Opinion statement
The cyclic hormonal underpinnings of catamenial seizure exacerbations are consistent with the neurophysiologic activity of estrogen and progesterone. For women with catamenial epilepsy who have regular menses, intermittent treatment approaches may be utilized. These interventions are targeted at adding or increasing anti-seizure treatments during established vulnerable days of the menstrual cycle, such as perimenstrually (C1 pattern), at ovulation (C2 pattern), and during the luteal phase (C3 pattern). The single large study of natural progesterone treatment showed benefit for women with clear perimenstrual seizure exacerbations (C1 pattern), but not for subjects with other catamenial patterns or for randomized women with epilepsy of reproductive age who did not have catamenial seizure exacerbations. In this protocol, natural progesterone was given at a high dose during the luteal phase and was generally well tolerated. Other intermittent cyclic treatments include benzodiazepine use, increasing the dose of an anti-seizure drug already in use, or acetazolamide. For women with irregular menses, or those in which the intermittent cyclic treatments are not effective, pharmacologically stopping the menstrual cycle altogether by using synthetic hormones such as medroxyprogesterone (Depo-Provera) or sustained oral contraceptives may be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catamenial epilepsy was first described by Sir Charles Locock in 1857; referred to as hysterical epilepsy, it “was confined to women and observed a regularity of return connected with the menstruation” [1, 2]. Since then, many other people have commented on the rhythmicity of seizures around the menstrual cycle in women. The term catamenial epilepsy does not refer to a specific epilepsy syndrome or localization, but rather to catamenial seizure exacerbations. Catamenial epilepsy is clearly associated with the neuroactive properties of reproductive hormones, termed “neurosteroids.” Seizure occurrence in women with epilepsy is influenced by the cyclic variations of these neurosteroids throughout the menstrual cycle due to their interaction with the epileptic substrate. Endogenous female reproductive neurosteroids alter neuronal excitability through interaction with GABAa receptors and neuronal membranes, and recent studies have shown a correlation of the serum estradiol/progesterone ratio with seizure frequency [3].
From the growing understanding of neurosteroid effects, treatment options specific to their cyclic fluctuations continue to be explored [4]. The classic nonsteroid interventions that are used intermittently throughout the cycle, such as acetazolamide and clobazem, lack large trials to support their use. Recent studies have focused on harnessing the hormonal fluctuations that cause catamenial epilepsy. Specifically, progesterone supplementation is a current area of focus and the only large-scale trial, the NIH Progesterone Trial, found a beneficial effect in a subset of women with catamenial epilepsy. Gonadotropin releasing hormone (GnRH) analogues have also been studied. The options for treatment are expanding, and this article serves to look at both the hormonal and non-hormonal options, and the data supporting their use.
Prior to any targeted intermittent treatment for catamenial epilepsy, the patient should document the timing of menstrual onset and seizure exact pattern across at least three menstrual cycles. Intermittent treatments are initiated within the menstrual cycle based on predicting when the menstrual onset will be, usually in order to augment treatment at the specific time of vulnerability. This is most often premenstrually, but may be at ovulation or over the entire luteal phase, as more fully outlined below. Therefore, a reasonably accurate prediction of the next menstrual onset is necessary. If menses are irregular, intermittent cyclic treatments may not be reasonable and treatments that suppress menstrual cycling altogether may be more appropriate. Irregular menses are defined as those that are shorter than 21 days or longer than 36 days.
Hormone fluctuations—their role in the nervous system and catamenial epilepsy
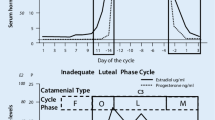
The average menstrual cycle is 28 days. Day 1 is considered the first day of menses and the mid-point, day 14, the first day of ovulation. The follicular phase of menstruation (days 1–14) involves growth of the ovarian follicles and ultimately release of a dominant follicle which becomes the oocyte. The luteal phase (days 15–28) starts after ovulation and involves the dominant follicle forming the corpus luteum, which produces progesterone. The luteal phase is always 14 days in length, and its occurrence within a menstrual cycle can only be reliably determined retrospectively, that is, by counting 14 days backwards from day 1, which is menstrual onset. This is in contrast to the follicular phase which can be variable in length. The normal hormonal fluctuations, which are thought to be associated with catamenial epilepsy, are the rapid and dramatic estrogen surge on day 13, which initiates ovulation, and the more gradual withdrawal of progesterone and estrogen on days 26–28 prior to menstruation. Figure 1 shows the hormone fluctuations throughout the normal menstrual cycle as well as in anovulatory cycles, also known as inadequate luteal phase [5]. In inadequate luteal phase cycles, there is a lack of progesterone during the entire luteal phase and therefore lack of ovulation, while the estrogen level remains high and wanes normally prior to menstruation. Therefore, during inadequate luteal phase cycles, the estradiol/progesterone ratio is high.
The graphs show menstrual cycles and corresponding catamenial seizure types. The top graph shows hormone fluctuations during normal menses with C1 catamenial seizures occurring during menses and C2 during ovulation. The bottom graph shows hormone fluctuations during inadequate luteal phase and C3 seizure clustering occurring during all phases except the follicular phase.
The clustering of seizures at certain times in the menstrual cycle is directly related to the fluctuations of these two hormones, specifically the anti-seizure effects of progesterone and pro-seizure effects of estrogen. The neurosteroid activity of progesterone is through its reduced metabolite, allopregnanolone. Allopregnanolone is a positive allosteric modulator of GABAa conductance, and binds to a site different than both barbiturates and benzodiazepines. Its main effect is to enhance the GABAergic affect by increasing inward chloride current. There is also a feedback mechanism that alters the subunit composition of the GABAa receptor, increasing expression of the α4-subunit in the hippocampus and decreasing responsiveness to benzodiazepines. This decreased responsiveness persists after progesterone withdrawal during the cycle and can affect the responsiveness to anti-seizure drugs and benzodiazepines, which was recently shown in a mouse kindling model [6]. In addition to the effect of progesterone and its metabolites on GABA receptors, there is some evidence that it may also decrease glutamate responsiveness by acting at the NMDA receptor [7, 8].
Estrogen exerts its neurosteroid activity of increasing neuronal excitability through several mechanisms. In rodents, estradiol increased the production and density of NMDA receptors on the dendritic spines of hippocampal CA1 pyramidal neurons [9, 10], as well as in Purkinje neurons of the cerebellum [11]. This increase in NMDA receptors increased calcium entry and created more excitatory inputs to pyramidal cells [12, 13]. Estrogen has also been shown to potentiate kainite-induced seizures in a dose-related manner in rat models as well [14].
Seizure clustering in catamenial epilepsy occurs at three specific times in association with normal estrogen and progesterone fluctuations across the cycle, and these patterns have been termed C1, C2, and C3. For women to meet the current criteria to fit into one of these patterns, their seizure frequency must increase twofold during the days encompassed by the pattern definition, compared to other days of their cycle. When determining a catamenial pattern designation, the date of ovulation is calculated by counting back 14 days prior to menstrual onset, or day −14. The perimenstrual, or C1 pattern, indicates seizure frequency increase of twofold during the cycle days around menses (days −3 to +3) compared to other days of the month. The C1 pattern neurophysiologically corresponds to the rapid withdrawal of the neuroinhibitory hormone, progesterone. This is the most common catamenial pattern and many women with this pattern may only have seizures during the premenstrual days of the month, but are otherwise seizure free. The periovulatory, or C2 pattern, occurs at ovulation (days 10 to −13) with women documenting an increase in seizure frequency during these days. This pattern neurophysiologically corresponds to the rapid rise of the neuroexcitatory hormone, estrogen. The final pattern, C3, occurs in inadequate luteal phase cycles, during which women report a two-fold increase in seizure frequency during the 14 days prior to menses, encompassing ovulation, the luteal phase, and menstrual onset. The neurophysiologic underpinning of this catamenial pattern may be the estradiol/progesterone ratio, which is most pronounced during anovulatory cycles [15]. Across the menstrual cycle, seizure frequency corresponds strongly with serum estradiol/progesterone levels, with the lowest ratios found in the mid-luteal phase and corresponding to the lowest frequency of seizures during ovulatory cycles [16].
Treatment—hormonal
The most thoroughly studied hormonal treatments for catamenial epilepsy are natural progesterone supplementation, synthetic progestogens, or menstrual-suppressive therapies.
Two open-label clinical trials of natural progesterone have been performed [16, 17]. They looked at women with complex partial seizures with catamenial characteristics and inadequate luteal phases to determine the affect of progesterone suppositories on seizure frequencies. The results showed a 68 % decline in seizure frequency (p < 0.05) in 6 of the 8 women studied, and none of the women showed an increase in frequency [17]. A second open-label trial looking at 25 women with both anovulatory and normal menstrual cycle exacerbations showed cyclic progesterone lozenges decreased seizure frequency in 18 women (72 % and p < 0.01) over a 3-month period. Average daily seizure frequency declined by 55 % (p < 0.01). The decrease in frequency was also noted to be greater in the women with inadequate luteal phase than perimenstrual exacerbation (59 versus 49 %). Average daily complex partial seizure (CPS) frequency decreased by 54 and 58 % for secondarily generalized tonic clonic (SGTC) [3, 18, 19••].
These positive studies led to a randomized, placebo-controlled, double-blind, clinical trial that looked at natural progesterone versus placebo therapy for treatment of women of reproductive age with focal epilepsy, stratified by the presence or absence of catamenial seizure patterns, the NIH Progesterone treatment Trial [18]. The investigators compared the proportion of ≥50 % responders in seizure frequency between a 3-month baseline and 3-month treatment phase. Treatment consisted of an optimized antiepileptic agent plus natural progesterone 200 mg lozenges or placebo. The lozenge was taken three times daily starting at ovulation on day −14 with a decremented taper starting on day 26 and with the treatment ending for that cycle on day 28. The findings showed no difference in partial seizure frequency when comparing the treatment arm or placebo.
Although adjunctive progesterone treatment did not significantly decrease seizure frequency in the overall study population, the subjects with a strong C1 pattern who had three times more seizures during the cycle days −3 to +3 were responders. This finding suggests that the approaches to treating catamenial seizure patterns should be specific for the pattern type. Significance of treatment for the C1 pattern was only achieved when there was at least a three-fold increase in seizure frequency during C1 days. Notably, there was no response for subjects with catamenial patterns type 2 or 3 or for the enrolled subjects without a catamenial pattern, who comprised slightly more than 50 % of the study population [20].
A further analysis of the NIH Progesterone Treatment Trial aimed to find evidence that the reduced progesterone metabolite, allopregnanolone (AP), the positive allosteric modulator of GABAa receptors [5] specifically mediates the reduction in seizure frequency discussed above. Using mid-luteal phase AP levels obtained during the study, a significant correlation was shown between reduction in seizure frequency and increases in the AP level for the C1 ≥ 3 group (C1 ≥ 3 progesterone treated r = −0.452, p = 0.035 versus C1 ≥ 3 placebo treated r = −0.367; C1 < 3 progesterone r = 0.099; C1 < 3 placebo r = 0.131; p = not significant) [21]. The study findings lent support to the idea that AP mediates the changes in seizure threshold seen with progesterone withdrawal and that cyclic progesterone supplementation in the subset of C1 catamenial epilepsy may be beneficial.
One case study provided preliminary evidence that seizure reduction in catamenial epilepsy occurred specifically through its reduced metabolites and not progesterone itself. A 34-year-old woman with intractable CPS and SGTC, which increased in severity during the luteal phase of her menstrual cycle, was treated with carbamazepine and cyclic progesterone. Her seizures decreased from several SGTC each month to none and only one CPS per month for several years. She was started on finasteride for baldness, which is a reductase enzyme inhibitor and would inhibit the metabolism of progesterone to its neurosteroid metabolite, AP. After addition of finasteride, seizure frequency increased from none to 3–6/month (p < 0.05), despite continued therapeutic levels of carbamazepine [22]. This is consistent with the reduced metabolite being the mediator of treatment rather than the progesterone itself.
Synthetic forms of progestogens such as medroxyprogesterone acetate (MPA) may also play a role in treatment of catamenial epilepsy. MPA has been widely used as a highly effective contraceptive, given as an intramuscular injection (Depo form) every 12 weeks and acts through suppressing ovulation. One study of 19 women added MPA as an adjunctive treatment to their current anti-seizure drug regimens delivered in the standard dose. Of the 19 women, 11 developed amenorrhea and, of these, 7 reported a 39 % decrease in seizures per month (p = 0.02) [23•]. While this study is small and nearly anecdotal, it has put MPA on the landscape of possible treatments for catamenial epilepsy. However, it is likely that medroxyprogesterone itself is not an inhibitory neurosteroid, as compared to natural progesterone; therefore, the seizure reduction in this report was likely due to suppression of menstrual cycling altogether [3]. A major caution exists regarding its use: MPA carries an FDA warning regarding its risk of bone density loss, which is consistently between up to 5 % within the first 2 years of use for women and adolescents [24].
Gonadotropin releasing hormone (GnRH) analogues will suppress menstruation and hormone fluctuations, and one report exists for use as a treatment for epilepsy. Adjunctive treatment of 10 female patients with triptorelin therapy (a synthetic GnRH analogue) which suppresses gonadotropin release allowed for 3 patients to become seizure free, and 4 patients had up to a 50 % reduction in seizure frequency. These results were seen rather quickly, within 1 to 2 months of beginning treatment. Only 2 patients showed no benefit with treatment and none had an increase in frequency (p < 0.02) [25]. This shows yet another mechanism in which suppression of hormonal fluctuations can treat seizure clustering throughout the menstrual cycle, but the safety and efficacy have not been established in larger studies.
Treatment—non-hormonal
Non-hormonal treatments such as acetazolamide have been used as treatment for catamenial epilepsy for much longer, but there are not large scale, randomized studies providing support of their efficacy [6]. Acetazolamide is a sulfonamide and inhibitor of carbonic anhydrase. It has been used since the 1950s as an anti-convulsant [26]. A retrospective analysis of women admitted to the Cleveland Clinic EMU from 1990 to 1991 looked at changes in seizure frequency and severity with acetazolamide therapy. Forty percent of the women claimed to have a decrease in seizure frequency, and 30 % claimed a decrease in severity. Acetazolamide was administered either daily or perimenstrually, and no significant difference in results was noted between dosing regimens [27]. The retrospective nature of the study creates limitations but nonetheless suggests some proof of efficacy in the decades-old therapy.
Clobazem is the only benzodiazepine that has been studied in daily usage to treat catamenial epilepsy. In a double-blind, cross-over study, doses between 20 and 30 mg were studied against placebo. Of the 18 patients who successfully completed the study, none had increased seizure frequency compared to placebo and 8 patients had a greater than 50 % reduction in seizure frequency [28]. This suggestion of efficacy with clobazam and the overall effectiveness of using benzodiazepines for seizure clustering have led to an acceptance of intermittent benzodiazepine use as a treatment approach for catamenial seizures.
Another possible avenue of seizure control may come in the form of oral contraceptives. There is no strong data showing this to be an effective treatment; however, given the evidence of hormonal neuroactivity as well as the relationship between hormone fluctuations and seizure clustering, it follows that modulating these fluctuations could reduce seizure frequency. The progestins in oral contraceptives act to suppress ovulation, and the combination pills containing both estrogen and progestins provide a stable hormonal milieu that suppresses the estrogen surge as well as the natural progesterone withdrawal. Dosage forms in which there is no withdrawal week for 3–12 month provide even less cyclic hormonal variation and are endorsed by some practitioners as a useful adjunct in the treatment of catamenial epilepsy.
As with any form of epilepsy, optimizing the patient’s anti-seizure therapies is another option. If the seizure pattern can be predicted based on the patient’s calendar, a temporary increase of 25–50 % of one anti-seizure medication may be useful. Of course, this should not be undertaken with phenytoin given its zero-order kinetics.
Conclusion
Like all epilepsy treatment approaches, there is no avenue that is effective for all patients with catamenial epilepsy. Figure 2 shows a basic algorithm that can assist in treating women with presumed catamenial seizure clustering based on the different subtypes. For women with a strong C1 pattern, natural progesterone may be considered. It should be kept in mind that the intermittent treatments are initiated within the menstrual cycle based on predicting when menstrual onset will be, in order to augment treatment on the vulnerable days. The treatment strategy should be tailored to the patient with considerations regarding the risks of intermittently increasing the background therapy, or with adding in a short benzodiazepine or acetazolamide course. Women with irregular menses may not be candidates for intermittent interventions. For these patients or for those who have failed the intermittent approaches, ongoing menstrual-suppressive therapies with MPA, oral contraceptives with infrequent withdrawal weeks may be appropriate. Tolerability for long-term menstrual cessation and the risks of decreased bone mineral density must be discussed.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Locock C. Discussion. In: Sieveking EH (ed) Analysis of fifty-two cases of epilepsy observed by the author. Med Times Gaz. 1857;14:524–6.
Foldvary-Schaefer N. Introduction. Atlas of epilepsies. London: Springer; 2010. p. 1295–9.
Herzog A, Fowler K, Sperling M, Massaro J, Group PTS. Distribution of seizures across the menstrual cycle in women with epilepsy. Epilepsia. 2015;56(5):e58–62.
Herzog A. Catamenial epilepsy: definition, prevalence, pathophysiology and treatment. Seizure. 2008;17:151–9.
Jones H. Luteal-phase defect: the role of Georgeanna Seegar Jones. Fert Ster. 2008;90(5):e5–7.
Reddy DS, Gould J, Gangisetty O. A mouse kindling model of perimenstrual catamenial epilepsy. J Pharm Exp Therap. 2012;341(3):784–93.
Harden C, Pennell P. Neuroendocrine considerations in the treatment of men and women with epilepsy. Lancet Neur. 2013;12:72–83.
Tauboll E, Sveberg L, Svalheim S. Interactions between hormones and epilepsy. Seizure. 2015;28:3–11.
Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306.
Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–683.
Smith SS. Estradiol administration increases neuronal response to excitatory amino acids as a long term effect. Brain Res. 1989;503:354–7.
Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-d-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7.
Finocchi C, Ferrari M. Female reproductive steroids and neuronal excitability. Neur Sci. 2011;32:31–5.
Nicoletti F, Speciale C, Sortino M, Summa G, Caruso G, Patti F, et al. Comparative effects of estradiol benzoate, the antiestrogen clomiphene citrate, and the progestin medroxyprogesterone acetate on kainic acid-induced seizures in male and female rats. Epilepsia. 1985;26(3):252–7.
Herzog A, Klein P, Ransil B. Three patterns of catamenial epilepsy. Epilepsia. 1997;38(10):1082–8.
Herzog A, Progesterone Trial Study Group. Distribution of seizures across the menstrual cycle in women with epilepsy. Epilepsia. 2015;56(5):e58–62.
Herzog A. Intermittent progesterone therapy and frequency of complex partial seizures in women with menstrual disorders. Neurology. 1986;36:1607–10.
Herzog A. Progesterone therapy in complex partial seizures and secondary generalized seizures. Neurology. 1995;45:1660–2.
Herzog AG, Fowler KM, Smithson SD, Kalayjian LA, Heck CN, Sperling MR, et al. For the Progesterone Trial Study Group. Progesterone vs placebo therapy for women with epilepsy: a randomized clinical trial. Neurology. 2012;78(24):1959–66. Major double blinded trial looking at progesterone treatment in women with different catamenial seizure types. Showed significant outcomes in post hoc analysis in C1 subtype with greater than 2 fold increase seizure frequency.
Herzog A. Catamenial epilepsy: update on prevalence, pathophysiology and treatment from the findings of the NIH progesterone treatment trial. Seizure. 2015;28:18–25.
Herzog A, Frye C. Allopregnanolone levels and seizure frequency in progesterone-treated women with epilepsy. Neurology. 2014;83:345–8.
Herzog A, Frye C. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–1.
Mattson R, Cramer J, Caldwell B, Siconolfi B. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology. 1984;34:1255–8. Strong evidence showing decrease in catamenial seizure exacerbations in women taking large enough amount of MPA to stop ovulatory cycle.
Clark M, Sowers M, Levy B, et al. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86(5):1466–74.
Bauer J, Wildt L, Flugel D, Stefan H. The effect of a synthetic GnRH analogue on catamenial epilepsy: a study in ten patients. J Neurol. 1992;239:284–6.
Bergstrom W. Observations on metabolic and clinical effects of carbonic anhydrase inhibitors in epileptics. Am J Dis Child. 1952;84:771.
Lim LL, Foldvary N, Mascha E, Lee J. Acetazolamide in women with catamenial epilepsy. Epilepsia. 2001;42(6):746–9.
Feely M, Calvert R and J Gibson. Clobazem in catamenial epilepsy: a model for evaluating anticonvulsants. The Lancet. 1982. 71–73
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Epilepsy
Rights and permissions
About this article
Cite this article
Navis, A., Harden, C. A Treatment Approach to Catamenial Epilepsy. Curr Treat Options Neurol 18, 30 (2016). https://doi.org/10.1007/s11940-016-0413-6
Published:
DOI: https://doi.org/10.1007/s11940-016-0413-6