Abstract
This study aimed to investigate the suitability of refined bleached deodorized palm olein oil (RBD POO) and Macadamia integrifolia oil (MO) blend as deep-fat frying substitute. Oxidative and hydrolytic stability of MO, POO and blends during 15 days of storage under accelerated oxidation condition (65 °C ± 1) studied by assessing free fatty acids (FFA), peroxide (PV), anisidine (AV) and TOTOX (TV) values. Blends formulated with POO: MO at 100:0, 75:25, 50:50, 25:75 and 0:100. Blending significantly affected the fatty acid profile, smoke point, FFA, PV, AV and TV of all samples (p < 0.05). Both independent variables (storage time and type of oil) and their interaction had significant effect on FFA, PV, AV and TV (p < 0.05). On day 15, the highest and the lowest FFA observed for MO and POO, respectively. The significant difference between FFA of MO and POO before incubation and day 15th was due to role of refining process in elimination of initial FFAs from POO. Results of PV, AV and TV showed that the highest and least changes were presented by POO and MO, respectively. Oil blends containing higher proportions of MO with a great percentage of monounsaturated and less polyunsaturated fats were more stable against oxidation. Also, presence of antioxidants played significant role against MO oxidation. From this research, blending POO with MO improved induction period of the blends and inhibited primary and secondary oxidation products formation. Blend 25:75 met the qualitative and nutritional criteria and suggested for formulation of a functional oxidative stable frying medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deep-fat frying is the immersion frying of the foods in edible oils at high temperature (150–200 °C) and under atmospheric pressure through which the simultaneous heat and mass transfer leads to the desirable taste and quality of fried foods (Yamsaengsung and Moreira 2002). During frying, variety of chemical reactions as oxidation, polymerization, hydrolysis, isomerization, and cyclization takes place. These reactions would result in the formation of insoluble, nonvolatile substances which increase the viscosity, darkening of the color, develop the foaming and decrease the smoke point of the oil (Kalogeropoulos et al. 2007). The volatile products such as aldehydes are vaporized and the non-volatile fraction remains in the frying oil and is absorbed by the food. The products of these reactions have been concerned with negative health effects, and as they destroy vitamins, prohibit enzymes and could cause mutations or gastrointestinal irritations (Clark and Serbia 1991). Therefore, the oxidative stability and nutritional quality are the main parameters which affect the frying quality and consumer satisfaction. To meet current market desire, frying oil mentioned to be low in saturated fat, not hydrogenated, low in linolenic acid and superior oxidation stable (Gupta 2005).
Palm oil can be fractionated into two fractions referred to as “olein” which is a low melting liquid and a high melting solid ‘stearin’. Palm olein oil (POO) is produced plentifully and has been particularly used as frying and cooking medium in tropical countries. POO has been known as a primary frying medium regarding its low polyunsaturation and the slip melting point which prevents this oil from immoderate waxiness. Although POO is less saturated than palm oil, it still consists of about 38% of palmitic acid (Enríquez-Fernández et al. 2011). In 2003, the world health organization (WHO) disclosed convincing evidence showing that myristic and palmitic acids could increase the risk of cardiovascular diseases (WHO 2003). In 2008, WHO introduced three important parameters for the nutritional assessment of edible oils including the ratio of saturated, mono- and poly-unsaturated fatty acids, the content of essential fatty acids and presence of antioxidants (WHO 2008). Further, application of palm oil with high content of saturated fats (SFAs) was associated with raising the total blood cholesterol level, increasing the ratio of LDL-C/HDL-C and development of cardiovascular disease, obesity, the metabolic syndrome, type 2 diabetes and cancer (Pedersen 2011). However, unlike SFAs, the diets rich in monounsaturated fats (MUFAs) mentioned to have defensive effects against cardiac disease by lowering the levels of LDL without adverse effects on the HDL levels (Pedersen 2011; Kris-Etherton et al. 1999).
Because of the lack of ideal oils which may satisfy both the frying stability and nutritional requirements, blending of fats and oil is considered as a cost-effective and a nondestructive technique through which new oils can be developed by changing the fatty acids composition, increasing the nutritional, physicochemical, organoleptic characteristics and oxidative stability (Choudhary et al. 2015). Prescha et al. (2014) introduced some cold pressed specialty oils as rich sources of bioactive lipids with strong radical scavenging properties and health-promoting. In addition, Choudhary et al. (2015) referred to the blending of specialty oils with conventional oils as a novel practice with different advantages including the oxidation stability enhancement. Among the new sources of specialty edible oils, macadamia oil is of interest and may play a major role in human health and nutrition. Macadamia oil (MO) from Macadamia integrifolia nut (with 59–78% w/w oil) is cultivated in Australia and the United States. It has a comparably high smoke point (198 °C) with a high content of oleic and palmitoleic acids. The Rancimat method applied to measure the oxidative stability of several varieties of MO suggested the induction periods of 3.6 h up to 19.8 h (Wall 2010). In addition, macadamia oil with low amounts of polyunsaturated fatty acids (PUFAs) is a non-drying oil (Iodine Index < 100) (Rengel et al. 2015). The MO has a great oxidative stability regarding its special fatty acid composition and the presence of valuable fat-soluble bioactives that are beneficial to human health. These compounds may protect the oil from oxidation degradation during storage and thus extend its shelf life (Wall 2010). The MO is referred to as the most highly monounsaturated fat (MUFA) oil available which possibly may help lowering blood cholesterol and to reduce the risk of heart disease (Ako et al. 1995). Decreasing the plasma LDL cholesterol and risk of cardiovascular disease were also attributed to macadamia oil due to its high content of MUFA and bioactive compounds (Garg et al. 2003; Kris-Etherton et al. 1999). Rengel et al. (2015) reported the existence of squalene in macadamia nut oil of four different cultivars. Squalene has been demonstrated as a hypo-cholesterolemic triterpene and a substrate for sterols to which antioxidant and cardioprotective properties have been associated (Sabeena et al. 2004). The most abundant phytosterol in MO reported to be β-Sitosterol (1506.7 μg/g) and the average level of squalene indicated to be 185.0 μg/g (Maguire et al. 2004). Wall (2010) detected significant amounts (30–92 μg/g oil) of tocotrienol homologs (δ-, γ-, α-T3) and squalene (72–171 μg/g oil) in MO, and stated that these phytochemicals may have antioxidant, anticancer, cholesterol-lowering properties to the consumers and also may protect the oil from oxidation rancidity. In comparison with olive oil, high-oleate safflower oil and canola oil, the high level of MUFA in macadamia (Approx. 60%) was mainly attributed into palmitoleic acid (16:1) (17–34%) (Kaijser et al. 2000; Wall 2010). The main objective of the present study was to investigate the oxidative stability of POO/MO blends during accelerated oxidation testing and to develop a healthier deep-fat frying medium. Although blending of the polyunsaturated oils with SAFs or MUFA has been studied extensively, the influence of partial replacement of POO with MO and for the purpose of oxidative and nutritional improvements has not yet been reported. MO with a relatively high smoke point, high content of MUFA and considerable amounts of bioactive compounds supposed to be appropriate for blending with POO and to formulate a more nutritive blend for deep-fat frying applications.
Materials and methods
Oils and reagents
Macadamia cold press virgin oil was purchased from Jedwards International, Inc. (Braintree, MA). Refined, bleached and deodorized (RBD) palm olein was supplied from AAK USA Inc. (Port Newark, NJ). Fatty acid methyl esters (FAME) standard mixture and sodium methoxide (0.5 M) were purchased from Sigma-Aldrich (St Louis, MO, USA). Disposable pre-vialed analytical reagents for measurement of free fatty acids, peroxide value and Anisidine value were donated by CDR Foodlab Company.
Preparation of the oil blends
Palm olein oil (POO) with a semi-liquid creamy appearance was gently warmed up at 50 °C to obtain clear liquid oil. POO was blended with Macadamia oil (MO) in varying proportions (POO:MO; 0:100, 75:25, 50:50, 25:75, 0:100). Pure RBD POO (100%, w/w) and pure MO were considered as control samples. Each samples proportion was carefully weighed in a 200 ml beaker to reach into a total weight of 100 gr. Oil blends were thoroughly mixed at 50 °C for 3 min using a magnetic stirrer for uniform blends.
Analytical methods
Determination of fatty acid composition by gas chromatography
The fatty acid profile of the fresh samples was assessed by conversion of fatty acids into fatty acid methyl esters. 2 mL of n-hexane was added to 90 mg of the oil followed by addition of 2 mL sodium methoxide (2 M). The mixtures were vortexed for 5 s and allowed to settle in a test tube warmer (Isotherm dry bath 147, Fisher Scientific, USA) at 73 °C for 30 min. After cooling, 4 mL of deionized water was added, vortexed for 5 s and allowed to settle for phase separation. Then the organic top layer was dried over anhydrous sodium sulfate. Then dried layer was transferred into amber auto-sampler GC vials (1.5 mL) for fatty acid gas chromatography (GC) analysis. The GC (Model 2014, Shimadzu Corporation, Kyoto, Japan) was equipped with an auto-injector (AOC-20i), a flame-ionization detector and a polar capillary column (BPX70 0.25) with 0.32 mm internal diameter, 60 m length and 0.25 μm film thickness (SGE Incorporated, USA) to obtain individual peaks of fatty acid methyl esters. The detector temperature was 240 °C and the column temperature was held at 110 °C for 1 min and increased at the rate of 8 °C/min to 220 °C and held for 1 min. The runtime was 45 min and fatty acid methyl esters were identified by comparing the retention time of each peak with those of standards. The percentage corresponding to each fatty acid was measured based on the peak area of each fatty acid species to the total peak area of all fatty acids in the oil sample.
Measurement of the blends smoke points
Smoke points of fresh samples were analyzed in accordance with AOCS Official Method Cc 9a-48 (AOCS, re-approved 2017), and by using Cleveland open cup flash tester, ASTM D. 92 (Fisher Scientific, USA).
Schaal oven test
To estimate the oxidation rate of the blends during heating, an accelerated Schaal oven test was conducted by incubation of samples at 65 ± 1 °C (Gomes et al. 2010). Oil blends were filled up to the volume in six similar open glass beakers and stored in an oven with enforced–air circulation at 65 °C to distribute the heat evenly and in absence of the light. The peroxide value (PV), p-anisidine value (AV) and free fatty acids (FFA) content of the samples were measured once before the incubation (day 0) and over the incubation period (days 3, 6, 9, 12 and 15).
Free fatty acids (FFA), peroxide value (PV) and p-anisidine value (AV)
Determination of FFA, PV, and AV of the samples was performed following CDR FOODLAB rapid testing procedures. The mentioned parameters are indicators of the state of oil deterioration. CDR FOODLAB apparatus (Model SLB222, Florence, Italy) and the related analytical kits provide accelerated conditions for precise quality control of the oils. The accuracy of CDR methods has been already validated by obtaining a high correlation between individual curves from CDR method and the results from respective American Oil Chemists’ Society (AOCS) official methods (i.e., Ca 5a-40, Cd 8b-90 and Cd 18–90) (R2 > 0.99).
TOTOX value (TV)
Measurement of TOTOX value has been performed extensively to estimate the oxidative rancidity of fats and oils. A combined determination of primary oxidation products through PV and the secondary oxidation compounds via measurement of AV is commonly expressed as TOTOX. TV is giving a value of the total oxidation status in oil and is equal to TV = 2PV + AV. The reason for the duplication of PV is that PV has a more obvious effect on the oil stability than the AV. TOTOX value is an empirical parameter and corresponds to the addition of two parameters with different dimensions where an increase in one PV unit corresponds to increase in two AV units: TV = 2PV + AV (Shahidi and Wanasundara 2002).
Statistical analysis
All measurements (GC, Smoke point, FFA, PV, AV, TV) were replicated three times to improve the reliability of the results and result reported as the mean ± SD. Data analysis conducted using Minitab software (Release 14; Minitab Inc., State College, PA, USA). Significant differences were established for a p value of < 0.05. The two-way ANOVA was performed to determine the significance of the effects of two main independent variables (storage time and oil type) and their interaction on oxidation parameters (PV, AV, TOTOX, and FFA). One-way ANOVA and Tukey’s pair-wise were done to find the significance of the differences between the independent variables’ levels. Where the absolute F ratio became larger and the p value was smaller, the effect of corresponding variables was regarded as more significant (p < 0.05) (Montgomery 2001).
Results and discussion
Fatty acid profile of samples
Fatty acid profile of palm olein oil (POO), Macadamia oil (MO) and their blends are presented in Table 1.
The GC profile of POO and MO was consistent with previous reports (Rengel et al. 2015; Maguire et al. 2004). Palmitic acid (C16:0) was the predominant saturated fatty acid (SFA) in all samples. According to Table 1, POO (100%) included the highest amount of SFAs (C16:0 and C18:0) and linoleic acid (C18:2) together with the lowest content of MUFAs (C16:1 and C18:1). Unlikely, MO (100%) presented the highest and the lowest percentages of MUFAs and SFAs, respectively (p < 0.05). Partial replacement of POO with MO (POO: MO; 75:25, 50:50, 25:75) lead to increasing in MUFAs as well as decreasing SFAs and linoleic acid. In agreement with earlier reports, the total amount of PUFAs (18:2 and 18:3) in MO (100%) was relatively lower than that of other samples (Table 1) (Rengel et al. 2015). POO and MO displayed the lowest and the highest MUFAs/SFAs ratios, respectively. Increasing the ratio of MO in blends resulted in a significant decrease in total SFAs and the increase of MUFAs/SFAs ratio (p < 0.05). The results of GC confirmed Mariod et al. (2005) statements who mentioned that blending may significantly change the fatty acid composition of the oils. In the present research, the ratio of MUFAs/SAFs which was 0.97 in POO before blending increased to 1.92 and 2.81 for 50:50 and 25:75 (POO: MO) blends, respectively. Based on WHO (2008), the oils with MUFAs/SFAs > 1.5 regarded as healthy. Further, the 25:75 blend met the highest ratio of MUFA/SAFs (2.81) compared to other blends (p < 0.05).
The smoke point of the oils and their binary blends
The smoke point mentioned being the temperature ultimate at which the oil can still be used and regarded as a suitable parameter for evaluation of the oils’ heat tolerance (Sarwar et al. 2016). At this temperature, the oil starts to burn and begins breaking down into free fatty acids and glycerol. Further, toxic acrolein may result from cleavage of glycerol and this point also introduced as the sign for both flavor and nutritional degradation (Gunstone 2011). Smoke points of the oil samples (POO:MO) of 100:0, 75:25, 50:50, 25:75 and 0:100 were 239 ± 3, 230 ± 2, 224 ± 2, 221 ± 2 and 212 ± 2 °C, respectively. As can be observed, by increasing the ratio of MO in blends the smoke point of the blends decreased in a descending order. The smoke point of refined POO was the highest compared to the rest. However, the lowest smoke point observed for cold press virgin MO with the highest initial FFA (p < 0.05). One reason could be the role of the refining process in the removal of FFA and other impurities from initial POO which mentioned to promote the oil to smoke. These results were in agreement with previous reports which correlated the smoke point into the presence of minor components and especially to FFAs with a direct effect on smoke point reduction (Gunstone 2011). However, Arens et al. (1977) declared that although the relevance of FFA content was more pronounced in decreasing the smoke point than the composition of fatty acids, the increase of unsaturated fatty acids in oils result in the decrease of smoke and the flashpoints. In accordance with Arens et al. (1977), this research also confirmed the relationship between fatty acid composition and smoke points of the samples. POO with the highest level of SFAs (Table 1) showed the highest smoke point and MO with the lowest level of SFAs presented the lowest smoke point among other samples (p < 0.05). Thus, another interpretation could be the oils rich in SFAs, offer a higher temperature and oxidation stability than those with high contents of unsaturated fatty acids. The smoke points of the POO, MO and their blends were significantly different (p < 0.05) except for 50:50 and 25:75 (p > 0.05). All samples including 25:75 blend showed smoke points above 200 °C and met the standard regulation limit for suitable frying oils (> 200 °C) (Gunstone 2011). Sarwar et al. (2016) stated that although the smoke point declines after each frying cycle due to the cleavage of FFA, a suitable frying medium needs to be still applicable during a number of frying cycles. They suggested the smoke point of used frying oils must be at least 170 °C and not different from the temperature of the fresh fat by more than 50 °C so that it can still be usable (Sarwar et al. 2016).
Free fatty acids content
Free fatty acids (FFA) are forming during hydrolysis through the cleavage of triacylglycerols. Thus, the measurement of FFA level is an important indicator of oils’ hydrolytic deterioration. This type of rancidity which occurs in the presence of moisture, hydrolytic enzymes, and metal ion may result in off-flavor of fats and oils (Tan 1994). Therefore, evaluation of FFA is one of the important tests to determine the life of frying oil. Reports indicated that higher concentrations of FFA usually limit the shelf life of the oils (Nadeem et al. 2015). It was mentioned that FFA affects the smoke point and thus, oils with higher FFA content are known to have lower smoke points (Gunstone 2011). Some reports referred to the surfactant nature and foaming effect of FFA that may affect the oil oxidation (Kittipongpittaya et al. 2014). In addition, FFA was known to be effective in increasing the risk of coronary heart disease through raising the cholesterol levels (Ascherio and Willett 1997).
Table 2 is indicating the significance of each variable’s effect and their interaction on FFA, PV, AV, and TV. As shown, there were significant differences between the FFA of samples in terms of the type of oil, storage time and their interaction effects (p < 0.05). The results indicated that FFA was significantly influenced by the independent variable and their interactions (p < 0.05). Type of frying oil and further the storage time displayed greater significant effects (p < 0.05) on FFA and due to their magnitude of F-ratios compared to their interaction effect (Montgomery 2001) (Table 2).
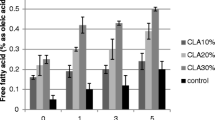
Figure 1 is showing the rates of FFA changes during 15 days of incubation at 65 °C for POO and MO and their blends (POO: MO, 100:0,75:25, 50:50, 25:75 and 100:0). The initial FFA value of cold press virgin MO (0.35% ± 0.01) was significantly higher than that of POO (0.04% ± 0.01) due to its unrefined condition. As could be seen, there were significant increases between FFA values from day 0 (before incubation) and day 15 for all samples (p < 0.05) (Fig. 1). These results were consistent with earlier literature which declared that FFA of vegetable oils increases during storage time (Nadeem et al. 2015). Regarding the slope of each curve, the least and the greatest changes in FFA values were correlated with POO (100:0) and MO (0:100), respectively.
Since FFA value was referred to as an indicator of hydrolysis in fats, the increase of FFA during storage time could be mainly attributed to the degree of hydrolysis in the oils. However, Tan (1994) expressed that the formation of free fatty acids can also be caused by the oxidation pathways. Present results showed that % FFA of samples after 15 days of storage at 65 °C ranged from 0.09 ± 0.01% for POO (100:0) to 0.45 ± 0.01% for MO (0:100). Although the MO was indicating the greatest FFA, this value was still lower than the standard limit of FFA (≤ 4%) for cold pressed and virgin oils. The blend ratio of 25:75 with FFA at 0.42 ± 0.01% after 15 days of incubation also met the standard limit (FFA ≤ 4%) (Codex amended 2015).
Peroxide value (PV)
Determination of PV or hydroperoxides which are primary lipid oxidation products was considered as an indicator of oxidation progress during the early stages (Shahidi and Wanasundara 2002). As PV is the degree of initial oxidation, a higher PV indicated a lower chemical stability of lipids. Figure 2 is presenting the PV changes in samples over the period of 15 days of storage at 65 °C.
According to Table 2, both independent variables (storage time and type of oil) and their interaction had a significant effect on PV (p < 0.05). The time and the interaction of the type of oil and time were the most and the least significant effect, respectively. The PV of all samples was increased during 15 consecutive days of incubation (p < 0.05) (Fig. 2). Similar to FFA, PV of cold press virgin MO (1.05 ± 0.01 meq O2/kg) was greater than that of refined bleached deodorized POO (0.40 ± 0.01 meq O2/kg) (p < 0.05) caused by the role of refining process in decreasing the hydroperoxides and other impurities. In the primary stage of oxidation (day 0–6) oil blends of 50:50 and 25:75 indicated higher PVs compared to POO, 75:25 and MO. However, in later oxidation stages (day 6–15), a sharper increase observed in PV of POO (p < 0.05) (Fig. 2). It could be interpreted that POO and 75:25 with more SFAs as well as less initial PV and FFA, were more stable against oxidation in the primary stage of oxidation. However, ratios of 50:50 and 25:75 with more initial FFA showed less oxidative stability than POO and 75:25.
The present results were also consistent with Aubourg (2001) reports regarding the catalytic effect of FFAs on lipid oxidation. Aubourg (2001) indicated that the carboxylic group of FFAs promotes the hydroperoxides decomposition which is required for the initiation of the oil oxidation. The further theory explained that FFAs were surface-active compounds due to the presence of un-esterified carboxylic groups and thus, may improve the oxygen transfer into the oil (Kittipongpittaya et al. 2014). In addition, the better oxidative stability of MO compared to other samples could be attributed to its considerable amounts of anti-oxidative compounds (Rengel et al. 2015; Wall 2010; Kaijser et al. 2000).
Surprisingly, this study revealed that the high content of powerful antioxidants existed in MO had a strong effect on retarding the oxidation development and even was effective in surpassing the pro-oxidative role of FFAs. From day 6 to 15, the oxidation rate of samples presented to be directly proportional to the amount of POO in blends and was in ascending order. The samples containing higher ratios of POO with higher linolenic acid content (C18:2) and the lower percentage of MUFA exhibited the greater PVs (Fig. 2). The sharper increase in PV of POO and the blends may be due to the degree of unsaturation to some extent. This finding was in agreement with earlier results which indicated the oils with more unsaturated fatty acids were more vulnerable to oxidation (Kerrihard et al. 2015; Nadeem et al. 2015). On last day of storage (day 15), the lowest and the greatest PV was observed for MO (4.27 meq O2/Kg) and POO (29.03 meq O2/Kg), respectively (p < 0.05). Pure POO containing the highest linolenic acid amount (9.48%) and the lowest MUFA (44.1%) showed the least stability against oxidation rancidity (p < 0.05) (Table 1 and Fig. 2). However, the lowest content of linolenic acid (2.29%) together with the highest amount of MUFA (75.39%) in MO explains why the level of peroxides in this oil was quite low (Kaijser et al. 2000). In conjunction with the special fatty acid composition of MO (high MUFA and low PUFA), the minor compounds in MO displayed an important role on the overall oxidative stability of the MO containing samples. Tocotrienols (T3) and squalene may contributed to oxidative stability of MO. Thus, the blends formulated with more MO and less POO showed lower PVs and the higher stabilities within day 6–15 (Rengel et al. 2015; Wall 2010 and Maguire et al. 2004). The oxidative stability of the samples in term of PVs (meq O2/Kg) on last day of storage (day 15) was as the followings (p < 0.05):
On day 15th the PVs of all samples except for MO were above the rancidity limit of 10 (Gunstone 2008). However, the PV of 25:75 in day 9th (9.47 ± 0.03) met the criteria mentioned by Gunstone (2008). This study showed blending POO with MO resulted in improvement of oxidative stability of the binary blends due to the antioxidative effect of minor compounds presented in MO. Although these compounds were not able to eliminate the entire oxidation reactions, they had an important role on the enhancement of the oil shelf life by extension of the induction period. Our results were in accordance with previous literature which mentioned the great values of fat-soluble bioactive compounds in MO may protect it from oxidation deterioration (Rengel et al. 2015; Wall 2010; Maguire et al. 2004; Kaijser et al. 2000). Oxidative results also agreed with Redondo-Cuevas et al. (2018) statements. They explained although the effect of type of fatty acid on oxidative stability of oils and fats is considerable, their role was to a lesser extent than that was mentioned by Kerrihard et al. (2015). Redondo-Cuevas et al. (2018) referred to minor bioactive compounds presented in unrefined oils with great impacts on oxidative stability. Besides the anti-oxidative function, phytochemicals with radicals scavenging effect displayed significant roles in the prevention of cancer, inflammation and cardiovascular diseases.
p-anisidine value (AV)
Although PV is a useful biomarker to show the rate of oxidation in initial stages at the room temperature, determination of PV alone is not a sufficient indicator to estimate the extent of the oils stability of at high temperatures (Shahidi and Wanasundara 2002). At high temperatures, hydroperoxides decompose and form aldehydes which are the secondary oxidation products and include almost 50% of volatile compounds (Przybylski and Eskin 1995). Unsaturated aldehydes are responsible for the off-flavors of oils regarding their low sensory threshold values and volatility (Shahidi and Wanasundara 2002; Przybylski and Eskin 1995). Since aldehydes are more heat-stable than the hydroperoxides, the p-anisidine value (AV) considered as the most reliable test for estimation of advanced oxidative rancidity in oils. Based on Table 2, both individual variables (the type of oil and storage time) and their interaction affected the AV (p < 0.05). However, type of the oil had the most significant effect followed by the storage time. The changes in the AV of samples during storage are shown in Fig. 3. The lowest initial AV (day 0) was observed for MO (1.14); while the RBD POO (2.53) showed the highest initial AV (p < 0.05). This indicated that although the refining process was effective to reduce the FFAs and hydroproxides in POO, this process was not sufficient to remove the secondary oxidation compounds.
During the incubation and due to the oxidation, AV of all samples increased from day 0 to day 15 (p < 0.05). From day 0 to day 15, the AV of POO increased from 2.53 to 3.82; while the AV of MO and blends (POO: MO, 25:75, 50:50 and 75:25) increased from 1.14 to 1.37, 1.34 to 2.17, 2.10 to 2.7 and 2.21 to 3.21, respectively. On day 15th, POO (100%) and MO (100%) indicated the highest and lowest AVs, respectively. Samples with higher amounts of POO showed less oxidative stability (greater AV) compared to the other blends. Indeed, the increase in PUFA content (C18:2) and/or decrease in MUFA (C18:1 and C16:1) led to increasing of the AV in the oil blends. This finding was in conformity with Kaijser et al. (2000) who indicated the rate of oxidation is proportional to the degree of unsaturation. They mentioned that the oxidation rate of C18:2 and C18:3 with more double bonds would be 12 and 25 times greater than C18:1. Although POO was containing the highest amount of SAFs (Table 1), samples with more POO contained higher amounts of linoleic acid (C18:2), and thus they were more sensitive to oxidation compared to others. This result offered that the oxidative stability of the oils during storage depends not only on the SFAs quantity but also on amount mono- and polyunsaturated fatty acids. 25:75 blend containing 25% (w/w) POO and 75% (w/w) MO showed the lowest AV and the highest oxidative stability as compared to the other blends. This could be attributed to the higher proportion of MO including more MUFA instead of PUFA together with the presence of great values of natural antioxidants with the protective role against the development of oxidation secondary by-products formation (Rengel et al. 2015; Wall 2010 and Maguire et al. 2004).
TOTOX value (TV)
Determination of AV is used together with PV to assess the oxidative rancidity (Shahidi and Wanasundara 2002). The TV is a parameter showing the sum of primary and secondary products of oxidation and thus, indicates both the oxidation history and potentials of oils for further rancidity. The TV measured and changes are indicated in Fig. 4.
Based on Table 2 and similar to PV, TVs of the samples were significantly affected by the individual factors (the type of oil and storage time) and their interaction effects (p < 0.05). The variables with the most and the least effect on TV were the time and the interaction of time with the oil type, respectively. TV of all treatments increased during the incubation process (p < 0.05). On day 15th of storage, TVs of samples (POO: MO) at ratios of 100:0, 75:25, 50:50, 25:75 and 0:100 increased to 61.88, 48.30, 45.65, 36.96 and 9.91, respectively. Therefore, POO and MO displayed the lowest and the highest oxidative stability. The magnitude of TV decreased with increasing MO content; whereas increasing the amount of POO in samples resulted in development of TV. Due to the higher oxidative stability of MUFAs and natural antioxidants presented in MO, increasing the MO content in blends improved the oxidative stability and thus, TV be decreased. Conversely, the higher the content of POO with more PUFAs led to a decrease in the oils’ stability and an increase of TV. The present study also validated the previous statements of Mariod et al. (2005) who demonstrated that blending of a high linoleic acid oil with a rich MUFA oil may result in enhancement of oxidative stability of the blend compared to the original linoleic oil.
Conclusion
Addition of the Macadamia integrifolia oil into palm olein improved the oxidative stability of the binary blends during 15-days of storage at 65 °C. The blend containing 75% Macadamia oil and 25% palm olein indicated the superior stability compared to pure palm olein and the other blends and met the standard smoke point limit of the frying oils. The great oxidative strength of 75% Macadamia oil blend together with its health promoting effects was due to the special and high level of mono-unsaturated fatty acids with the cardiovascular protective function and the presence of bioactive compounds (squalene and T3) in Macadamia oil. This study also reconfirmed that the stability of the oils against rancidity depends not only on saturated fatty acid content but also on the degree of the unsaturation and the amount of natural antioxidant. Although the market price of Macadamia oil blended with palm olein (75:25) could be higher than that of pure palm olein, including the Macadamia oil in the blend formulation would result in increasing the frying shelf-life of the final blend. Therefore, the frying process cost could be reduced by decreasing the number of the frying oil replenishment during the frying cycles.
References
Ako H, Okuda D, Gray D (1995) Healthful new oil from Macadamia nuts. Nutrition 11:286–288
American Oil Chemists’ Society (2017) AOCS Official method Cc 9a-48, smoke, flash and fire points cleveland open cup method. Official methods and recommended practices of the AOCS, 7th edn. Champaign ILL, American Oil Chemists’ Society
Arens VM, Ghur G, Waibel J (1977) Bestimmung des rauchpunktes zur beurteilung von bratund seidefetten. Fette Seifen Anstrichmittel 79:256–261
Ascherio A, Willett W (1997) Health effects of trans fatty acids. Am J Clin Nutr 66(supplement):1006S–1010S
Aubourg SP (2001) Fluorescence study of the pro-oxidant effect of free fatty acids on marine lipids. J Sci Food Agr 81:385–390
Choudhary M, Grover K, Kaur G (2015) Development of rice bran oil blends for quality improvement. Food Chem 173:770–777
Clark LW, Serbia GW (1991) Safety aspects of frying fats and oils. Food Technol 45:84–94
Codex Stan (2015) Codex alimentarius international food standards. Standard for amendment vegetable oils. CODEX STAN 210-1999, pp 1–16
Enríquez-Fernández BE, y Yañez LDLC, Sosa-Morales ME (2011) Comparison of the stability of palm olein and a palm olein/canola oil blend during deep-fat frying of chicken nuggets and French fries. Int J Food Sci Tech 46:1231–1237
Garg ML, Blake RJ, Wills RBH (2003) Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr 133:1060–1063
Gomes T, Caponio F, Bruno G et al (2010) Effects of monoacylglycerols on the oxidative stability of olive oil. J Sci Food Agric 90:2228–2232
Gunstone FD (2008) Oils and fats in the food industry (Chapter 8). Wiley-Blackwell, Hoboken
Gunstone FD (2011) Production and trade of vegetable oils. In: Gunstone FD (ed) Vegetable oils in food technology: composition, properties and uses, 2nd edn. Wiley, Hoboken
Gupta MK (2005) Frying oils. In: Shahidi F (ed) Bailey’s industrial oil and fat products, vol 4, 6th edn. Wiley, Hoboken
Kaijser A, Dutta P, Savage G (2000) Oxidative stability and lipid composition of macadamia nuts grown in New Zealand. Food Chem 71:67–70
Kalogeropoulos N, Salta FN, Chiou A et al (2007) Formation and distribution of oxidized fatty acids during deep- and pan-frying of potatoes. Eur J Lipid Sci Technol 109:1111–1123
Kerrihard AL, Nagy K, Craft BD et al (2015) Oxidative stability of commodity fats and oils: modeling based on fatty acid composition. J Am Oil Chem Soc 92:1153–1163
Kittipongpittaya K, Panya A, McClements DJ et al (2014) Impact of free fatty acids and phospholipids on reverse micelles formation and lipid oxidation in bulk oil. J Am Oil Chem Soc 91:453–462
Kris-Etherton PM, Pearson TA, Wan Y et al (1999) High monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am J Clin Nutr 70:1009–1015
Maguire LS, O’Sullivan SM, Galvin K et al (2004) Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the Macadamia nut. Int J Food Sci Nutr 55:171–178
Mariod A, Matthaus B, Eichner K et al (2005) Improving the oxidative stability of sunflower oil by blending with Sclerocarya birrea oil and Aspongopus viduatus oils. J Food Lipids 12:150–158
Montgomery DC (2001) Design and analysis of experiments. Wiley, New York, pp 455–492
Nadeem M, Waqar Azeem M, Rahman F (2015) Assessment of transesterified palm olein and Moringa oleifera oil blends as vanaspati substitutes. J Food Sci Technol 52:2408–2414
Pedersen JI (2011) Health aspects of saturated fatty acids. In: Talbot G (ed) Reducing saturated fats in foods, vol 221. Woodhead Publishing series in food science, technology and nutrition. Woodhead Publishing, Cambridge, pp 77–97
Prescha A, Grajzer M, Dedyk M et al (2014) The antioxidant activity and oxidative stability of cold-pressed oils. J Am Oil Chem Soc 91:1291–1301
Przybylski R, Eskin NAM (1995) Measurement and significance of volatile compounds. In: Warner K, Eskin NAM (eds) Methods to assess quality and stability of oils and fat-containing foods. AOCS Press, Champaign, pp 107–133
Redondo-Cuevas L, Castellano G, Torrens F et al (2018) Revealing the relationship between vegetable oil composition and oxidative stability: a multifactorial approach. J Food Compos Anal 66:221–229
Rengel A, Pérez E, Piombo G et al (2015) Lipid profile and antioxidant activity of macadamia nuts (Macadamia integrifolia) cultivated in venezuela. Nat Sci 7:535–547
Sabeena F, Anandan R, Senthil K et al (2004) Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmaco Res 50:231–236
Sarwar A, Vunguturi SH, Ferdose A (2016) A study on smoke point and peroxide values of different widely used edible oils. Int Engine Tech Sci Res 5:271–273
Shahidi F, Wanasundara UN (2002) Methods for measuring oxidative rancidity in fats and oils. In: Akoh CC, Min DB (eds) Food lipids: chemistry, nutrition and biotechnology, 2nd edn. Marcel Dekker, New York, pp 465–482
Tan YA (1994) Analytical techniques in palm oil and palm kernel oil specifications. In: Selected readings on palm oil and its uses, technical committee of 1994 palm oil familiarization program, palm oil research institute of Malaysia, Kuala Lumpur, Malaysia, pp 78–90
Wall MM (2010) Functional lipid characteristics, oxidative stability, and antioxidant activity of macadamia nut (Macadamia integrifolia) cultivars. Food Chem 121:1103–1108
WHO (2003) Diet, nutrition and the prevention of chronic diseases. Technical report series 916 Geneva, pp 1–104
WHO (2008) Interim summary of conclusions and dietary recommendations on total fat and fatty acids. In: The joint FAO/WHO expert consultation on fats and fatty acids in human nutrition, Geneva, WHO TRS 916
Yamsaengsung R, Moreira R (2002) Modeling the transport phenomena and structural changes during deep fat frying. J Food Eng 53:11–25
Acknowledgements
This study was supported by the Process Engineering R&D Center (PERDC), Fats and Oils Program, Texas A&M University, College Station, TX, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koohikamali, S., Alam, M.S. Improvement in nutritional quality and thermal stability of palm olein blended with macadamia oil for deep-fat frying application. J Food Sci Technol 56, 5063–5073 (2019). https://doi.org/10.1007/s13197-019-03979-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03979-0