Abstract
Purpose of review
To provide an updated summary on the diagnosis and treatment of patients with malignant cerebral edema after ischemic stroke.
Recent findings
The risk of malignant middle cerebral artery (MCA) stroke is highest in young patients with large vessel occlusion and unsuccessful revascularization. Several scores are available for risk stratification. Treatment includes supportive care, close neurologic monitoring, and hyperosmolar therapy. Yet, the main therapeutic decision is whether to proceed with decompressive craniectomy. Multiple randomized clinical trials and several meta-analyses have demonstrated that decompressive hemicraniectomy is the single most important intervention associated with survival. Survivors may face severe disability regardless of surgical treatment, and the definition of acceptable outcome in this context remains elusive.
Summary
Malignant MCA infarcts are life-threatening and invariably cause disability, most often severe. Neurologic deterioration requires airway management and hyperosmolar therapy. Decompressive hemicraniectomy is a lifesaving procedure; approximately 50% of surgically treated patients younger than 60 years can regain independent ambulation, and one nearly in five may become functionally independent at 1 year. Older patients face a much worse functional prognosis; surgical decisions in these patients should be assessed case by case.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Case
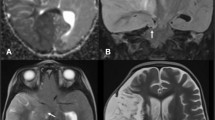
A patient in the fourth decade of life with no significant medical history was found down with global aphasia, right-sided hemiplegia, and homonymous hemianopsia. The patient had been seen normally 36 h prior. Initial non-contrast computed tomography (CT) revealed an established left middle cerebral artery territory infarction (Fig. 1B–C). The left internal carotid (ICA) and middle cerebral arteries (MCA) were occluded, as evidenced by the hyperdense arteries (arrows in Fig. 1A). A CT angiogram revealed an ICA dissection with thrombus propagation into the ipsilateral MCA (image not shown). Over the next 24 h, the patient developed progressive somnolence. A repeat CT head showed an evolving MCA infarct with increasing mass effect over the ipsilateral ventricular system (Fig. 1D–F). The following day, the patient’s level of consciousness further deteriorated, requiring sustained noxious stimulation to achieve eye-opening. A new CT head (Fig. 1G–I) showed increased cerebral edema and worsening mass effect, with a rightward midline shift of 11 mm. This was associated with marked compression of the left lateral ventricle and third ventricle. There were signs of early hydrocephalus with mild enlargement of the right temporal horn (Arrows in Fig. 1G and H). The patient was intubated, received a bolus of 23% hypertonic saline, and was emergently taken to the operating room for decompressive hemicraniectomy with no complications. A postoperative CT head showed appropriate cerebral decompression with a slightly improved midline shift. (Fig. 2A) The next day, the patient’s mental status improved significantly. Seven days later, he was discharged to inpatient rehabilitation, and home 1 month after. On discharge, the patient required assistance for transfers and bodily needs but could stand with help for 40 s (modified Rankin scale [mRS] 4). Severe hemiparesis, aphasia, and right homonymous hemianopia persisted. The patient continued to improve with outpatient rehabilitation and underwent cranioplasty without complications (Fig. 2B). One year after surgery, the patient could walk up to 4 blocks without assistance and communicate with others despite severe expressive aphasia (mRS 2). Unfortunately, the patient developed medically refractory epilepsy with numerous hospitalizations and severe spasticity requiring regular botulinum toxin injections.
Evolution of malignant MCA infarction. A–C Initial non-contrast CT scan. Arrows in A show the hyperdense ICA and MCA signs, suggesting acute arterial occlusion. D–F Non-contrast CT scan at 24 h. G–I Non-contrast CT scan at 48 h. Arrows in G and H point to the dilated temporal horn of the right lateral ventricle.
Introduction
The term malignant MCA infarction was introduced in 1996 to describe a group of patients with large hemispheric strokes who develop progressive cerebral edema leading to neurologic deterioration, brain herniation, and death [1]. This life-threatening complication affects about 10% of patients with hemispheric strokes within 5 days of presentation, and it is associated with a mortality rate as high as 80% [1, 2, 3•]. The risk increases with the size of infarcted brain tissue, and it is more frequent in young patients with “full brains” and lower cerebral compliance. Several observational studies have analyzed the risk factors for the development of malignant cerebral edema, and nine randomized clinical trials examined the value of decompressive hemicraniectomy in this population. Although the treatment is multifactorial, decompressive hemicraniectomy is by far the single most important intervention to improve survival [4••]. Yet, survivors may face severe disability. Defining acceptable outcomes in this context and identifying ideal candidates for surgery are still a matter of debate.
In this manuscript, we present an updated, evidence-based approach for the evaluation and treatment of malignant MCA infarction.
Malignant middle cerebral artery strokes: natural history
Space-occupying brain edema is the leading cause of death within the first week after a stroke.
Initially, patients present with a complete middle cerebral artery syndrome, including severe hemiparesis, hemianopia, and gaze palsy [1]. The presence of aphasia depends on the affected side and hemispheric dominance; hemineglect is often present, especially in right hemispheric strokes. As edema evolves, there is a progressive decline in the level of consciousness. Although maximal brain swelling occurs 3 to 5 days after the stroke, most patients experience neurologic deterioration within 24–48 h of the ischemic event [5]. Pupillary asymmetry (ipsilateral or less often contralateral) can be seen as soon as 17 h after stroke symptom onset, and it is often reversible with anti-edema measures, at least initially [1]. Without decompressive surgery, fixed pupillary dilation follows, usually between 1 and 7 days after symptoms onset (median 3 days). Bilateral permanent pupillary dilatation occurs approximately 24 h after initial pupillary changes. Bilateral motor signs are uncommon; they occur in 12% of these individuals at some point in their clinical evolution [1]. Earlier peak of brain edema (days 2–4 vs. 3–7) seems to be associated with higher mortality [1].
This clinical evolution has a clear pathophysiological correlation, with edema formation encompassing three distinct phases. Initially, energy failure alters the function of neuronal and glial ionic pumps causing cytotoxic edema of neurons and astrocytes. This also creates an ionic gradient between the vascular and interstitial compartments that ultimately drives water from the blood and cerebral spinal fluid (CSF) into the interstitium (ionic edema). If blood flow is not restored, blood–brain barrier breakdown follows 4–6 h later, allowing water and plasma proteins to leak into the interstitial compartment (interstitial edema). These processes are further exacerbated by dysfunction of the glymphatic system and transcellular and paracellular transport pathways which fail to clear interstitial solutes. Lastly, circulating leukocytes accumulate in the infarcted and adjacent tissues, releasing inflammatory factors that cause secondary blood–brain barrier disruption. In turn, neuroinflammation contributes to worsening edema formation [6].
Risk factors for the development of malignant MCA stroke
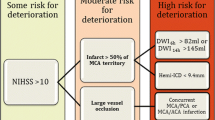
Patients with severe hemispheric syndromes from a distal ICA or proximal MCA occlusion who have ischemic changes involving > 50% of the MCA territory on CT within 12 h of symptoms onset are more likely to develop massive hemispheric edema in the following 24 to 72 h [1]. Also, diffusion-weighted magnetic resonance imaging (DWI-MRI) volumes > 80 mL within 6 h [7] and > 145 ml within 14 h of stroke onset predict a malignant evolution [8].
In a meta-analysis of 31 cohort and 7 case-control studies (n = 3278), younger age, higher admission National Institutes of Health Stroke Scale (NIHSS) scores (17–20 for patients with malignant cerebral edema, as compared to 5–15 for those without this complication), and hypoattenuation involving > 50% of the MCA territory on initial CT (OR = 5.33; 95% CI, 2.93–9.68) were associated with the development of malignant cerebral edema [2]. CT hypoattenuation was reliable as early as within 6 h and up to 40 h after symptoms onset. Depressed level of consciousness, gaze palsy, nausea or vomiting, and the need for mechanical ventilation were additional clinical features associated with a higher risk of malignant cerebral edema [2]. Further analysis of brain imaging revealed that more proximal arterial occlusions (OR, 4.89; 95% CI, 2.77–8.64), longer extension of the occlusion (OR, 3.78; 95% CI, 1.96–7.28), and tandem occlusions (OR = 4.05; 95% CI, 2.24–7.34) are additional risk factors [2]. On the other hand, arterial revascularization (OR = 0.37; 95% CI, 0.24–0.57) and brain atrophy were associated with a lower risk of malignant edema. Although age-related brain atrophy possibly mediates the protective effect of age on the development of brain edema, the exact age cutoff for protection and whether age is an independent protective factor for malignant edema deserve further research [2].
Scoring systems
Most available prediction models for malignant MCA stroke are limited by their retrospective design, small sample sizes, and lack of external validation [9,10,11,12,13] (Table 1). The DASH score, for example, requires MRI and quantification of collateral flow, information that may not be widely available [13]. The INTEP-AR score was developed in a multicenter prospective study and addressed most of these limitations [3•]. Among 2183 patients with CT within 24 h, 232 (10.6%) developed malignant brain edema. In the derivation cohort (n = 1627, 11.7% developed malignant edema), higher NIHSS (OR = 1.09, 1.06–1.12), large infarct defined as ≥ 50% of the territory of MCA, anterior cerebral artery (ACA) or posterior cerebral artery (PCA) (OR = 40.90, 95% CI 20.20–82.80), pneumonia (OR = 2.47, 1.53–3.97), intravenous thrombolysis (OR 2.11, 1.18–3.78), and endovascular treatment (OR = 2.87, 1.47–5.59) were associated with the development of malignant MCA infarcts. Brain atrophy (OR = 0.57, 0.37–0.86) and arterial recanalization (OR = 0.36, 0.17–0.75) were associated with a reduced risk [3•]. The score was built with these variables ranging from − 1 to 20, and a score ≥ 10 had good discrimination and calibration for malignant MCA infarct in the derivation (n = 1627, 11,6% had malignant edema, AUC 0.89, 0.87–0.92; sensitivity 0.95, 0.92–0.98) and validation (n = 556 patients, 7.7% had malignant brain edema, AUC 0.88, 0.82–0.95; sensitivity 0.84, 0.73–0.95) cohorts [3•]. The analysis of estimates for each variable indicates that stroke size is the single most important variable associated with increased risk for the development of malignant edema.
All predictive models that include imaging data are built on a one-time assessment of initial CT or MRI. Reduction in CSF volume from baseline to follow-up CT scan is closely related to midline shift at nadir [14]. Remarkably, the majority of CSF volume reduction occurs within 24 h from stroke onset, providing earlier insights than the midline shift that typically peaks much later. The reduction in CSF volume is significantly associated with the onset of malignant cerebral edema [14]. In one study, the risk of cerebral edema increased by 76%, and the risk of poor outcome and neurological deterioration increased by 34% for every 10% change in CSF volume within 24 h [15].
Radiomics based on the texture analysis of CT images that quantifies the heterogeneity of macroscopic cerebral tissue [16] and neural networks utilizing the ratio of cerebral spinal fluid volume displacement between the two hemispheres over time [17] appear as promising prognostic tools but need further development and validation. Machine-learning models combining CSF volume, CSF-to-cranial volume ratio, midline shift, and infarct-related hypodensity volume at baseline and 24 h may also increase the identification of patients at higher risk of malignant cerebral edema [18].
Treatment
General measures
Malignant MCA infarcts progress fast and usually require airway management; hence, patients at high risk should be monitored and treated in an intensive care unit. General measures for all patients include head-of-bed elevation to optimize intracranial venous drainage, normonatremia, normothermia, normocarbia, and adequate pain control. Intracranial hypertension compromises cerebral perfusion pressure. Thus, although treatment of extreme arterial hypertension may be needed, aggressive blood pressure lowering is ill-advised. Hourly neurologic examinations are required to identify a decline in mental status and early pupillary changes. Automated pupillometry can detect subtle changes imperceptible to the human eye. The neurological pupil index (NPi) quantifies pupillary size, latency, and velocity of contraction in a single number that ranges from 0 to 5; a score of < 3 indicates abnormal pupillary reactivity. This measurement has been associated with the development of malignant cerebral edema in post-thrombectomy patients [19]. Also, constriction and re-dilation velocities are slower, and the percentage change is smaller in patients who develop malignant cerebral edema after mechanical thrombectomy [20].
Serial CT scans complete the clinical evaluation by allowing assessment of the progression of brain edema, midline shift, and development of obstructive hydrocephalus. Midline shift at the level of the pineal gland indicates brainstem compression and has been shown to have the strongest correlation with decreased level of consciousness [21]. Imaging data in combination with the patient’s age, comorbidities, clinical evolution (particularly including the time from symptom onset), and personal preferences should be considered when deciding whether to proceed with surgical decompression and, if surgery is offered, when to take the patient to the operating room.
Intracranial pressure monitoring
Invasive intracranial pressure (ICP) monitors often fail to detect early neurologic deterioration in patients with focal hemispheric lesions. This was first demonstrated by Jeffrey Frank in 19 patients with hemispheric stroke (14 had complete MCA and five complete ICA infarcts). ICP monitors were ipsilateral intraparenchymal in 12 cases, contralateral intraventricular in five, and ipsilateral epidural in two. Only five patients had ICP > 15 mmHg at the time of initial deterioration of consciousness [22]. Schwab et al. [23] confirmed these findings in 48 patients with malignant MCA/ICA infarcts (all had ipsilateral epidural monitors, and seven had additional contralateral epidural monitors). The mean initial ICP was 18.9 mmHg. Patients with midline shifts greater than 10 mmHg had ICP recordings > 30 mmHg and were unlikely to survive. Most patients had pupillary asymmetry before ICP increased to > 25 mmHg, and clinical signs of herniation were always present before a major ICP increase [23]. Unilaterally or bilaterally dilated and fixed pupils preceded ICP increases above 35 mmHg. The existence of cranial compartments explains the development of pressure gradients from unilateral lesions causing cerebral herniation prior to a global increase in ICP. Hence, ICP monitors are not routinely recommended for the monitoring of patients with hemispheric strokes [24]. When ICP monitors are used, ICP measurements should supplement but never replace serial neurological examinations and brain imaging.
Hyperosmolar therapy
Hyperosmolar therapy is safe and effective for the treatment of intracranial hypertension from different etiologies [25]. In patients with malignant hemispheric strokes, it can reverse initial brain herniation and ICP surges, and it is usually combined with other measures such as hyperventilation (target PCO2 30–35 mmHg for 30 min) and sedation (propofol, midazolam, barbiturates). However, its effectiveness is unlikely to be sustained without timely cerebral decompression. In essence, it primarily serves as a life-saving measure to mitigate brain herniation until surgical intervention can be pursued [1]. Based on our experience, administration of serial boluses of hypertonic saline or mannitol may be sufficient to avoid herniation without decompressive surgery in some selected patients with gradual worsening of brain edema who manifest mild decline in their level of consciousness by days 4–5. However, meticulous patient selection, vigilant monitoring, and a well-defined rescue surgical strategy are imperative. Also, hyperosmolar therapy may need to be continued until brain edema eases in patients who experience neurologic decline after decompressive hemicraniectomy. We favor intermittent boluses over continuous infusions to mitigate neuronal adaptation to a sustained hyperosmolar state. This process involves the increase of osmotically active particles (such as electrolytes, organic substances, and idiogenic osmoles) within neurons, thus reducing the osmotic gradient between the vascular and intracellular compartments. The adaptation begins a few hours after initiation of hyperosmolar therapy, and it is maximally effective 24–48 h later [26]. For the same reason, and to avoid rebound intracranial hypertension, we taper hyperosmolar therapy when used regularly for 48 h or more.
There is no data to support the superiority of hypertonic saline over mannitol or vice versa, particularly for patients with ischemic brain edema. Most studies addressing this matter included patients with traumatic brain injury; they are limited by their retrospective nature, small sample sizes, and the absence of equiosmolar doses of both substances for direct comparison [27]. The intact blood–brain barrier is impermeable to both substances, as indicated by coefficients of reflection of 0.9 for mannitol and 1 for hypertonic saline (with 0 indicating complete permeability and 1 indicating complete impermeability). Hence, in clinical practice, the choice depends on availability, convenience of administration (central access historically required for hypertonic saline), and side effects [28]. Mannitol is classically associated with volume depletion and increased risk of renal failure (which can be minimized with careful volume repletion and by maintaining an osmolar gap below 20 mOsm/kg), and hypertonic saline with congestive heart failure, hypernatremia, and hyperchloremic acidosis [28]. The theoretical concerns that osmotic agents may accumulate within areas of the injured brain tissue, due to the increased blood–brain barrier permeability in those regions, and consequently exacerbate deleterious pressure gradients, are not substantiated by clinical experience.
Decompressive hemicraniectomy
The first case series about decompressive hemicraniectomy for the treatment of malignant middle cerebral artery infarction was published in 1956 [29]. Since then, this approach has emerged as the foundation for the treatment of malignant MCA stroke. The surgical technique has been refined and several studies have proven the lifesaving effect of timely brain decompression. For optimal results, it is typically recommended to perform a frontal–temporal-parietal decompressive hemicraniectomy with durotomy, measuring at least 12 cm in length and 9 cm in height. While temporal lobectomy can be added, it is not routinely performed [30].
Between 2007 and 2021, nine randomized clinical trials have studied the value of decompressive hemicraniectomy in patients with malignant middle cerebral artery infarctions [31,32,33,34,35,36,37,38] (Table 2). One of them, the Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery: a Randomized, Controlled Trial in a Turkish Population (DEMITUR) trial, was completed in 2007, published in 2021, and withdrawn shortly after [39]. Hence, it will not be discussed here. The DEMITUR trial had the largest sample size of all trials (n = 151) and included a significant proportion of patients aged 60 years or more (n = 88) who had remarkably good functional outcomes (> 66% achieved a mRS ≤ 3), affecting the results of subsequent metanalysis that included its results [4••, 40••]. The remainder 8 trials differ in their inclusion criteria (age, time from symptom onset to randomization, imaging findings, severity of symptoms at randomization, and pre-stroke disability) and primary outcome. All but 2 studies [31, 35] (likely due to their small sample sizes) consistently showed robust mortality benefits with hemicraniectomy. Individually, most trials failed to detect a benefit to achieve the primary outcome when it was defined as functional independence (mRS ≤ 3) at 3 to 12 months. The exceptions were those trials that included mRS 4 in the good outcome category [33, 34]. The two most recent meta-analyses have corroborated an 80% reduction in mortality and moderate (mRS > 3) and severe (mRS > 4) disability associated with surgical intervention [4••, 40••]. A patient-level data metanalysis also reported a benefit of surgical treatment to achieve functional independence (mRS ≤ 3) at 1 year (absolute risk difference, 21%; 95% CI, 9–33; adjusted OR, 2.95; 95% CI, 1.55–5.60) [4••]. The benefit of decompressive hemicraniectomy in people younger than 60 years is clear. An individual patient data metanalysis of the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial (HAMLET), sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL), and Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY) trials (n = 93) showed that the number needed to treat (NNT) to save one life is 3 and to help one patient to achieve functional independence 4. Overall, decompressive surgery doubles the chances of functional independence (mRS ≤ 3 43% vs 21%) [4]. In meta-analyses, the benefits were not offset by age (> 60 years), time from ictus, presence of aphasia, or involvement of an additional vascular territory [4••, 40••].
Yet, age does matter when it comes to functional outcomes after decompressive hemicraniectomy for massive hemispheric strokes. Real-world data of 188 patients who underwent decompressive hemicraniectomy for malignant MCA infarcts from 8 neurosurgical departments revealed that age > 50 years had an unfavorable impact on survival and favorable outcome; 35% of the patients ≤ 50 years of age achieved a Glasgow outcome scale (GOS) score of > 3 (severe disability), as compared with 12% of the older population [42]. In a systematic review of observational studies, 80% of 75 post-hemicraniectomy patients older > 50 years of age were dead or severely disabled at a minimum follow-up of 4 months compared with 32% of 63 patients ≤ 50 years of age [43]. Per protocol, four randomized clinical trials allowed patients older than 60 years of age, but only (Table 2) the Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery II (DESTINY II) had adequate sample size to draw conclusions. In this trial, only 6% of patients aged over 60 years achieved a mRS of ≤ 3 post-surgery [4••]. Although 2 meta-analyses claim the benefit of decompressive hemicraniectomy to achieve functional independence irrespective of age, their results should be analyzed with caution given the inclusion of the withdrawn DEMITUR trial. Also, in these studies, age was handled as a continuous variable and the estimates of treatment outcome in higher age decades were imprecise due to the low number of observations [4••, 33].
The ideal timing for hemicraniectomy has also been extensively debated. Although most decompressive hemicraniectomy studies included patients in the first 48 h after stroke symptoms onset, they do not negate the benefit in patients who declined later. This is supported by retrospective data indicating a benefit of surgical treatment up to 72 h after symptoms onset [44]. In this study, the associations between the timing of surgery and outcomes were only present in patients with cerebral herniation [44]. In other words, hemicraniectomy should be considered after 48 h if herniation has not occurred.
Decompressive hemicraniectomy has complications in up to 54% of patients [45, 46]. When insufficiently large, brain herniation occurs causing tissue damage and venous compression with venous infarcts and hemorrhages [45]. The prevalence of seizures in post-craniectomy patients can be as high as 60% [47]. The syndrome of trephined or sinking skin flap syndrome presents with sensorimotor language or cognitive deterioration after craniectomy and can include paradoxical herniation when most severe. Although its pathogenesis is not completely understood, the effect of atmospheric pressure on the brain seems to play a role. Its incidence ranges widely from 1% in older studies to 65% in more recent prospective ones, a difference that is likely due to the use of more inclusive diagnostic criteria in the newer studies [48•]. The acute treatment consists of placing the patient in reverse Trendelenburg and discontinuing CSF diversion if ongoing; these interventions are only a bridge to cranioplasty, which should be pursued as soon as feasible. Communicating hydrocephalus, subdural, epidural, and extracranial fluid collections and CSF leaks can also occur in these cases, generating additional mass effects, impairing wound healing, and increasing the infection risk [45, 46]. Between 5 and 15% of patients with decompressive craniectomy require ventriculoperitoneal shunt placement, which is also associated with long-term risk of dysfunction and infection [46]. The reported complication rates after cranioplasty can be as high as 35%, and one in four patients may require revision surgery due to infection, bone resorption, abnormal wound healing, and hematomas [49]. The ideal timing of cranioplasty is unclear, with some observational studies showing a higher complication rate with early (within 8–12 weeks) versus late (> 12 weeks) bone replacement [50].
Prognosis
Hemispheric strokes represent profoundly debilitating conditions, irrespective of the development of malignant cerebral edema. Without decompressive hemicraniectomy, the mortality rate ranges between 68% in metanalyses of randomized clinical trials and 78% in prospective cohorts, despite maximal medical treatment. A real-world practice multicenter study of 232 patients with malignant MCA stroke (18% received tPA, 39% had endovascular treatment [60% achieved successful recanalization] and 10% decompressive hemicraniectomy) showed that 65% had died by 30 days. The occurrence of unfavorable outcomes (mRS ≥ 3) at 3 months and 1 year was 97% and 95%, respectively [3•]. In people younger than 60 years without other major comorbidities and with good pre-stroke functional status, hemicraniectomy increases survival from 20 to 80% and doubles the odds of walking without assistance while increasing 10 times the odds of requiring assistance to walk and attend to own body needs; it does not increase the odds of severe disability (defined as being bedridden, incontinent, and requiring constant cares) [41]. In people older than 60, hemicraniectomy increases survival from 20 to 60%, but the odds of walking unassisted do not improve; the odds of requiring assistance to walk and attend own body needs triples, and the odds of severe disability doubles [33].
In this context, the definition of a good outcome for malignant MCA stroke survivors is a highly debated topic, with some advocating to include mRS 4 as a good outcome given the devastating nature of this condition. In a multicenter survey of 355 stroke survivors and 199 relatives, around 80% of respondents considered mRS ≤ 2 an acceptable outcome. Yet, a mRS of 3, 4, and 5 was also considered acceptable by 56%, 24%, and 7% of respondents, respectively. Relatives usually considered acceptable higher mRS scores than patients, and older respondents more often declined decompressive hemicraniectomy as a treatment option [51]. A similar survey among 627 nurses found that 90% considered mRS ≤ 2 a good outcome, while 60%, 15%, and 1.6% considered mRS 3, 4, and 5 as acceptable, respectively. Only 30% would consider hemicraniectomy as a treatment option, and older nurses more often declined the option of hemicraniectomy, irrespective of the presence of aphasia [52]. Randomized clinical trials and subsequent metanalyses suggest a consistent benefit from hemicraniectomy regardless of hemispheric dominance and the presence of aphasia [4••, 40••]. However, outcome assessment by the mRS score does not fully address several quality-of-life domains or the impact of aphasia. This is relevant because aphasia is independently associated with increased length of stay and complications during acute stroke admissions and is more likely to produce a poor functional outcome than hemiparesis [53]. In different surveys, aphasia was cited as an important factor when deciding whether to accept hemicraniectomy by 46% of stroke survivors and 39% of their relatives [51], as well as one-third of 627 nurses [52]. On the other hand, aphasia can recover significantly, though it requires longer rehabilitation time; young age has been reported to be the single most important predictor of aphasia improvement after hemicraniectomy [54].
In a systematic review of post-hemicraniectomy stroke survivors (n = 156, 41% had mRS ≤ 3 and 47% had mRS ≤ 4 at a mean follow-up of 19 months) quality of life decreased by 45%, while 56% had moderate and 25% severe depression [55]. Yet, 77% of patients or caregivers were satisfied with the outcome and would give consent for hemicraniectomy again [55]. Patients older than 60 years of age seem to have a lower quality of life in comparison with younger patients [56].
The subject is complex, and no study can completely outline what a good outcome is, mostly considering that the definition of an acceptable outcome differs among patients and cultural backgrounds. Hence, an individualized approach and shared decision-making are imperative.
For all these reasons, we clearly explain to patients and decision-makers the specific deficits expected from the stroke including aphasia, neglect, sensory deficits, weakness, hemianopia, and cognitive changes, regardless of potential mRS benefits of the surgical intervention. We also reinforce the need for extensive rehabilitation efforts to achieve the best functional state and describe the potential complications of the surgery.
Conclusions
Malignant MCA infarcts cause progressive neurologic deterioration and herniation, with high mortality and disability. Patients at high risk of this complication (young patients with large artery occlusions and high NIHSS within 48 h of stroke onset) should be monitored in an intensive care unit and have serial neurologic examinations and head CT. Neurologic deterioration requires airway management and hyperosmolar therapy. Decompressive hemicraniectomy is a lifesaving procedure and patients younger than 60 years can achieve meaningful functional recovery. Recovery is less favorable in older patients, and the decision of whether to proceed with surgical decompression should be assessed carefully on a case-by-case basis in these instances.
References and Recommended Readings
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hacke W. “Malignant” middle cerebral artery territory infarction. Arch Neurol. 1996;53(4):309. https://doi.org/10.1001/archneur.1996.00550040037012.
Wu S, Yuan R, Wang Y, et al. Early prediction of malignant brain edema after ischemic stroke. Stroke. 2018;49(12):2918–27. https://doi.org/10.1161/STROKEAHA.118.022001.
• Wu S, Wang Y, Yuan R, et al. Predicting the emergence of malignant brain oedema in acute ischaemic stroke: a prospective multicentre study with development and validation of predictive modelling. EClinicalMedicine. 2023;59:101977. Multicenter prospective study with the most accurate predictive score for malignant MCA infarction.
•• Reinink H, Jüttler E, Hacke W, et al. Surgical decompression for space-occupying hemispheric infarction. JAMA Neurol. 2021;78(2):208. https://doi.org/10.1001/jamaneurol.2020.3745. Individual patient data meta-analysis of decompressive hemicraniectomy trails. Results should be interpreted with caution due to inclusion of a study that was withdrawn after publication.
Qureshi AI, Suarez JI, Yahia AM, et al. Timing of neurologic deterioration in massive middle cerebral artery infarction: a multicenter review. Crit Care Med. 2003;31(1):272–7. https://doi.org/10.1097/00003246-200301000-00043.
Chen S, Shao L, Ma L. Cerebral edema formation after stroke: emphasis on blood-brain barrier and the lymphatic drainage system of the brain. Front Cell Neurosci. 2021;15:716825. https://doi.org/10.3389/fncel.2021.716825.
Thomalla G, Hartmann F, Juettler E, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: a prospective multicenter observational study. Ann Neurol. 2010;68(4):435–45. https://doi.org/10.1002/ana.22125.
Oppenheim C, Samson Y, Manaï R, et al. Prediction of malignant middle cerebral artery infarction by diffusion weighted imaging. Stroke. 2000;31(9):2175–87. https://doi.org/10.1161/01.STR.31.9.2175.
Cheng Y, Wu S, Wang Y, et al. External validation and modification of the EDEMA score for predicting malignant brain edema after acute ischemic stroke. Neurocrit Care. 2020;32(1):104–12. https://doi.org/10.1007/s12028-019-00844-y.
Ong CJ, Gluckstein J, Laurido-Soto O, Yan Y, Dhar R, Lee JM. Enhanced detection of edema in malignant anterior circulation stroke (EDEMA) score. Stroke. 2017;48(7):1969–72. https://doi.org/10.1161/STROKEAHA.117.016733.
Kasner SE, Demchuk AM, Berrouschot J, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001;32(9):2117–23. https://doi.org/10.1161/hs0901.095719.
Jo K, Bajgur SS, Kim H, Choi HA, Huh PW, Lee K. A simple prediction score system for malignant brain edema progression in large hemispheric infarction. PLoS ONE. 2017;12(2):e0171425. https://doi.org/10.1371/journal.pone.0171425.
Shimoyama T, Kimura K, Uemura J, et al. The DASH score: a simple score to assess risk for development of malignant middle cerebral artery infarction. J Neurol Sci. 2014;338(1–2):102–6. https://doi.org/10.1016/j.jns.2013.12.024.
Dhar R, Yuan K, Kulik T, et al. CSF volumetric analysis for quantification of cerebral edema after hemispheric infarction. Neurocrit Care. 2016;24(3):420–7. https://doi.org/10.1007/s12028-015-0204-z.
Dhar R, Chen Y, Hamzehloo A, et al. Reduction in cerebrospinal fluid volume as an early quantitative biomarker of cerebral edema after ischemic stroke. Stroke. 2020;51(2):462–7. https://doi.org/10.1161/STROKEAHA.119.027895.
Wen X, Li Y, He X, et al. Prediction of malignant acute middle cerebral artery infarction via computed tomography radiomics. Front Neurosci. 2020. https://doi.org/10.3389/fnins.2020.00708.
Foroushani HM, Hamzehloo A, Kumar A, et al. Accelerating prediction of malignant cerebral edema after ischemic stroke with automated image analysis and explainable neural networks. Neurocrit Care. 2022;36(2):471–82. https://doi.org/10.1007/s12028-021-01325-x.
Foroushani HM, Hamzehloo A, Kumar A, et al. Quantitative serial CT imaging-derived features improve prediction of malignant cerebral edema after ischemic stroke. Neurocrit Care. 2020;33(3):785–92. https://doi.org/10.1007/s12028-020-01056-5.
Dowlati E, Sarpong K, Kamande S, et al. Abnormal neurological pupil index is associated with malignant cerebral edema after mechanical thrombectomy in large vessel occlusion patients. Neurol Sci. 2021;42(12):5139–48. https://doi.org/10.1007/s10072-021-05218-x.
Kossel CS, Kobus F, Borutta MC, et al. Pupillometry in the follow-up of patients undergoing EVT - prediction of space-occupying hemispheric infarction. J Neurol. 2023;270(9):4507–17. https://doi.org/10.1007/s00415-023-11797-w.
Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314(15):953–8. https://doi.org/10.1056/NEJM198604103141504.
Frank JI. Large hemispheric infarction, deterioration, and intracranial pressure. Neurol. 1995;45(7):1286–90. https://doi.org/10.1212/WNL.45.7.1286.
Schwab S, Aschoff A, Spranger M, Albert F, Hacke W. The value of intracranial pressure monitoring in acute hemispheric stroke. Neurol. 1996;47(2):393–8. https://doi.org/10.1212/wnl.47.2.393.
Wijdicks EFM, Sheth KN, Carter BS, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling. Stroke. 2014;45(4):1222–38. https://doi.org/10.1161/01.str.0000441965.15164.d6.
Ropper AH. Hyperosmolar therapy for raised intracranial pressure. N Engl J Med. 2012;367(8):746–52. https://doi.org/10.1056/NEJMct1206321.
Diringer MN. New trends in hyperosmolar therapy? Curr Opin Crit Care. 2013;19(2):77–82. https://doi.org/10.1097/MCC.0b013e32835eba30.
Chen H, Song Z, Dennis JA. Hypertonic saline versus other intracranial pressure-lowering agents for people with acute traumatic brain injury. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD010904.pub3.
DeHoff G, Lau W. Medical management of cerebral edema in large hemispheric infarcts. Front Neurol. 2022. https://doi.org/10.3389/fneur.2022.857640.
Scarcella G. Encephalomalacia simulating the clinical and radiological aspects of brain tumor. J Neurosurg. 1956;13(4):278–92. https://doi.org/10.3171/jns.1956.13.4.0278.
Agarwalla PK, Stapleton CJ, Ogilvy CS. Craniectomy in acute ischemic stroke neurosurgery. 2014;74(Supplement 1):S151–62. https://doi.org/10.1227/NEU.0000000000000226.
Frank JI, Schumm LP, Wroblewski K, et al. Hemicraniectomy and durotomy upon deterioration from infarction-related swelling trial. Stroke. 2014;45(3):781–7. https://doi.org/10.1161/STROKEAHA.113.003200.
Chua A, Buckley B, Lapitan M, Jamora R. Hemicraniectomy for malignant middle cerebral artery infarction (HeMMI): a randomized controlled clinical trial of decompressive surgery with standardized medical care versus standardized medical care alone. Acta Med Philipp. 2015;49(1):28–33.
Jüttler E, Unterberg A, Woitzik J, et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014;370(12):1091–100. https://doi.org/10.1056/NEJMoa1311367.
Zhao J, Su YY, Zhang Y, et al. Decompressive hemicraniectomy in malignant middle cerebral artery infarct: a randomized controlled trial enrolling patients up to 80 Years Old. Neurocrit Care. 2012;17(2):161–71. https://doi.org/10.1007/s12028-012-9703-3.
Slezins JKVBRMAVESJMO. Preliminary results of randomized controlled study on decompressive craniectomy in treatment of malignant middle cerebral artery stroke. Medicina (Kauna). 2012;48(10):96.
Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8(4):326–33. https://doi.org/10.1016/S1474-4422(09)70047-X.
Jüttler E, Schwab S, Schmiedek P, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY). Stroke. 2007;38(9):2518–25. https://doi.org/10.1161/STROKEAHA.107.485649.
Vahedi K, Vicaut E, Mateo J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke. 2007;38(9):2506–17. https://doi.org/10.1161/STROKEAHA.107.485235.
Kumral E, Sirin H, Sağduyu A, Güler A, Özdamar N, Köse T. WITHDRAWN: decompressive surgery in patients with malignant middle cerebral artery infarction: a randomized, controlled trial in a Turkish population (Demitur trial). Int J Stroke. 2021. https://doi.org/10.1177/17474930211007671.
•• Dower A, Mulcahy M, Maharaj M, et al. Surgical decompression for malignant cerebral edema after ischemic stroke: Cochrane review. Stroke. 2023. https://doi.org/10.1161/STROKEAHA.122.042260. Metanalysis of decompressive hemicraniectomy trails. Results should be interpreted with caution due to inclusion of a study that was withdrawn after publication.
Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215–22. https://doi.org/10.1016/S1474-4422(07)70036-4.
Uhl E, Kreth FW, Elias B, et al. Outcome and prognostic factors of hemicraniectomy for space occupying cerebral infarction. J Neurol Neurosurg Psychiatry. 2004;75(2):270–4.
Gupta R, Connolly ES, Mayer S, Elkind MSV. Hemicraniectomy for massive middle cerebral artery territory infarction. Stroke. 2004;35(2):539–43. https://doi.org/10.1161/01.STR.0000109772.64650.18.
Dasenbrock HH, Robertson FC, Vaitkevicius H, et al. Timing of decompressive hemicraniectomy for stroke. Stroke. 2017;48(3):704–11. https://doi.org/10.1161/STROKEAHA.116.014727.
Gopalakrishnan MS, Shanbhag NC, Shukla DP, Konar SK, Bhat DI, Devi BI. Complications of decompressive craniectomy. Front Neurol. 2018. https://doi.org/10.3389/fneur.2018.00977.
Lin J, Frontera JA. Decompressive hemicraniectomy for large hemispheric strokes. Stroke. 2021;52(4):1500–10. https://doi.org/10.1161/STROKEAHA.120.032359.
Brondani R, de Garcia Almeida A, Abrahim Cherubini P, et al. High risk of seizures and epilepsy after decompressive hemicraniectomy for malignant middle cerebral artery stroke. Cerebrovasc Dis Extra. 2017;7(1):51–61. https://doi.org/10.1159/000458730.
• Sveikata L, Vasung L, El Rahal A, et al. Syndrome of the trephined: clinical spectrum, risk factors, and impact of cranioplasty on neurologic recovery in a prospective cohort. Neurosurg Rev. 2022;45(2):1431–43. https://doi.org/10.1007/s10143-021-01655-6. Prospective evaluating the incidence, clinical spectrum, and risk factors for the syndrome of the trephined in patients with decompressive hemicraniectomy.
Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 2009;26(6):E9. https://doi.org/10.3171/2009.3.FOCUS0962.
Borger V, Schuss P, Kinfe TM, Vatter H, Güresir E. Decompressive craniectomy for stroke: early cranioplasty is a predictor for postoperative complications. World Neurosurg. 2016;92:83–8. https://doi.org/10.1016/j.wneu.2016.04.113.
Neugebauer H, Schnabl M, Lulé D, Heuschmann PU, Jüttler E. Attitudes of patients and relatives toward disability and treatment in malignant MCA infarction. Neurocrit Care. 2017;26(2):311–8. https://doi.org/10.1007/s12028-016-0362-7.
Neugebauer H, Malakou F, Uttner I, Köpke M, Jüttler E. Attitudes of nurses toward disability and treatment in space-occupying middle cerebral artery stroke. Neurocrit Care. 2019;30(1):132–8. https://doi.org/10.1007/s12028-018-0586-9.
Boehme AK, Martin-Schild S, Marshall RS, Lazar RM. Effect of aphasia on acute stroke outcomes. Neurol. 2016;87(22):2348–54. https://doi.org/10.1212/WNL.0000000000003297.
Kastrau F, Wolter M, Huber W, Block F. Recovery from aphasia after hemicraniectomy for infarction of the speech-dominant hemisphere. Stroke. 2005;36(4):825–9. https://doi.org/10.1161/01.STR.0000157595.93115.70.
Rahme R, Zuccarello M, Kleindorfer D, Adeoye OM, Ringer AJ. Decompressive hemicraniectomy for malignant middle cerebral artery territory infarction: is life worth living? J Neurosurg. 2012;117(4):749–54. https://doi.org/10.3171/2012.6.JNS111140.
van Middelaar T, Nederkoorn PJ, van der Worp HB, Stam J, Richard E. Quality of life after surgical decompression for space-occupying middle cerebral artery infarction: systematic review. Int J Stroke. 2015;10(2):170–6. https://doi.org/10.1111/ijs.12329.
Author information
Authors and Affiliations
Contributions
MAH wrote the main manuscript. AAR reviewed and edited the manuscript. Both authors reviewed and agree with the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Maximiliano A. Hawkes and Alejandro A. Rabinstein declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hawkes, M.A., Rabinstein, A.A. Treatment of Malignant Cerebral Edema in Acute Ischemic Stroke. Curr Treat Options Neurol 26, 243–259 (2024). https://doi.org/10.1007/s11940-024-00793-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11940-024-00793-8