Abstract
The main aim of this work was to assess the frying strength of the enzymatically synthesized palm-based medium- and long-chain triacylglycerols (MLCT) oil with the aid of different antioxidants under deep-frying conditions. Palm-based MLCT oil in the presence of synthetic or natural antioxidants showed significantly better (P < 0.05) thermal resistance and oxidative strength than refined, bleached, and deodorized (RBD) palm olein throughout the five consecutive days of frying. Rancimat induction period, free fatty acid content, anisidine value, \(E^{{\text{1\% }}} _{1{\text{cm}}} \) at 232 and 268 nm, color, percentage of oil uptake, and viscosity measurement can be used as oil quality parameters to indicate the degree of oil deterioration under continuous stressed frying conditions. No significant changes (P > 0.05) in the saturated/unsaturated fatty acids ratio across frying periods indicated good oxidative stability of the palm-based MLCT oil. Due to the polarity of medium- and long-chain triacylglycerols in palm-based MLCT oil, total polar compounds determination may not be a suitable oil quality measures. Sensory evaluation of fried chips showed no significant differences (P > 0.05) between chips fried in RBD palm olein and palm-based MLCT oil over the 3-month storage period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipid quality in foods is significantly influenced by its oxidative status. Oxidation of lipids is a slow process that may take over several months at low temperature. Therefore, accelerated oxidation tests such as the “Rancimat”, “oxidative stability instruments,” and oxidative stability at elevated temperature index are frequently used to predict the oxidative stability of oils and fats as they give an indication of the length of shelf life (Gertz et al. 2000; Verleyen et al. 2004). However, these accelerated oxidative tests which were carried out with excess oxygen at 100–120 °C are totally different from normal frying conditions. Nevertheless, these methods can be used to compare the degree of oil deterioration resulting from deep frying. The onset of rancidity as determined by human sensory evaluation is the ultimate test to determine the oxidative status of oils and fats. Only sensory analysis can detect off-flavor formation by oxidative and non-oxidative degradation reactions as a consequence of the low odor thresholds of volatile products of lipid oxidation (Villière et al. 2007). Sensory analysis of frying oil and fried-food quality may be conducted by analytical descriptive method using trained and experienced panelists or hedonic scale sensory attributes using untrained panelists (Melton 1996).
Deep-fat frying, known as immersion frying, has gained popularity because of its simplicity, convenience, and ability to supply desirable flavor, golden color, and crisp texture. The frying process is a complex process, as the frying oils themselves undergo extensive physical and chemical changes at high temperature condition. Oxidation and polymerization reactions are more prevalent than hydrolytic reaction during deep-fat frying, leading to the oil decomposition to form a wide variety of volatile and non-volatile decomposition products, which ultimately alter the nutritive and functional properties of the oil (Warner 2002; Stier 2004a). The degradation products formed as a result of oil breakdown is an irreversible process. These materials affect the heat transfer at the oil–food interface and act to reduce the surface tension between two immiscible materials, which are also known as surface-active agent. In general, there are three proposed mechanisms used to describe the oil uptake process during deep-fat frying, which include water replacement, cooling phase effect, and surface-active agents (Blumenthal 1991; Mehta and Swinburn 2001; Mellema 2003; Stier 2004a). The first mechanism describes that the oil uptake of relatively large voids in the fried food was largely determined by the moisture content of food. The second mechanism explains how the product surface characteristics and oil viscosity affecting the amount of oil absorbed when the food is removed from the fryer. The presence of surface-active agent in oil caused an increased contact between food and oil, subsequently resulting in more oil being absorbed by the food. Nevertheless, Dana and Saguy’s (2006) review article showed that surface-active agents’ formation provides only a limited explanation for the increased oil uptake during prolonged frying. Higher oil uptake after extended frying time is probably related to higher oil viscosity caused by polymerization reactions and oil adherence to the product surface.
A considerable number of papers on structured lipids that contained both medium- and long-chain fatty acids in the same glycerol backbone (Matsuo et al. 2001; Kasai et al. 2003; Matsuo and Takeuchi 2004) had been published, and most of these papers reported on the effects of a medium- and long-chain triacylglycerols (MLCT) diet supplement on body fat accumulation. The decreased accumulation of body fat after MLCT ingestion was related to the increased thermic effect of MLCT. The dietary study of Kasai et al. (2003) on healthy humans suggested that about 2 g of medium-chain fatty acids (MCFA) per day (~12% MCFA in MLCT) was sufficient to accelerate lipid metabolism. MLCT has better cooking properties compared to physical mixtures of medium-chain triacylglycerols and long-chain triacylglycerols, as it has higher smoke point and less foaming tendency than that of the latter (Matsuo et al. 2001; Negishi et al. 2003).
To date, there are no continuous frying study carried out using palm-based MLCT oil in high temperature operations. This paper is focused on the deep frying performance of the enzymatically synthesized palm-based MLCT oil under deep frying conditions. The main objective was to investigate the frying strength of palm-based MLCT oil with different antioxidants under continuous frying conditions. The oxidative stability and organoleptic quality of fried potato chips stored for 3 months at ambient temperature were also determined.
Materials and Methods
Materials
A commercial oleoresin sage extracts (Herbalox seasoning, type S-O) in liquid form was donated by Kalsec Inc., USA (Gulf Chemical Sdn. Bhd., Petaling Jaya). tert-Butylhydroquinone (TBHQ; Eastman Chemical, USA) and refined, bleached, and deodorized (RBD) palm olein cooking oil were provided by Golden Jomalina Food Industries Sdn. Bhd. Fresh potato (Russet var.) were bought from a local supermarket. All chemicals and solvents used were either of analytical grade or high-performance liquid chromatography (HPLC) grade.
Rancimat Analysis on Fresh Oil Samples
A preliminary rancimat analysis on RBD palm olein, palm-based MLCT oil, palm-based MLCT oil with 200 ppm TBHQ, and palm-based MLCT with 1,000 ppm oleoresin sage extracts were conducted to check the induction period of the oil samples prior to frying experiment. The thermal-resistant oxidation strength of four different oil samples was determined with a 743 Rancimat Apparatus (Metrohm, Switzerland) by measuring the induction period of oils based on conductiometric method. The principle of this test is to purge the stream of air through heated oil and then monitor continuously the conductivity of water in which this effluent gas was trapped. Three grams of oil was heated to 120 °C under an air flow rate of 20 l/h (Matthäus 2006). All analyses were carried out in duplicate.
Sample Preparations
MLCT oil was produced through esterification synthesis of capric, oleic acid, and glycerol using Lipozyme RM IM lipase in a 16-l packed-bed bioreactor. The reaction was conducted at 70 °C for 14 h with 10% (w/w) enzyme packed in column under vacuum conditions to remove excess water using the optimum parameters determined in our previous study (Koh et al. 2008). The product was then purified through physical refining and was found to contain 76% MLCT. MLCT oil was mixed with 50% RBD palm olein to have at least 12% MCFA in palm-based MLCT oil blends. RBD palm olein, which is commonly used as cooking oil, was used as control and denoted as system I. Two types of antioxidants were added separately into palm-based MLCT oil: 200 ppm TBHQ and 1,000 ppm oleoresin sage extracts, denoted as systems II and III, respectively.
Fresh potatoes were peeled and sliced to a thickness of 1 mm using mechanical slicer (Model F-377, Mini Wonder, Malaysia). They were blanched with hot water (85 °C, 3 min) to prevent browning and blotted dry with tissue paper before weighing 100 g per batch for frying experiment. The mass ratio of oil to blanched potato slice was 2.2:1. The diameter of the potato slice used in this experiment was in the range of 4–6 cm.
Frying Experiment
Frying experiments were conducted in three oil systems: (a) RBD palm olein (system I); (b) palm-based MLCT oil with 200 ppm TBHQ (system II), and (c) palm-based MLCT oil with 1,000 ppm oleoresin sage extracts (system III). Each of the frying oil (2.2 kg) was put into a 2.7-l deep fryer (Model EDF 2715, Fiamma Sdn. Bhd., Kuala Lumpur, Malaysia). The temperature was raised to 180 °C and maintained for the first 20 min before frying. A batch of 100 g blanched potato slice was fried for 3 min at 20-min intervals for a period of 3.5 h per day with ten batches fried per day. A total of 50 frying batches for five consecutive frying days were conducted for three different oil groups. The fryer was left uncovered during the frying period to allow the evaporation of steam. At the end of the tenth frying, the fryer was switched off and the temperature allowed to cool to 60 °C. The oil (100 g) was collected in amber bottles and purged with nitrogen prior to storage at 4 °C for further oil analysis. Then, the oil was left to cool to ambient temperature before the lid of the fryer was put on for frying next day. A total of 250 g fresh oil was topped up to the frying vessels on following frying days to ensure sufficient oil for five consecutive days of frying experiments. Potato chips collected from the first to ninth batches of the first day frying were kept at ambient temperature for sensory evaluation.
Analyses of Oil
The quality assessments for oil samples, (a) RBD palm olein (system I), (b) palm-based MLCT oil with 200 ppm TBHQ (system II), and (c) palm-based MLCT oil with 1,000 ppm oleoresin sage extracts (system III), were monitored across the five consecutive days of deep frying experiments. All analyses were carried out in duplicate. Free fatty acids, given as % palmitic acid (FFA, AOCS 1997, Ca 5a-40), peroxide value (PV, AOCS 1997, Cd 8-53), anisidine value (AV, AOCS 1997, Cd 18-90), and oil uptake of fried potato chips (AOCS 1997, Ba 3-38) were determined using AOCS official methods. The totox value (TV) was defined as TV = 2PV + AV (Shahidi and Wanasundara 2002). The oil color (PORIM p4.1, 1995) was measured in 5.25-in cell in a Lovibond Tintometer (Salisbury, UK). The primary and secondary products of oxidation were analyzed through spectrophotometric measurement at the wavelengths of 232 and 268 nm, respectively, using International Union of Pure and Applied Chemistry (IUPAC 2.505) methods. The absorbance \(E^{{\text{1\% }}} _{1{\text{cm}}} \) at the various wavelengths is given by the formula:
where
- A λ :
-
the absorbance measured at wavelength λ
- c :
-
the concentration of the solution (in grams per 100 ml of solution)
- d :
-
the length of the cell (in cm).
Determination of Tocopherols and Tocotrienols Content by HPLC
The total tocopherols and tocotrienols content of RBD palm olein used in this experiment was analyzed with high-performance liquid chromatography system (Agilent 1100 series, USA) equipped with an Auto Injector (G1313A, Agilent) and high-pressure quaternary pump (G1311A, Agilent). A fluorescence detector (G1321A, Agilent) with the excitation wavelength set at 290 nm and emission wavelength at 330 nm was used (AOCS 1997, Ce 8-89). A pre-coated microparticulate silica HPLC column (Genesis Silica 120A, 25 cm × 4.6 mm) with 4-μm thickness was used as a stationary phase. The column temperature was set at 30 °C. The isocratic mobile phase consisted of isopropanol and n-hexane (0.5:99.5, v/v) with a flow rate of 1 ml/min. The total reaction time was 20 min. Sample preparations were made by dissolving 2 g oil in 25 ml n-hexane. An aliquot of 20 μl of the mixed tocopherols/tocotrienol standard working solution was injected into the column and the areas of the individual tocopherols/tocotrienols peaks recorded, followed by injection of 20 μl of the test solution into column. The total tocopherols and tocotrienols content present in RBD palm olein was identified with reference to the chromatograms obtained from tocopherols/tocotrienols standards from Malaysian Palm Oil Board. The tocopherols/tocotrienols content determined by this procedure can be expressed in micrograms per gram (μg/g) or parts per million (ppm). All analyses were carried out in duplicate.
Fatty Acid Composition of Fried Oils
The fatty acid analysis was performed by converting free and glyceride fatty acid to their corresponding methyl esters prior to the analysis by gas chromatography (GC; PORIM 1995, p. 3.4). Fatty acid methyl esters (FAME) were prepared by trans-esterification of oil (50 μl oil in 950 μl n-hexane) with sodium methoxide (0.5 N, 50 μl), and the samples were analyzed on a Perkin Elmer Clarus 500 instrument equipped with a flame ionization detector (FID). A capillary column DB-Wax of 0.25-mm internal diameter, 30-m length, and 0.25-μm film thickness was used. Nitrogen (99.995%) at a column head pressure of 20 psi was used as the carrier gas. The FID detector was set at 260 °C, while injector temperature was maintained at 250 °C. The GC split ratio was 1:50, and samples of about 0.5 μl were injected with a 10-μl GC syringes. The initial column oven temperature was 130 °C. Temperature was programmed to 170 °C at 20 °C/min, and then the heating rate was reduced to 10 °C/min until it reached 230 °C. The temperature was held for 5 min at 230 °C before heating at higher rates of 30 °C/min to 250 °C and held for 1 min until the analysis was completed. FAME peaks were verified using known lipid standard mixtures. The peak areas were calculated using Totalchrom Navigator software (version 6.3.1), and percentages of FAME were obtained as area percentages by direct normalization. All analyses were carried out in duplicate.
Rheological Analysis
Viscosity of fried oils was analyzed by means of a dynamic controlled stress rheometer (model RS600, Thermo Electron Corporation, Karlsruhe, Germany). The cone–plate geometry of a diameter of 35 mm with 2° angle was used to evaluate the rheological properties of the oil samples. The gap between the plate and cone was 0.105 mm. A programmable water bath (Model K20, Haake, Germany) with universal temperature controller (Haake) was used to ensure precise and stable control of the temperature during measurements. The rheometer and water bath were controlled using RheoWin 3 job manager, and the data obtained were analyzed using RheoWin 3 data manager provided by the manufacturer of the rheometer. The shear rates on oil samples were ramped from 0 to 100 s−1 and the temperature maintained at 30 °C. All the data collected are based on 20 repeated measurements of each ramp point at the interval of 30 s. The oil viscosity was obtained from the slope of the fit of experimental shear stress–shear rate data to Newton’s law of the viscosity equation (Eq. 1)
Total Polar Compounds
Total polar compounds of fried oil were analyzed using food oil sensor test kit (Model FOM 200, Ebro Electronic GmbH & Co. KG, Ingolstadt, Germany). In order to obtain optimum measuring result, the oil was heated up to 160 °C to 180 °C, and the temperature sensor was immersed up to the marked area. The total polar compounds were measured in percent. This sensor monitors the changes in the dielectric constant of degrading frying fats (Stier 2004b). The dielectric constant of the oil/fat increases with the increment of polar compounds. All analyses were carried out in duplicate.
Oil Turnover
The amount of fresh oil added was related to the turnover rate. It was defined as the ratio of oil weight in fryer and average weight of fresh oil added per hour (Banks 1996).
Sensory Evaluation on Fried Potato Chips
The organoleptic quality of potato chips on the first day of frying was used for sensory analysis. Nine-point scale hedonic sensory attributes was used to evaluate the taste, odor, crispiness, and overall acceptability of the fried potato chips by giving a score ranging from 1 to 9 (1 = like extremely, 3 = like moderately, 5 = neither like or dislike, 7 = dislike moderately and 9 = dislike extremely; Peryam and Pilgrim 1957; Che Man and Tan 1999). All these attributes were evaluated by 40 untrained panelists who are regular consumers of potato chips (female to male ratio = 1:1, age in between 24 and 30). Each sample was coded with a three-digit random number. All panelists were asked to read the instructions and the questionnaire before sensory begins. The meaning of each attribute was explained to the panelists to avoid misinterpretation.
Rancidity assessment was conducted based on the first day fried potato chips which were packed in aluminum-laminated bags and stored at ambient temperature (26–28 °C). For odor perception analysis, bags were sampled at the start of the storage period (0 month) for the duration of 3 months. At each interval of 1-month period, the chips were presented to a sensory panel comprising ten trained panelists with experiences in food rancidity from Research and Development Department, Golden Jomalina Food Industries Sdn. Bhd. (Malaysia) To enhance the odor perception analysis, chips (10 g) from each group were transferred to ten pieces of glass bottles (30 ml) with screw-capped lids and kept in oven at 50 °C for 1 h to develop odor in the headspace for better rancidity evaluation. Panelists were asked to remove the lid of the bottle and take three short sniffs. Then, panelists were instructed to take a sniff from a bottle containing lemon juice to refresh the nose sensor before proceeding to the following samples. All the panelists were asked to rate rancidity attributes of fried chips based on the scale ranging from 1 (very good, fresh) to 6 (very rancid, disagreeable off-flavor).
Statistical Analysis
Data were statistically analyzed by one-way analysis of variance procedure using SAS software (1989). Significant differences (P < 0.05) between means were determined by Duncan’s multiple-range test.
Results and Discussion
Rancimat Analysis on Fresh Oil Samples
The accelerated oxidative test based on rancimat induction period (IP) was conducted to evaluate the oxidative stability of four fresh oil samples prior to frying experiment. As the MLCT oil was enzymatically synthesized through esterification reaction, it did not contain any natural antioxidants and was susceptible to lipid oxidation. Blending of MLCT oil with 50% RBD palm olein increased rancimat IP in the palm-based MLCT oil (Table 1). However, the induction period of palm-based MLCT oil without addition of antioxidants was still low (IP 8.87 h) as compared to RBD palm olein (IP 13.14 h) and may be easily oxidized in the stressed frying conditions. There are no significant differences in rancimat IP (P > 0.05) between RBD palm olein and palm-based MLCT oil with 1,000 ppm oleoresin sage extracts (IP 13.44 h). RBD palm olein was chosen instead of palm-based MLCT oil to be used as control to study the deep frying performance and rancidity assessment of palm-based MLCT oil with different antioxidants. Although palm-based MLCT oil with 200 ppm TBHQ (IP 20.65 h) displayed the highest induction period among all, the effectiveness of this synthetic antioxidant against thermal oxidation at high temperature will be compared to natural antioxidants such as oleoresin sage extracts under continuous stressed frying conditions.
Changes in Color and Rancimat IP
Oil turnover, the ratio of the fryer’s volumetric capacity to the rate at which fresh frying oil was added to replenish the fryer (Banks 1996), is seen as a critical point to maintain frying oil quality. In this study, the frying experiments were conducted at relatively stressed conditions with oil turnover rate of 30.81 h and low ratio of oil to potato chips (2.2:1) to shorten the frying days. High turnover rate leads to the accumulation of degraded products in the frying oils, and their eventual incorporation in the fried food is a major concern in terms of nutritional and flavor. Measurement of the quality indicators of the fried oil collected at the end of each day was used to assess the thermal oxidative strength of the three different frying oils. Although this stressed deep frying experiment may not reflect the actual deep frying condition, it is a useful accelerated frying test to examine the frying strength of oil against high thermal oxidation threats in a short period of time.
Oil color was measured in the beginning and the subsequent days of frying and results are shown in Fig. 1. All three oil systems experienced oil darkening across five consecutive days of frying. RBD palm olein darkened quickly to an appreciable amount up to day 2, but very little after that. Although palm-based MLCT oil with oleoresin sage extracts has slightly darker color at the initial stage, the result obtained showed similar trends of color darkening as palm-based MLCT oil with TBHQ treatment throughout the five frying days. The darkening of palm olein could not be solely linked to oxidative deterioration of oil as reported by Augustin et al. (1987). The darkening of oil color was caused by many factors in different oils, for example, the presence of trace phenolic compounds, as a result of oil oxidation and formation of browning pigments from potato chips, etc. Many phenolic compounds are natural antioxidants, and its darkening effect on oil did not affect the quality of the chips. Only very dark color can indicate some kind of abuse on oil. Therefore, oil color changes were not a sensitive test for assessing the quality of different frying oils.
Rancimat induction measurements with excess of oxygen at 120 °C was done to predict the oxidative strength of the three oil systems as shown in Table 2. RBD palm olein (system I), naturally rich in vitamin E, containing about 860 ppm mixtures of tocopherol and tocotrienol has similar induction period with palm-based MLCT oil with 1,000 ppm oleoresin sage extracts (system III), which was about 13 h before frying. However, the induction period of system I decreased at a faster rate than system III across 5 days of frying, indicating a higher degree of oil degradation in system I than in system III. On the contrary, system II (palm-based MLCT with 200 ppm TBHQ) had a remarkably high induction period initially as compared to systems I and III. Nevertheless, the IP decreased drastically after first day of frying, followed by the gradual decrease in rancimat induction period throughout five consecutive days of frying. This phenomenon showed that the rancimat measurement was a good oil quality parameter to compare the degree of oil deterioration resulting from continuous frying process.
Changes in FFA, PV, AV, TV, and \(E^{{\text{1\% }}} _{1{\text{cm}}} \) at 232 and 268 nm
The extent of oil deterioration was best reflected in the changes of FFA, PV, AV, TV, and \(E^{{\text{1\% }}} _{1{\text{cm}}} \) at 232 and 268 nm which showed significant differences (P < 0.05) over the five consecutive frying days (Table 2). The oil system using RBD palm olein as control showed a greater degree of oil deterioration than oil systems using palm-based MLCT oil with antioxidants. Although palm-based MLCT oil with oleoresin sage extracts (system III) was significantly different (P < 0.05) compared to palm-based MLCT oil with TBHQ for all the parameters, the degree of oil deterioration was comparatively much smaller compared to RBD palm olein.
Free fatty acids content is the most common indicator of oil quality in industry, as it contributes to the development of off-flavor in products. Oils developed acidity during frying mainly due to oil hydrolysis. The fatty acids level in the fryer represents the balance between the rate of its formation and the rate of its removal by distillation. A significant change (P < 0.05) in FFA content throughout the frying periods in all three oil systems indicated that the rate of oil decomposition was much faster than the rate of its removal through distillation. No smoke haze was observed throughout 5 days of frying in all oil systems. The smoke haze was mainly due to the contribution of free fatty acids produced in the frying operation that has considerable effect on its smoke point. All three oil systems have FFA content less than 0.5%, although the oil systems have been subjected to five consecutive days of frying. The results showed that palm-based MLCT oils with addition of antioxidants was stable and suitable to be used as deep fat frying oils.
Peroxide value is an indication of primary oil oxidation, mainly due to the formation of hydroperoxide compounds. It is a good indicator of lipid oxidation under normal condition. The PV of three oil systems was not easily predicted throughout the five frying days. In general, PV decomposes at high temperatures and forms the secondary oxidation products. This phenomenon has been observed in most deep-fat frying studies (Pantzaris 1997; Makhoul et al. 2006). Even though PV rose and fell throughout frying periods, the PV was significantly higher (P < 0.05) in RBD palm olein (system I) than both palm-based MLCT oil (systems II and III), indicating that RBD palm olein was less thermal-resistant to oxidation (Table 2).
The AV of all systems significantly increased (P < 0.05) with the frying days (Table 2). A drastic increase in AV over the whole frying period showed that AV was a more meaningful test than PV for frying oils, as it measures aldehyde products, which were more stable during frying than peroxides products. Accumulation of the aldehyde products during five consecutive days of frying in all oil systems was one of the major reasons for the oil rancidity and off-flavor development. The AV of RBD palm olein increased drastically during the frying process as compared to both palm-based MLCT oil systems (systems II and III), indicating significant differences (P < 0.05) on the degree of oil deterioration. The changes of AV in both palm-based MLCT oil systems (systems II and III) were significantly (P < 0.05) smaller than RBD palm olein.
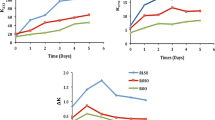
TV is another parameter used to monitor oil quality. It is considered to have the advantage of combining both evidence of past history of oil (as reflected in the AV) with its present state (as reflected in the PV). Although PV has higher experimental error than AV under heat-abuse condition, TV still able to give a general view on the oxidative stability of different frying oils. Figure 2 showed the changes in TV trends as a function of frying days in three different oil systems. All oil systems exhibited a steady increase trend of TV for the first 4 days of frying. RBD palm olein experienced rapid increase in TV compared to both palm-based MLCT oil systems (systems II and III). Both systems II and III showed similar increasing trends of TV throughout the 5 days of frying.
The changes in \(E^{{\text{1\% }}} _{1{\text{cm}}} \) at 232 and 268 nm of the oil can be used as a relative measurement of oxidation (Gray 1978). This method was based on the determination of the absorbance in the ultraviolet spectrum of oils and showed an indication of oil purity and deterioration. Conjugated dienes as a result of linoleic hydroperoxide composition showed an absorption band at 232 nm, while the secondary oxidation products, particularly ethylenic diketones, showed an absorption band at 268 nm. In general, all three oil systems showed significant (P < 0.05) increases in absorbance reading at 232 and 268 nm across five consecutive days of frying (Table 2). During the first 3 days of frying, the rapid changes in the \(E^{{\text{1\% }}} _{1{\text{cm}}} \) at 232 and 268 nm indicated that a large amount of primary and secondary oxidation products were formed. However, these two readings showed small increments in the last 2 days, indicating an equilibrium between the rate of formation of conjugated dienes and ethylenic diketones and the rate at which these compounds were converted into polymers (Warner 2002).
Changes in TPC, Viscosity, and Oil Uptake
Total polar compounds (TPC) is well recognized as an objective method for assessing the degradation status of frying oils, and it correlates well with the content of oxidized fatty acids (Sanibal and Mancini-Filho 2004). Many European countries have established regulatory limit of TPC of 25–27% as the discard point for frying oil (Firestone 1993; Mellema 2003; Sanibal and Mancini-Filho 2004). The test kit of food oil sensor (FOS) was developed to measure the degradation state of in-process frying oils, and it has been widely used by industrial frying plants and food catering restaurants. The study of Hein et al. (1998) showed a good correlation between FOS value and TPC, indicating that FOS is suitable to be used to substitute the determination of polar components by column chromatography. It is a fast and convenient way to monitor oil quality by monitoring the changes in the dielectric constant of degraded frying fats without the use of hazardous chemicals (Stier 2004b). Under the stressed frying conditions, all three oil systems experienced significant increase (P < 0.05) in TPC with frying times (Table 2). The TPC are composed of degradation products, non-volatile oxidized derivatives, polymeric and cyclic substances produced in the course of deep frying, and soluble components from food fried in the oil. RBD palm olein reached the discard point for TPC after 5 days of frying. Palm-based MLCT oils have inherently higher levels of polar compounds, mainly due to the structure of triacylglycerols which are composed of medium- and long-chain triacylglycerols and also the presence of partial glycerides (Shimizu et al. 2004). These products are not the harmful products formed during the frying process. In this case, the TPC may not be a good quality indicator for the palm-based MLCT oil. Generally, all three oil systems showed significant increase (P < 0.05) in TPC with frying days. Both palm-based MLCT oils (systems II and III) showed no significant difference (P > 0.05) in TPC under the continuous frying conditions.
The relationship between viscosity and frying days is shown in Fig. 3a,b. The oil viscosity is directly related to the degradation products which are mainly polymer formed during frying processes. Figure 3a showed an example of shear stress to shear rate graph of RBD palm olein (system I) collected after five consecutive days of frying. Similar shear stress changes as a function of shear rate were also observed in both palm-based MLCT oil systems (systems II and III). The linear relationship of shear stress to shear rate indicates that all oil samples exhibited a Newtonian behavior. Consequently, the viscosities of oil samples were obtained from the equation given by Newton’s law of viscosity. For all cases, the regression coefficients (R 2) obtained from the equation were greater than 0.999. Figure 3b summarizes the viscosity data for three oil systems based on the rheological measurement of fried oils collected at the end of each frying day. As expected, there was a noteworthy increase in viscosity of fried oil with increasing frying days as a result of the formation of high-molecular-weight compounds in oil samples. Majority of the dimeric and polymeric compounds are intermolecularly formed from carbon to carbon linkages and only minor dimers formed by carbon–oxygen–carbon bridges, and these molecules continue to cross-link as the oil was continuously exposed to high temperatures (Warner 2002; Kochhar and Gertz 2004). As the polymerized products increased in the frying oil, the viscosity of oil also increased in the same manner. RBD palm olein, which suffered greater oil deterioration as discussed in previous sections, showed a drastic increase in oil viscosity as compared to both palm-based MLCT oils (systems II and III). The oil viscosity is a sensitive indicator for oil degradation and can be used as an alternative method for the measurement of high-molecular-weight compounds in frying oils with no hazardous chemicals used.
a Comparison of shear stress–shear rate profile for RBD palm olein (30 °C) obtained from different frying days. b Effect of frying days on viscosity profile of different frying oil systems. System I: RBD palm olein; System II: palm-based MLCT oil with 200 ppm TBHQ; System III: palm-based MLCT oil with 1,000 ppm oleoresin sage extracts
A quantitative determination of total oil uptake was determined using Soxhlet extraction method in which the oil was extracted from the fried product using organic solvents like petroleum ether. In general, all three frying oil systems showed significant increment (P < 0.05) in percent oil uptake during the five frying days (Table 2). Increase in the degradation products (total polar compounds) and viscosity have been associated with a greater fat absorption by the fried food (Blumenthal 1991; Gertz 2004). An oil with higher viscosity will increase the contact between the food and oil, resulting in a slow oil release from the food into the oil surrounding. This causes excessive oil absorption by the food when it is removed from the frying medium (Bouchon et al. 2003; Moyano and Pedreschi 2006). For the three frying oil systems, the oil uptake results showed significant differences (P < 0.05) among the three oil systems for the first 3 days of frying and then remained indifferent (P > 0.05) among all systems at fourth and fifth days of frying. In this study, the excessive oil uptake by the fried potato chips was observed mainly due to the pretreatment on potato slices which was blanched in hot water to remove surface starch, indirectly causing more oil to penetrate in the cooked core, and that the microstructure of the crust is the main determining factor in oil uptake (Mellema 2003; Moyano and Pedreschi 2006)
Changes in SFA/USFA ratio and Fatty Acid Composition
The oxidative stability of frying oils is greatly influenced by the degree of unsaturation, particularly by the content of linoleic and linolenic acids as well as the presence of natural antioxidants such as tocopherols. The shelf life of frying oil can be enhanced by the inclusion of synthetic antioxidants such as TBHQ or natural antioxidants such as oleoresin sage extracts. The changes of SFA/USFA ratio across the 5 days of frying for three frying oil systems are shown in Table 2. No significant difference (P > 0.05) in the SFA/USFA ratio was observed between both palm-based MLCT oil systems (systems II and III) throughout the five consecutive days of frying. This phenomenon can be explained by a slight decrease in medium-chain fatty acids and a marginal decrease in unsaturated fatty acids under the stressed frying condition (Table 3). For system I, the SFA/USFA ratio showed a significant change (P < 0.05) from day 2 to day 5 of frying. It was found that the main causes for this observation was a decrease in linoleic acid (C18:2) and an increase in palmitic acid (C16:0) during the frying periods. Linoleic acid is more susceptible to oxidation, while palmitic acid is highly stable. Therefore, the ratio of C18:2 and C16:0 has been used as an indicator for the extent of oil deterioration (Che Man and Tan 1999).
Sensory Evaluation of Fried Potato Chips
A nine-point hedonic scale (1 = like extremely to 9 = dislike extremely) with 40 consumer-type panels were used to evaluate the acceptability of potato chips fried in three different oil systems. Table 4 shows the sensory scores of fried potato chips for odor, taste, crispiness, and overall acceptability. Melton (1996) found that the flavor likeability of the fried food was dependent on consumer perception and affected by the type of oil used for frying. However, in this case, the sensory data showed no significant differences (P > 0.05) in terms of odor, taste, crispiness, and overall acceptability of potato chips fried in all three different oil systems. The addition of oleoresin sage extracts did not affect the consumer perception on the odor of the fried potato chips, although it did contribute to a mild flavor on the fried potato chips. The sensory data generally showed that potato chips fried in palm-based MLCT oils were not significantly different (P > 0.05) from potato chips fried in RBD palm olein.
Oxidative Stability of Fried Potato Chips
The results for the rancid scores of stored potato chips during storage at ambient temperature are presented in Table 5. An odor perception scoring point system was selected on a scale of 1–6, whereby fresh, very good was rated as 1 and disagreeable off-flavor, very rancid was rated as 6. A value of 3 was generally accepted as the acceptability limit for potato chips. In this study, statistical analysis of sensory scores for rancid odor showed that there were no significant (P > 0.05) differences in organoleptic quality of potato chips fried in either RBD palm olein (system I) or palm-based MLCT oils (systems II & III). No significant changes (P > 0.05) in rancid scores for potato chips fried in three different frying oil systems throughout the storage period were observed. The rancid odor score point for potato chips fried in three frying oil systems was consistently low throughout 3 months of storage. The storage study of fried potato chips showed that both TBHQ and oleoresin sage extracts showed the similar trend of rancid odor development for potato chips fried in palm-based MLCT oil.
Conclusions
Exposure to high temperature frying operations for the five consecutive frying periods showed that palm-based MLCT oils have better thermal and oxidative strength than RBD palm olein with the aid of synthetic antioxidants, TBHQ, or natural antioxidant like oleoresin sage extracts. In summary, rancimat induction period, free fatty acid content, anisidine value, \(E^{{\text{1\% }}} _{1{\text{cm}}} \) at 232 and 268 nm, color, percent of oil uptake, and viscosity measurement can be used as oil quality parameters to indicate the degree of oil deterioration under continuous stressed frying conditions. Due to the polarity of the medium- and long-chain triacylglycerols structures and partial glycerides in palm-based MLCT oils, the total polar compounds determination may not be a suitable oil quality measurement, as it may provide wrong information on the oil deterioration level as a result of the polarity structures of the oil. Sensory evaluation on fried potato chips revealed no significant differences (P > 0.05) between RBD palm olein and palm-based MLCT oils in terms of odor, taste, crispiness, and overall acceptability of fried chips. Rancidity assessment of fried chips over 3 months of storage periods indicated stable organoleptic quality of chips fried with either RBD palm olein or palm-based MLCT oils with different antioxidant treatments.
References
AOCS. (1997). Official methods and recommended practices of the American Oil Chemist’s Society (5th ed.). Champaign, USA: American Oil Chemist’s Society Press.
Augustin, M. A., Lee, K. H., & Yan, K. T. (1987). Comparison of the frying performance of market samples of palm olein, corn oil and soya oil in Malaysia. Pertanika, 10, 295–304.
Banks, D. (1996). Food service frying. In E. G. Perkins, & M. D. Erickson (Eds.), Deep frying (pp. 245–257, 2nd ed.). Champaign, USA: American Oil Chemist’s Society Press.
Blumenthal, M. M. (1991). A new look at the chemistry and physics of deep-fat frying. Food Technologist, 45, 68–74.
Bouchon, P., Aguilera, J. M., & Pyle, D. L. (2003). Structured oil-absorption relationships during deep-fat frying. Journal of Food Science, 68, 2711–2716. doi:10.1111/j.1365-2621.2003.tb05793.x.
Che Man, Y. B., & Tan, C. P. (1999). Effects of natural and synthetic antioxidants on changes in refined, bleached, and deodorized palm olein deep-fat frying of potato chips. Journal of the American Oil Chemists’ Society, 76, 331–339.
Dana, D., & Saguy, I. S. (2006). Mechanism of oil uptake during deep-fat frying and the surfactant effect-theory and myth. Advances in Colloid and Interface Science, 128–130, 267–272. doi:10.1016/j.cis.2006.11.013.
Firestone, D. (1993). Worldwide regulation of frying fats and oils. Informatik, 4, 1366–1371.
Gertz, C. (2004). Optimising the baking and frying process using oil-improving agents. Eur J Lipid Sci Technol., 106, 736–745. doi:10.1002/ejlt.200401015.
Gertz, C., Klostermann, S., & Kochhar, S. P. (2000). Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. European Journal of Lipid Science and Technology, 102, 543–551. doi:10.1002/1438-9312(200009)102:8/9<543::AID-EJLT543>3.0.CO;2-V.
Gray, J. I. (1978). Measurement of lipid oxidation—A review. Journal of the American Oil Chemists’ Society, 55, 539–546.
Hein, M., Henning, H., & Isengard, H. (1998). Determination of total polar parts with new methods for the quality survey of frying fats and oils. Talanta, 47, 447–454. doi:10.1016/S0039-9140(98)00148-9.
Kasai, M., Nosaka, N., Maki, H., Negishi, S., Aoyama, T., Nakamura, M., et al. (2003). Effect of dietary medium- and long-chain triacylglycerols (MLCT) on accumulation of body fat in healthy humans. Asia Pacific Journal Of Clinical Nutrition, 12, 151–160.
Kochhar, S. P., & Gertz, C. (2004). New theoretical and practical aspects of the frying process. European Journal of Lipid Science and Technology, 106, 722–727. doi:10.1002/ejlt.200400996.
Koh, S. P., Tan, C. P., Lai, O. M., Arifin, N., Yusoff, M. S. A., & Long, K. (2008). Enzymatic synthesis of medium- and long-chain triacylglycerols (MLCT): Optimization of process parameter using response surface methodology. Food Bioprocess Technology. doi:10.1007/s11947-008-0073-y.
Makhoul, H., Ghaddar, T., & Toufeili, I. (2006). Identification of some rancidity measures at the end of the shelf life of sunflower. European Journal of Lipid Science and Technology, 108, 143–148. doi:10.1002/ejlt.200500262.
Matsuo, T., & Takeuchi, H. (2004). Effects of structured medium- and long-chain triacylglycerols in diets with various levels of fat on body fat accumulation in rats. British Journal of Nutrition, 91, 219–125. doi:10.1079/BJN20031041.
Matsuo, T., Matsuo, M., Taguchi, N., & Takeuchi, H. (2001). The thermic effect is greater for structured medium- and long-chain triacylglycerols versus long-chain triacylglycerols in healthy young women. Metabolism, 50, 125–130.
Matthäus, B. (2006). Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. European Journal of Lipid Science and Technology, 108, 200–211. doi:10.1002/ejlt.200500249.
Mehta, U., & Swinburn, b. (2001). A review of factors affecting fat absorption in hot chips. Critical Reviews in Food Science and Nutrition, 14, 133–154. doi:10.1080/20014091091788.
Mellema, M. (2003). Mechanism and reduction of fat uptake in deep-fat fried foods. Trends in Food Science & Technology, 14, 364–373. doi:10.1016/S0924-2244(03)00050-5.
Melton, S. L. (1996). Sensory evaluation of frying fat and deep-fried products. In E. G. Perkins, & M. D. Erickson (Eds.), Deep frying (pp. 311–322, 2nd ed.). Champaign, USA: American Oil Chemist’s Society Press.
Moyano, P. C., & Pedreschi, F. (2006). Kinetics of oil uptake during frying of potato slices: Effect of pretreatments. LWT, 39, 285–291. doi:10.1016/j.lwt.2005.01.010.
Negishi, S., Itakura, M., Arimoto, S., Nagasawa, T., & Tsuchiya, K. (2003). Measurement of foaming of frying oil and effect of the composition of TG on foaming. Journal of the American Oil Chemists’ Society, 80, 471–474.
Pantzaris, T. P. (1997). Criteria for comparing frying oils. In S. P. Kochhar (Ed.), New developments in industrial frying (pp. 73–90). UK: PJ Barnes & Associates.
Peryam, D. R., & Pilgrim, F. J. (1957). Hedonic scale method of measuring food preferences. Food Technologist, 11, 9–14.
Test Methods PORIM. (1995) Palm Oil Research Institute of Malaysia, Ministry of Primary Industries, Malaysia.
Sanibal, E. A. A., & Mancini-Filho, J. (2004). Frying oil and fat quality measured by chemical, physical and test kit analyses. Journal of the American Oil Chemists’ Society, 81, 847–852.
Shahidi, F., & Wanasundara, U. N. (2002). Methods for measuring oxidative rancidity in fats and oils. In C. C. Akoh, & D. B. Min (Eds.), Food lipids (pp. 465–487). New York: Marcel Dekker.
Shimizu, M., Moriwaki, J., Nishide, T., & Nakajima, Y. (2004). Thermal deterioration of diacylglycerol and triacylglycerol oils during deep-frying. Journal of the American Oil Chemists’ Society, 81, 571–576.
Stier, R. F. (2004a). Frying as a science—An introduction. European Journal of Lipid Science and Technology, 106, 715–721. doi:10.1002/ejlt.200401065.
Stier, R. F. (2004b). Tests to monitor quality of deep-frying fats and oils. European Journal of Lipid Science and Technology, 106, 766–771. doi:10.1002/ejlt.200401049.
Verleyen, T., Van Dyck, S., & Adams, C. A. (2004). Use of accelerated oxidation tests to evaluate antioxidant activity. Lipid Technology, 16, 39–41.
Villière, A., Rousseau, F., Brossard, C., & Genot, C. (2007). Sensory evaluation of the odour of a sunflower oil emission throughout oxidation. European Journal of Lipid Science and Technology, 109, 38–48. doi:10.1002/ejlt.200600084.
Warner, K. (2002). Chemistry of frying oils. In C. C. Akoh, & D. B. Min (Eds.),Food lipids (pp. 205–221). New York: Marcel Dekker.
Acknowledgments
The authors gratefully acknowledge the R&D staff from Golden Jomalina Food Industries Sdn. Bhd., Selangor, Malaysia for their kind technical assistance. They would like to thank Golden Hope Research Sdn. Bhd. for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koh, S.P., Arifin, N., Tan, C.P. et al. Deep Frying Performance of Enzymatically Synthesized Palm-Based Medium- and Long-Chain Triacylglycerols (MLCT) Oil Blends. Food Bioprocess Technol 4, 124–135 (2011). https://doi.org/10.1007/s11947-008-0138-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0138-y