CO3, 60 mg/mL) solution was added and kept for 30 min at room temperature. The color change was determined by reading the wavelength at 765 nm (Thermo 4001/4 UV–Vis Spectrophotometer, Thermo Fisher Scientific). The gallic acid standard curve prepared at different concentrations (25–500 ppm) and TPC of the extract was expressed as mg gallic acid equivalent per gram of extract.
High Performance Liquid Chromatography Analysis
The determination of the major phenolic compounds in B. hispida seed extracts was performed by a HPLC using a Water 600 pump Controller and a 9486 tunable absorbance UV detector. Efficient chromatographic separation was obtained by an Eclipes XDR- C18 reversed-phase column (25 cm × 4.6 mm × 5 μm, Supelco, USA) with solvent A (triflouroacetic acid, 2.5 pH in deionized water) and solvent B (pure methanol, HPLC grade). The major bioactive phenolic compounds were quantified using regression equations from their respective standard curves. The injection volume was 20 μL, and all of the main flavonoid compounds were identified by matching their retention time against those of available standard compounds including GA, CA, NA, MY, and QU.
Preparation of Fatty Acid Methyl Esters
In order to obtain the fatty acid methyl esters (FAMEs), the extracts were brought to temperature of 50–60 °C and homogenized thoroughly before taking a test sample. A 100 μL of the test sample was mixed with 1 mL n-hexane in a 2-mL vial. A 1-μL aliquot of sodium methoxide was added to the vial. The mixture first became clear, and then turbid as sodium glyceroxide was precipitated. The clear upper layer of methyl ester was pipetted off and injected in the GC for fatty acid composition analysis (Zaidul et al. 2007).
Gas Chromatography Analysis
Fatty acid composition analysis was carried out in a Hewlett-Packard 6890 GC (Wilmington, DE) equipped with a flame ionization detector (FID) and a GC column BPX70 (30 m × 0.25 mm × 0.25 μm, Victoria, Australia). Oven temperature was programmed isothermally to 115 °C during 2 min, then it was raised at 4 °C/min to 163 °C and then at 1 °C/min to 170 °C. Finally, temperature increased to 200 °C at 10 °C/min and held at this temperature for 2 min. Helium was used as a carrier gas which flowed at a rate of 1 mL/min. The injection volume was 1 μL. Standard methyl esters of fatty acids were used as authentic samples. The fatty acid determination was duplicated for each sample.
Experimental Design and Statistical Analysis
Extraction process was carried out using RSM to maximize the CEY. Central composite design (CCD) with axial points was used for designing the experimental conditions. This generated 20 treatments with six replications at the center point to estimate the repeatability of the method (Montgomery 2001). The levels of the independent variables are shown in Table 1. Randomizing order of experiments minimized the effect of unexplained variability induced by extraneous factors on the responses (Liyana-Pathirana and Shahidi 2005). Moreover, it was assumed that the nature and shape of response surface are not affected by blocks (Mirhosseini et al. 2008).
The second-order polynomial model applied to predict the dependent variable as a function of independent variables is as follows:
Where Y i is the predicted response; β 0 is the offset term; β 1, β 2, and β 3 are the regression coefficients for linear effect terms; β 11, β 22, and β 33 are quadratic terms; and β 12, β 13, and β 23 are interaction terms. In this model, X 1, X 2, and X 3 represent the coded value of pressure, holding time (HT), and continuous extraction time (CT), respectively.
Optimum level of process variables, aiming to maximize the CEY value, was obtained using graphical and numerical optimization procedures. For graphical optimization, three-dimensional (3D) response surfaces were plotted to visualize the relationship between the significant interaction effects of variables and response variables. Numerical optimization through response optimizer was applied to predict the exact optimum level of independent variables leading to the desirable response. Verification of the final model adequacy was performed by comparing experimental and predicted data. In addition, the quality of the fit between the experimental and predicted data was determined according to values of the mean relative deviation modulus (E). The criteria can be calculated as follows:
Where V exp and V pre are the experimental and predicted values, respectively, and n is the number of experimental data. The experimental design matrix, data analysis, regression coefficients, generation of 3D graphs, and optimization procedure were conducted by using Minitab V. 16 statistical software (Minitab Inc., PA, USA). Treatment means were compared using Student’s t test at a 5 % significance level (p < 0.05).
Results and Discussion
Response Surface Analysis
Extraction pressure, HT, and CT were considered as the most important independent variables that significantly (p < 0.05) affect the SCE-PST process. The significant terms in the model were found by ANOVA for response based on F ratio and p value. It was found that the interaction effect between HT and CT was not significant (p > 0.05). Therefore, it was dropped from the initial model to obtain the final reduced model. The regression coefficients and significant probabilities of linear, quadratic, and interaction terms of independent variables studied on the CEY of B. hispida seeds are shown in Table 2. Second-order polynomial model for predicting CEY was rendered by multiple linear regression analysis technique. The effect of different variables on CEY could be predicted by following equation:
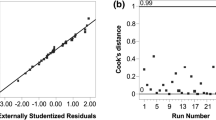
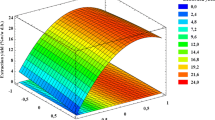
Where X 1 is the pressure, X 2 is HT, and X 3 is CT. The model adequacy was determined using model analysis, lack of fit test (p > 0.05), coefficient of determination (R 2 = 0.99), adjusted R 2(0.99), and E value (0.30 %) (Table 2). The p value of the model was less than 0.05, indicating that the model is statistically significant. To visualize the combined effects of two independent variables on a particular response, the response surface plot was generated as the function of two independent variables, while keeping the other variable at the central value. Two different response surface plots are depicted in Fig. 3a, b.
Effect of Process Variables on the Crude Extraction Yield
The CEY values obtained under the conditions studied varied widely ranging from 153.42 ± 0.14 to 230.80 ± 0.19 mg g−1. The SCE without PST (un-interrupted process) was performed as control condition at minimum, moderate, and maximum levels of independent variables (Table 3). The results showed significant (p < 0.05) increase on the CEY values using SCE-PST (Table 3). This enhancement of extraction could be attributed to the effective role of pressurization-depressurization defined as PST. This finding is in agreement with those reported by Smith Jr.et al. (2003) and Zaidul et al. (2007). Volume enlargement of the compounds in contact with CO2 most likely happened during pressurization and holding time (Martinez 2008). Reduction of the solutes viscosity and improvement of mass transfer are other phenomena that probably happened due to the expansion of the liquid phase. It should be noted that the rate of depressurization following an extraction affects the breakage of the seed coats, as demonstrated for pecan kernels (Martinez 2008). When the sample vessel was depressurized slowly, no breakage was observed, whereas a significant amount of breakage occurred during faster depressurization. When the extraction vessel was opened immediately after depressurizing the equipment, the particles jumped around, suggesting that most of breakup occurred as CO2-saturated particles were depressurized. The breakage of the seeds occurred due to the phase change of CO2. As mentioned earlier, a rapid depressurization to atmospheric pressure forms liquid CO2 as well as dry ice. Therefore, CO2 trapped inside the solid matrix expands, causing breakage of the cells (Martinez 2008).
The response surface plots, which present the effects of independent variables as well as their interactions on the CEY of B. hispida seeds, are shown in Fig. 3a, b. It was shown that during 45 min of CT, the CEY increased with increasing pressure and holding time up to a certain value (Fig. 3a). There is no significant (p > 0.05) improvement in recovery of extract using higher pressures at moderate HT (9 min) and CT (45 min), indicating there is an optimal value for pressure. As stated by Liza et al. (2010), the density of the supercritical carbon dioxide (SC-CO2) will increase. As the density increases, the distance between molecules decreases and the interaction between compounds and CO2 increases which leads to greater solubility of target compounds in CO2. However, applying higher pressure not always improve the results. As shown in Fig. 3a, b, using higher pressure levels led to a slight reduction in the extraction efficiency. One possible explanation of low efficiency of SCE process at high pressure is the B. hispida seed coat that would reduce the ability of the solvent to diffuse into the seed particles. A similar behavior was observed by Luengthanaphol et al. (2004) for extraction of antioxidants from sweet Thai tamarind seed. Furthermore, this unexpected reduction can probably be related to the reduced diffusion rates of the solutes from the sample matrix to the supercritical fluid medium at higher pressure (Rezaei and Temelli 2000; Kazzazi et al. 2007). The increment of CEY with increasing HT could also be seen in Fig. 3a and gradually remain constant at high levels of HT. Increasing HT from 6 to 9 min led to an increase of CEY from 193.75 to 226.27 mg g−1 at constant pressure of 162.50 bar during 45 min of CT. This could be due to the strong effect of HT for expansion of the compounds in contact with the solvent used, which resulted in higher CEY. A similar behavior was reported by Zaidul et al. (2007) for the extraction of palm kernel oil.
It was observed that the CEY slightly increased with CT and remained constant after 50 min (Fig. 3b). Further increases in CT after 50 min resulted in little change in the value of B. hispida seed CEY. The CEY value increased during 60 min of CT at lower pressures due to the lower CO2 density. However, at higher pressure levels, the extraction process completed during shorter CT. This is due to the improvement of cellular compounds solubility which caused by increased CO2 density with the rise of pressure (Liza et al. 2010).
Verification of the Final Reduced Model
The optimum conditions were found by numerical optimization procedure as 181.35 bar of pressure, 9.93 min of HT, and 50.14 min of CT. The statistical analysis revealed that there were no significant (p > 0.05) differences between the experimental (235.70 ± 0.11 mg g−1) and predicted (235.06 ± 0.14 mg g−1) results under the optimized condition. The validity of the final reduced model was statistically verified by comparison between experimental and predicted values (Table 1). It could be observed that only small deviations were found between the experimental and predicted values which were not significant (p > 0.05). Thus, the rendered model could be applied to optimize the B. hispida seed extraction using SCE-PST.
Antioxidant Activity
Results of un-interrupted SCE at given optimal conditions were used to find out the effective role of pressurization and depressurization steps. The RSA of extract obtained using SCE-PST (64.42 ± 0.16 % inhibition of DPPH· radicals and 67.36 ± 0.28 % inhibition of ABTS·+ radicals) was significantly (p < 0.05) higher compared with those obtained from un-interrupted SCE (47.40 ± 0.12 % inhibition of DPPH· radicals and 56.12 ± 0.17 % inhibition of ABTS·+ radicals) at optimal conditions. This could be attributed to the key role of PST to improve the extraction of compounds with antioxidant activity. As stated by Smith Jr. et al. (2003) pressure swing results in swelling phenomenon which increases the surface area of the sample to the bulk CO2 phase and would result in higher solute extraction.
Total Phenolic Content of Extracts
The TPC of extract obtained under optimum conditions of SCE-PST (42.77 ± 0.40 mg gallic acid equivalent/g extract) was significantly (p < 0.05) higher compared with that obtained from un-interrupted SCE at given optimal conditions (30.04 ± 0.20 mg gallic acid equivalent/g extract). This finding also confirmed the effectiveness of combining SCE with PST to enhance the separation of valuable bioactive phenolic compounds. The current result revealed that the B. hispida seed is a potential source of valuable bioactive phenolic compounds.
Identification and Quantification of Fatty Acid
Fatty acid analysis of the extract obtained using optimal SCE-PST conditions revealed that the B. hispida seed extract contains a high content (84.34 %) of unsaturated fatty acids (UFAs) including palmitoleic acid (16:1), oleic acid (18:1), linoleic acid (18:2), and linolenic acid (18:3). Table 4 shows the major fatty acid in the SCE-PST extract was linoleic acid (67.33 ± 0.22 %), followed by oleic acid (14.15 ± 0.33 %). The results indicated that the extract was rich in essential fatty acids (EFAs), which constituted 67.88 % of the total amount. EFA such as linoleic acid (LA) and α-linolenic acid (ALA) must be obtained from food because they are not synthesized by the human body. These valuable compounds are associated with healthy cell membranes formation and better performance of brain and nervous system (Sánchez-Vicente et al. 2009). Furthermore, disorders like atherosclerosis, coronary heart disease, and high blood pressure can be prevented by consumption of the dietary fats rich in α-linolenic acid (Wilkinson et al. 2005).
Palmitic acid (9.50 ± 0.26 %) was the main saturated acid in the extract followed by stearic acid (5.25 ± 0.18 %) and myristic acid (1.36 ± 0.38 %). Generally, the fatty acid content and its composition are influenced by age, species, season, and geographical regions (Wei et al. 2009).
Identification and Quantification of Phenolic Compounds
Polyphenols are one of the most used groups of bioactive compounds and have been extensively used for decades as food additives due to their well-known radical scavenging activities (Luengthanaphol et al. 2004). Furthermore, it has been proved that these valuable bioactive compounds have protective effects against diseases related to free radical formation such as cancer and cardiovascular diseases. After extraction applying different conditions of SCE-PST, the extracts were investigated for their qualitative and quantitative content of flavonoid compounds by reversed-phase HPLC and the results were summarized in Table 5. The extracts obtained using un-interrupted SCE (without applying HT) compared with those obtained using SCE-PST to investigate the effect of PST on extraction of phenolic compounds. According to Table 5, the significant (p < 0.05) differences between different extraction conditions are evident. All valuable bioactive phenolic compounds including GA, CA, NA, MY, and QU found in B. hispida seed extracts are present in higher amounts in extracts obtained using SCE-PST compared with those detected in extracts obtained by un-interrupted SCE. Regarding the SCE-PST, optimal conditions were more efficient than minimum and maximum levels of variables studied for obtaining bioactive phenolic compounds. GA had the highest concentration (0.668 ± 0.34 mg g−1) among the other phenolic compounds in the extract obtained under SCE-PST optimal conditions, whereas its concentration was 0.252 ± 0.05 mg g−1 in the extract obtained by un-interrupted SCE. This could imply the ability and potentiality of PST to extract more bioactive valuable compounds from B. hispida seeds.
Comparison of Different Extraction Methods
The efficiency of different environment friendly techniques including UAE (Bimakr et al. 2012), SCE (Bimakr et al. 2013), and SCE-PST were compared for separation of valuable compounds from winter melon (B. hispida) seeds (Table 4). Furthermore, these techniques were compared with the conventional Soxhlet extraction (CSE) (Bimakr et al. 2012) as a reference to determine preferable process conditions to obtain the highest CEY, RSA in terms of DPPH˙ and ABTS˙+ free radical inhibition and TPC of extracts. Application of various extraction techniques resulted in extracts that differ in quality and quantity of crude extract. Since a primary task in production of natural product is lowering the economic costs, higher extraction yield is favored. The RSA of the extracts obtained using SCE and SCE-PST techniques were significantly higher (p < 0.05), whereas CSE gave the highest value of CEY (250.00 ± 1.30 mg g−1) among other techniques. It was found that UAE was the fastest extraction method (∼36 min) compared with SCE-PST (∼50 min), SCE (∼97 min), and CSE (∼360 min). The extraction efficiency of UAE in terms of CEY was lower than the other techniques. However, it should be kept in mind that the quality of the extract in terms of antioxidant activity was significantly (p < 0.05) higher than CSE. The disadvantages of CSE were low content of valuable compounds and long extraction time (360 min). By applying SCE-PST, it could be possible to obtain crude extract in better quantity and quality compared with un-interrupted SCE process. In addition, it was found that application of different extraction methods including CSE, UAE, SCE, and SCE-PST resulted in extracts with different fatty acid contents. It should be noted that the linoleic acid was dominant (∼67 %) in all extracts obtained by UAE, SCE, and SCE-PST. Furthermore, EFAs contributed to high percentage (∼68 %) of total fatty acids, which confirmed the valuability of the extracts. Hence, it was observed that the fatty acid compositions of extracts were hardly affected by the application of UAE, SCE, and SCE-PST. As a conclusion, remarkable variations in CEY, RSA, and TPC of B. hispida seeds extract were found depending on the extraction technique. Higher CEY could be obtained by CSE with lower separation of bioactive compounds as they are thermo sensitive compounds and may be degraded during CSE. In contrast, using UAE, SCE, and SCE-PST lead to lower quantity of CEY with better quality in terms of valuable bioactive compounds separation.
Conclusion
This study showed, for the first time, the potential application of SCE combined with PST as a promising and effective extraction technique to obtain extracts from B. hispida seeds with good yield and rich in valuable compounds. The results revealed that application of pressurization-depressurization before continuous extraction time had a significant effect on the extraction efficiency. Furthermore, RSM was an effective tool for optimizing SCE-PST of B. hispida seeds. Second-order polynomial models were developed for predicting CEY. The combined level of 181.35 bar of pressure, 9.93 min of HT, and 50.14 min of CT were found as optimal conditions for obtaining the highest value of CEY (235.70 ± 0.11 mg g−1). Under these conditions, the experimental values agreed with the predicted values indicating a high goodness of fit of the model used. Furthermore, the extract obtained under optimal conditions of SCE-PST was rich in flavonoid compounds (GA, CA, NA, MY, and QU) and EFAs. Therefore, PST can be introduced to improve the efficiency of SCE process for the extraction of bioactive compounds from B. hispida seeds. In relation to the comparison results, promising methods for extraction of valuable compounds from B. hispida seeds are UAE, SCE, and SCE-PST. It can be stated that further studies could be performed to develop extraction processes and to assess the quality of extracts. In addition, more research is needed for exploiting industrial applications of novel green extraction techniques.
References
Al-Khalifa, A. S. (1996). Physicochemical characteristics, fatty acid composition, and lipoxygenase activity of crude pumpkin and melon seed oils. Journal of Agricultural and Food Chemistry, 44, 964–966.
Al-Naqeeb, G., Ismail, M., & Al-Zubairi, A. S. (2009). Fatty acid profile, α-tocopherol content and total antioxidant activity of oil extracted from Nigella sativa seeds. International Journal of Pharmacology, 5, 244–250.
Bas, D., & Boyaci, I. H. (2007). Modeling and optimization I, usability of response surface methodology. Journal of Food Engineering, 78, 836–845.
Bhattacharjee, P., Singhal, R. S., & Tiwari, S. R. (2007). Supercritical carbon dioxide extraction of cottonseed oil. Journal of Food Engineering, 79, 892–898.
Bimakr, M., Rahman, R. A., Taip, F. S., Adzahan, N. M., Sarker, M. Z. I., & Ganjloo, A. (2012). Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasa hispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules, 7, 11748–11762.
Bimakr, M., Rahman, R. A., Taip, F. S., Adzahan, N. M., Sarker, M. Z. I., & Ganjloo, A. (2013). Supercritical carbon dioxide extraction of seed oil from winter melon (Benincasa hispida) and its antioxidant activity and fatty acid composition. Molecules, 18, 997–1014.
Cao, X., & Ito, Y. (2003). Supercritical fluid extraction of grape seed oil and subsequent separation of free fatty acids by high-speed counter-current chromatography. Journal of Chromatography A, 1021, 117–124.
Ensminger, M. E., Oldfield, J. E., & Heinemann, W. W. (1990). Feeds and nutrition. 2nd edition. Ensminger, Clovis
Kazzazi, H., Rezaei, K., Ghotb-Sharif, S. J., Emam-Djomeh, Z., & Yamini, Y. (2007). Supercritical fluid extraction of flavors and fragrances from Hyssopus officinalis L. cultivated in Iran. Food Chemistry, 105, 805–811.
Khan, M. K., Abert-Vian, M., Fabiano-Tixier, A. S., Dangles, O., & Chemat, F. (2010). Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chemistry, 119, 851–858.
Lee, W. Y., Cho, Y. J., Oh, S. L., Park, J. H., Cha, W. S., Jung, J. Y., & Choi, Y. H. (2000). Extraction of grape seed oil by supercritical CO2 and ethanol modifier. Food Science and Biotechnology, 9, 174–178.
Liu, G., Xu, X., Hao, Q., & Gao, Y. (2009). Supercritical CO2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. LWT--Food Science and Technology, 42, 1491–1495.
Liyana-Pathirana, C., & Shahidi, F. (2005). Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chemistry, 93, 47–56.
Liza, M. S., Abdul Rahman, R., Mandana, B., Jinap, S., Rahmat, A., Zaidul, I. S. M., & Hamid, S. (2010). Supercritical carbon dioxide extraction of bioactive flavonoid from Strobilanthes crispus (Pecah Kaca). Food and Bioproducts Processing, 88, 319–326.
Luengthanaphol, S., Mongkholkhajornsilp, D., Douglas, S., Douglas, P. L., Pengsopa, L., & Pongamphai, S. (2004). Extraction of antioxidants from sweet Thai tamarind seed coat—preliminary experiments. Journal of Food Engineering, 63, 247–252.
Machmudah, S., Kawahito, Y., Sasaki, M., & Goto, M. (2007). Supercritical CO2 extraction of rosehip seed oil: fatty acids composition and process optimization. Journal of Supercritical Fluids, 41, 421–428.
Mandana, B., Russly, A. R., Farah, S. T., Noranizan, M. A., Zaidul, I. S., & Ali, G. (2012). Antioxidant activity of winter melon (Benincasa hispida) seeds using conventional Soxhlet extraction technique. International Food Research Journal, 19, 229–234.
Mariod, A. A., Ahmed, Y. M., Matthäus, B., Khaleel, G., Siddig, A., Gabra, A. M., & Abdelwahab, S. I. (2009). A comparative study of the properties of six Sudanese cucurbit seeds and seed oils. Journal of the American Oil Chemists' Society, 86, 1181–1188.
Martinez, J. L. (2008). Supercritical fluid extraction of nutraceuticals and bioactive compounds. New York: United States of America, CRC Press.
Mingyu, D., Mingzhang, L., Qinghong, Y., Weiming, F., Jianxiang, X., & Weiming, X. (1995). A study on Benincasa hispida contents effective for protection of kidney. Jiangsu Journal of Agricultural Sciences, 11, 46–52.
Mirhosseini, H., Tan, C. P., Hamid, N. S. A., & Yusof, S. (2008). Effect of Arabic gum, xanthan gum and orange oil on flavour release from diluted orange beverage emulsion. Food Chemistry, 107, 1161–1172.
Montgomery, D. C. (2001). Design and analysis of experiments (5th ed.). New York: Wiley.
Nyam, K. L., Tan, C. P., Lai, O. M., Long, K., & Che Man, Y. B. (2009). Physicochemical properties and bioactive compounds of selected seed oils. LWT - Food Science and Technology, 42, 1396–1403.
Oliveira, R., Rodrigues, M. F., & Bernardo-Gil, M. G. (2002). Characterization and supercritical carbon dioxide extraction of walnut oil. Journal of the American Oil Chemists' Society, 79, 225–230.
Rezaei, K., & Temelli, F. (2000). Using supercritical fluid chromatography to determine diffusion coefficients of lipids in supercritical CO2. Journal of Supercritical Fluids, 17, 35–44.
Rezzoug, S. A., Boutekedjiret, C., & Allaf, K. (2005). Optimization of operating conditions of rosemary essential oil extraction by a fast controlled pressure drop process using response surface methodology. Journal of Food Engineering, 71, 9–17.
Salto, S. (1995). Research activities on supercritical fluid science and technology in Japan—a review. Journal of Supercritical Fluids, 8, 177–204.
Sánchez-Vicente, Y., Cabañas, A., Renuncio, J. A. R., & Pando, C. (2009). Supercritical fluid extraction of peach (Prunus persica) seed oil using carbon dioxide and ethanol. Journal of Supercritical Fluids, 49, 167–173.
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Smith, R. L., Jr., Malaluan, R. M., Setianto, W. B., Inomata, H., & Arai, K. (2003). Separation of cashew (Anacardium occidentale L.) nut shell liquid with supercritical carbon dioxide. Bioresource Technology, 88, 1–7.
Stévigny, C., Rolle, L., Valentini, N., & Zeppa, G. (2007). Optimization of extraction of phenolic content from hazelnut shell using response surface methodology. Journal of the Science of Food and Agriculture, 87, 2817–2822.
Thana, P., Machmudah, S., Goto, M., Sasaki, M., Pavasant, P., & Shotipruk, A. (2008). Response surface methodology to supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis. Bioresource Technology, 99, 3110–3115.
Triveni, R., Shamala, T. R., & Rastogi, N. K. (2001). Optimised production and utilisation of exopolysaccharide from Agrobacterium radiobacter. Process Biochemistry, 36, 787–795.
Valcárcel, M., & Tena, M. T. (1997). Applications of supercritical fluid extraction in food analysis. Fresenius Journal of Analytical Chemistry, 358, 561–573.
Wang, L., & Weller, C. L. (2006). Recent advances in extraction of nutraceuticals from plants. Trends in Food Science and Technology, 17, 300–312.
Wang, L., Yang, B., Du, X., & Yi, C. (2008). Optimisation of supercritical fluid extraction of flavonoids from Pueraria lobata. Food Chemistry, 108, 737–741.
Wei, Z. J., Liao, M. A., Zhang, H. X., Liu, J., & Jiang, S. H. (2009). Optimization of supercritical carbon dioxide extraction of silkworm pupal oil applying the response surface methodology. Bioresource Technology, 100, 4214–4219.
Wilkinson, P., Leach, C., Ahsing, E. E., Hussain, N., Miller, G. J., & Millward, D. J. (2005). Influence of α-linolenic acid and fish-oil on markers of cardiovascular risk in subjects with an atherogenic lipoprotein phenotype. Atherosclerosis, 181, 115–124.
Zaidul, I. S. M., Norulaini, N. A. N., Omar, A. K. M., Sato, Y., & Smith, R. L., Jr. (2007). Separation of palm kernel oil from palm kernel with supercritical carbon dioxide using pressure swing technique. Journal of Food Engineering, 81, 419–428.
Zaini, N. A. M., Anwar, F., Hamid, A. A., & Saari, N. (2011). Kundur [Benincasa hispida (Thunb.) Cogn.]: a potential source for valuable nutrients and functional foods. Food Research International, 44, 2368–2376.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bimakr, M., Rahman, R.A., Ganjloo, A. et al. Characterization of Valuable Compounds from Winter Melon (Benincasa hispida (Thunb.) Cogn.) Seeds Using Supercritical Carbon Dioxide Extraction Combined with Pressure Swing Technique. Food Bioprocess Technol 9, 396–406 (2016). https://doi.org/10.1007/s11947-015-1636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1636-3