Abstract
Purpose of review
Peripartum cardiomyopathy (PPCM) is a potentially catastrophic form of heart failure caused by left ventricular systolic dysfunction that develops during pregnancy or in the early postpartum period. After the initial diagnosis and treatment, many women desire another pregnancy; however, risks of a subsequent pregnancy need to be considered, as PPCM may lead to significant adverse outcomes for both mother and fetus. The goal of this review is to provide information about risk stratification prior to subsequent pregnancy, strategies to mitigate the risk of subsequent pregnancy, and long-term maternal outcomes.
Recent findings
The degree of myocardial recovery is currently the most effective predictor of heart failure relapse and adverse outcomes during a subsequent pregnancy. Women with persistent left ventricular systolic dysfunction have worse maternal and fetal outcomes during subsequent pregnancy. Pharmacologic options for the acute management of heart failure during pregnancy are limited to diuretics, beta-blockers, hydralazine, isosorbide dinitrate, and digoxin. After delivery, however, most guideline-directed heart failure medications can be used safely, including in women who are breastfeeding. Because of the risks of subsequent pregnancy, options for contraception should be discussed with women with PPCM. Finally, women with PPCM should be under the care of a multidisciplinary cardio-obstetrics team for preconception counseling and management during a subsequent pregnancy.
Summary
An essential component of caring for women with PPCM includes detailed counseling about the risks of a subsequent pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overview of peripartum cardiomyopathy
Peripartum cardiomyopathy (PPCM) is an idiopathic cardiomyopathy in which left ventricular (LV) systolic dysfunction develops during pregnancy or in the early postpartum period [1, 2•, 3]. The incidence of PPCM in the USA is estimated to be about 1 in 968 live births [4]. African-American women are more commonly affected than other racial groups [5]. Older maternal age, multifetal pregnancies, hypertension, and preeclampsia are also risk factors for PPCM [2, 6]. Outcomes are variable, ranging from prompt complete recovery, to persistent myocardial dysfunction and symptomatic heart failure, to severe heart failure with need for advanced therapies. In one study, PPCM was associated with a 25% risk of major adverse events, including death, cardiac arrest, heart transplantation, mechanical circulatory support, fulminant pulmonary edema, thromboembolism, and implantable cardioverter defibrillator or pacemaker implantation [7].
Rates of myocardial recovery are variable, ranging from 45 to 96% [8, 9•, 10•]. The likelihood of recovery of left ventricular ejection fraction (LVEF) remains challenging to predict. Recovery may be impacted by initial LVEF, race, medical treatment, delays in diagnosis, concomitant preeclampsia, and additional factors [9•, 11,12,13, 14•]. In the Investigations of Pregnancy-Associated Cardiomyopathy (IPAC) study, the overall rate of LVEF recovery was 72%; however, the rate of LVEF recovery in African-American women was lower at 59%, despite similar rates of guideline-directed medical therapy [9•]. Improvement in LVEF typically occurs within 2 to 6 months of the diagnosis, although recovery may occur up to 2 years later [6, 15].
PPCM presents unique challenges because it not only impacts the health of the mother and her child (if diagnosed during pregnancy), but future pregnancies are associated with recurrent heart failure, deterioration of LVEF, and additional adverse maternal and fetal outcomes. One of the most frequently asked questions by women with a diagnosis of PPCM is about subsequent pregnancies [16]. In this review, we will illustrate some of the important points to consider when counseling women with a history of PPCM about subsequent pregnancies.
Contraception
In order to avoid unplanned pregnancies, the discussion about another pregnancy needs to begin with discussion of contraception. An unplanned pregnancy in the presence of significant LV dysfunction leads to difficult conversations and shared decision-making about the need to terminate a pregnancy. Unplanned pregnancies should also be avoided in order to allow for thorough counseling about the risks, and certain medications (such as ACE-inhibitors, aldosterone receptor blockers, aldosterone antagonists, sacubitril-valsartan) may need to be stopped prior to pregnancy; therefore, counseling about risks of pregnancy should also include a discussion of contraception. Findings from a survey of 177 women with PPCM underscored the importance of this counseling; while 74% of the women surveyed reported wanting more biological children, 25% reported not receiving information regarding contraception and the risks of subsequent pregnancies [16]. Additionally, despite being sexually active, 27% of women with PPCM reported never or rarely using contraception, and close to 35% reported using non-hormonal barriers, withdrawal, or the rhythm method, which are associated with high failure rates [16, 17]. These findings highlight an important patient-provider mismatch in priorities and counseling.
Tepper et al. performed a systematic review of the safety of pharmacologic contraception in women with cardiac disease. They included 3 relevant articles that prospectively studied 169 women with cardiac disease, including 5 women with cardiomyopathy, who were on pharmacologic methods of contraception, including progesterone-only pills, combine oral contraceptives, depot medroxyprogesterone acetate, and copper intrauterine device; in this study, the intrauterine device use was associated with the least cardiac adverse effects [18]. Of note, this older study did not include the levonorgestrel intrauterine device which is also very safe and highly effective [19]. In women at high risk of pregnancy complications, the most important consideration in choosing a method of contraception is finding an effective method that a woman is willing to use reliably. Contraception options for women with PPCM are listed in Table 1 [19, 20].
Risk assessment
Counseling regarding maternal outcomes
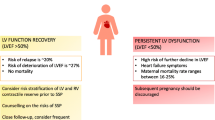
Currently, the degree of LVEF recovery is the most effective predictor of heart failure relapse during subsequent pregnancies [21]. In particular, a recovered LVEF of at least 50% is associated with significantly lower rates of relapse of heart failure, maternal mortality, and premature delivery. Elkayam et al. performed a retrospective review of 44 women who had PPCM and 60 subsequent pregnancies [22]. In this study, normal LV systolic function was defined as a LVEF of at least 50%. In the first subsequent pregnancies, 28 women had normal LV systolic function (group 1, average LVEF of 56 ± 7%) and 16 women had persistent LV systolic dysfunction (group 2, average LVEF of 36 ± 9%). In both groups, there was a decrease in LVEF during the subsequent pregnancy (group 1: 56 ± 7% to 49 ± 10%, p = 0.002; group 2: 36 ± 9% to 32 ± 11%, p = 0.08). Pre-pregnancy normal LV systolic function was associated with less heart failure symptoms (21 vs. 44%) and a lower incidence of a substantial (> 20%) decrease in ejection fraction (21 vs. 25%) during subsequent pregnancy. Normal LV systolic function pre-pregnancy also was associated with significantly lower maternal mortality after the subsequent pregnancy (0 vs. 19%) and less long-term LV systolic dysfunction (14 vs. 31% at a mean of 72 months) [22]. Several small earlier studies corroborated the conclusion that a pre-pregnancy LVEF of at least 50% is associated with lower rates of heart failure relapse, persistent LV systolic dysfunction, and maternal mortality [23,24,25]. Data from more recent studies since 2010 are summarized in Table 2 [26,27,28, 29•]. Our suggested approach to counseling patients with PPCM on subsequent pregnancy is outlined in Fig. 1.
Approach to counseling patients with peripartum cardiomyopathy about subsequent pregnancy. * Would assess LV ejection fraction after 3 months off ACE/ARB/spironolactone/sacubitril-valsartan. If normal LV function, would consider assessment for subclinical dysfunction using strain imaging and/or exercise stress echocardiogram to assess contractile reserve and functional capacity. LV, left ventricular; LVEF, left ventricular ejection fraction
Many studies have used an LVEF of 50% or greater as the definition of myocardial recovery. However, a stricter definition for recovery would be a LVEF of 55% or greater, and may be associated with lower rates of relapse. In a study by Fett et al., 56 women with PPCM had 61 subsequent pregnancies [26]. Relapsed heart failure was defined as a decrease in LVEF to 45% or less, a decrease in LVEF by 10% in women with a baseline LVEF of 45% or less, or a change in the New York Heart Association (NYHA) classification. There were 18 heart failure relapses (29.5%), and the rate of relapse was significantly different based on the LVEF before subsequent pregnancy. The rate of relapse was 67% for women with a LVEF of less than 45%, 35% for women with a LVEF of 45–54%, and 17% for women with a LVEF of 55% or higher (p < 0.001) [26]. This data suggests that the definition of normal recovered LV systolic function should likely be a LVEF of 55% or more, which is more consistent with the American Society of Echocardiography guidelines for normal LVEF in women [30].
This data highlights that when counseling women with PPCM about subsequent pregnancy, it is important to discuss that even if a woman’s recovered LV systolic function is normal, a subsequent pregnancy is not risk-free. The data from Elkayam et al. and Fett et al. suggest that even with LVEF recovery pre-pregnancy, the risk of relapsed heart failure and persistent LV systolic dysfunction is about 15–20% [22, 26]. Close monitoring of symptoms and LVEF by echocardiography is necessary during subsequent pregnancies to detect heart failure and deterioration in cardiac function. Furthermore, women may not recover again after a subsequent pregnancy and may have ongoing heart failure requiring lifelong heart failure medications.
Women with PPCM and persistent LV systolic dysfunction need to be counseled that the risk of subsequent pregnancy also includes maternal mortality. Because of these risks, the European Society of Cardiology (ESC) guidelines state that subsequent pregnancy is not recommended in women with PPCM and residual LV systolic dysfunction [31••]. In real-world clinical practice, patients and providers often have more nuanced discussions of risks and priorities, but for women with persistent LV systolic dysfunction, other options for subsequent pregnancy should be discussed, including surrogacy and adoption.
Counseling regarding fetal outcomes
When counseling women with PPCM about subsequent pregnancy, the risk of adverse fetal outcomes should also be addressed. In the cohort of women reviewed by Elkayam et al., persistent LV systolic dysfunction was associated with higher rates of abortion and premature delivery [22]. In the group with a LVEF of at least 50%, one therapeutic abortion occurred (4%). The rate of premature delivery, which was defined as delivery at less than 37 weeks, was 13%. In the group with a LVEF less than 50%, 4 therapeutic abortions occurred (25%). The rate of premature delivery was significantly higher at 50%. In this group, premature delivery occurred in 6 women (2 women at 30 weeks, 1 at 34 weeks, 1 at 35 weeks, and 2 at 36 weeks). In both groups, there was no perinatal mortality [22].
Assessment of subclinical dysfunction for risk assessment
While recovery of LVEF pre-pregnancy is associated with better maternal and fetal outcomes during subsequent pregnancy, many women with recovered LVEF still develop recurrent heart failure. Because of this, other methods for risk stratification have been proposed, such as assessing myocardial contractile reserve by exercise stress echocardiography, strain, cardiac magnetic resonance imaging (MRI), and maintenance of LV systolic function after withdrawal of heart failure medications.
Lampert et al. assessed the contractile reserve of patients with PPCM and normal LV size and systolic function with matched controls via dobutamine challenge [32]. They found that the contractile reserve was significantly reduced in patients with recovered PPCM (p < 0.03). These findings provide a possible explanation for why patients with a history of PPCM have worse outcomes during subsequent pregnancies, even if the LV size and systolic function have returned to normal. Fett et al. prospectively identified 56 patients with PPCM who underwent 61 subsequent pregnancies. In this population, the overall rate of relapsed heart failure was 29.5%. The rate of relapsed heart failure was significantly lower in patients with recovered LVEF (17.1 vs. 46.2%, p < 0.001). Nine of 29 mothers with recovered LVEF underwent exercise stress echocardiography and all demonstrated adequate contractile reserve. These 9 women did not experience recurrent heart failure in subsequent pregnancies [26]. These findings suggest that assessing contractile reserve could help identify women at greater risk for complications with subsequent pregnancies, but further studies are needed.
Deformation indices, such as strain, have been shown to be sensitive markers of subclinical LV dysfunction in many conditions, including PPCM [33]. LV global longitudinal strain is routinely used to monitor patients receiving cardiotoxic chemotherapies as the decrease in strain precedes a decrease in LVEF [34]. Decreased LV global longitudinal strain has prognostic value in other diseases as well, such as asymptomatic severe aortic stenosis [35]. Goland et al. assessed 29 patients with PPCM and LVEF recovery and found significantly lower LV global longitudinal strain compared with controls (− 19.1 vs. − 22.7, p < 0.001) [36]. This imaging technique may provide additional prognostic information in patients with PPCM, but additional studies are needed to determine whether it has a role in counseling women with PPCM about subsequent pregnancy.
Late gadolinium enhancement (LGE) on cardiac MRI has prognostic value in nonischemic dilated cardiomyopathy and is associated with an increased risk of sudden cardiac death and cardiovascular mortality [37]. The prognostic value of cardiac MRI in PPCM remains unclear. Schelbert et al. recruited 40 women from the IPAC cohort to undergo cardiac MRI and only found LGE in 2 women (5%). Furthermore, the presence of LGE was not predictive of LVEF, NYHA class, or mortality [38]. Ersboll et al. found a similarly low prevalence of LGE, present in only 1 of 28 women with PPCM [39]. Additional studies are needed to determine whether cardiac MRI findings can help with risk stratification in PPCM.
Genetic testing may also prove useful for counseling women about their risk of relapse during a subsequent pregnancy. Several studies have identified genetic mutations in women with PPCM that overlap with those of idiopathic dilated cardiomyopathy [40,41,42,43]. Truncating variants in the TTN gene are over-represented in patients with PPCM [43], as well as chemotherapy- and alcohol-related cardiomyopathies [44, 45]. While it is possible that women with TTN truncating variants may be at increased risk for relapse with a subsequent pregnancy, it is also important to note that > 90% of individuals with TTN truncating variants do not develop dilated cardiomyopathy or PPCM [46]. Therefore, additional research is needed before genetic testing can be reliably used for preconception risk stratification.
Finally, maintenance of normal LV systolic function after withdrawal of heart failure medications is an important consideration. Fett et al. found that in the 9 women who had a recovered LVEF of at least 55%, maintained a LVEF of at least 55% after withdrawal of heart failure medications, and demonstrated adequate contractile reserve by exercise stress echocardiography, there were no instances of relapsed heart failure with subsequent pregnancy [26]. During pregnancy, ACE-inhibitors, aldosterone receptor blockers, mineralocorticoid antagonists, and sacubitril-valstartan are contraindicated. Therefore, discontinuation of these medications and reassessment of LVEF after approximately 3 months is often recommended to ensure the LVEF remains normal.
Potential treatments to mitigate risk during subsequent pregnancy
Use of beta-blockers
Options for heart failure therapy during pregnancy are limited due to potential adverse effects on the fetus. The prophylactic use of beta-blockers in patients with PPCM during subsequent pregnancies has been proposed, although data to support this practice is limited, and they are associated with lower adjusted fetal birth weight [47]. Codsi et al. performed a retrospective review of 25 patients with PPCM and 43 subsequent pregnancies. Beta-blockers were used during 19 of 43 subsequent pregnancies. During these pregnancies, six women (31.6%) had relapsed heart failure and there was no intrauterine growth retardation [29•]. This limited data suggests that beta-blockers are relatively safe to use but the benefit in preventing recurrent heart failure is unclear. Generally, beta-blockers are frequently continued if a woman was taking this prior to pregnancy. The most frequently used beta-blocker during pregnancy is metoprolol tartrate, but other beta-blockers may also be used, with the exception of atenolol which has the highest risk of intrauterine growth restriction.
Use of bromocriptine, considered experimental
A pathologic fragment of prolactin has been implicated in the development of PPCM in mouse models [48]. Because of this association, there has been interest in whether bromocriptine, a dopamine agonist that inhibits the release of prolactin, could optimize recovery from PPCM. The initial pilot study showed promise [49], but was limited by small sample size in a select population. Subsequently, a German study evaluated two regimens of bromocriptine and found similar rates of LVEF recovery and hospitalizations for heart failure in both groups, but no control group was included so the benefit over standard heart failure therapy remains unclear [50]. The risks of bromocriptine are important to consider. Bromocriptine causes lactation suppression, so the downstream effects of not breastfeeding should be discussed with patients. Additionally, bromocriptine is associated with thrombotic complications, including myocardial infarction and stroke, so anticoagulation is recommended for patients taking bromocriptine [51,52,53,54]. Because of the unclear benefit, bromocriptine remains an experimental therapy in the USA.
Heart failure medications during pregnancy and after delivery
During pregnancy, options for heart failure medications are limited since angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone receptor antagonists are contraindicated because they are associated with fetal abnormalities [55]. Beta-blockers, hydralazine, isosorbide dinitrate, and diuretics can be used for the acute management of heart failure during pregnancy. After delivery, almost all guideline-directed heart failure medications can be used safely, including in women who are breastfeeding. Loop diuretics, beta-blockers, hydralazine, nitrates, digoxin, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone receptor antagonists can be used while breastfeeding. Sacubitril-valsartan and ivabradine have not been studied in humans but animal studies demonstrate the presence of the medication in milk [55].
Pregnancy is a hypercoagulable state. As such, in women with PPCM and a LVEF of less than 30–35%, anticoagulation should be considered [31, 56••]. During pregnancy, low-molecular weight heparin or intravenous heparin is preferred as warfarin crosses the placenta and has possible teratogenic effects. In the postpartum period, warfarin is safe to use with breastfeeding [55].
Breastfeeding
Breastfeeding provides numerous medical benefits for infants. Medical benefits of breastfeeding for mothers include lower rates of breast cancer, lower rates of metabolic syndrome, and improved birth spacing [57, 58]. From a practical standpoint, breastfeeding is crucial for infant nutrition when formula is not financially feasible or in areas of the world where access to clean water is not available [59, 60]. Since prolactin has been implicated in the development of PPCM, it has been hypothesized that breastfeeding would be associated with worse outcomes in women with PPCM. Based on this hypothesis, the 2010 ESC guidelines advised against breastfeeding in patients with suspected PPCM [1]. However, recent studies suggest that breastfeeding is safe in women with PPCM [61•, 62]. Koczo et al. measured prolactin levels in 100 women with PPCM. They found that while breastfeeding, women had higher prolactin levels than women who were not breastfeeding, but there was no significant difference in recovery of LV systolic function [61•]. This data is reassuring that breastfeeding should not impact the safety of subsequent pregnancies in most women.
Monitoring during subsequent pregnancy
Women with a history of PPCM should be under the care of a multidisciplinary team when proceeding with a subsequent pregnancy. Close monitoring of symptoms, repeat assessment of LV systolic function by echocardiography, and monitoring of B-type natriuretic peptide levels should occur each trimester, after delivery before discharge from the hospital, and at 1 month post-discharge, as well as if new symptoms develop. The degree of LV systolic dysfunction and symptom burden will influence the frequency of evaluation. Finally, vaginal delivery is usually preferred unless there is an obstetric reason for a cesarean delivery [2•].
Mental health
It is important to acknowledge the psychological toll of PPCM, as it often impacts otherwise healthy young women and significantly affects their quality of life and future family planning. In the survey by Rosman et al. of 177 women with PPCM, clinical depression was present in nearly one third of women. PPCM patients with depression were more likely to have a higher body mass index (BMI), require antihypertensive medications, and receive disability, and they were less likely to attend scheduled medical appointments [63]. Another survey of 149 women with PPCM found that 53% reported elevated generalized anxiety symptoms [64]. Therefore, when caring for women with PPCM, and especially when having difficult conversations about subsequent pregnancies, attention to mental health, stress, anxiety, and depression are essential components of counseling.
Racial disparities in outcomes
Multiple studies have shown that non-Hispanic black women have worse outcomes during pregnancy and suffer significantly higher rates of heart failure, acute renal failure, and acute respiratory distress syndrome during delivery when compared with non-Hispanic white women [65]. African American women with PPCM have been found to be diagnosed later in the postpartum period, present with more severe dysfunction (LVEF less than 30%), and are less likely to have LVEF recovery when compared with non-African American women [11]. These disparities may be related to health care delivery, socioeconomic differences, and the multi-faceted effects of structural racism. Studies are needed to examine whether racial disparities also exist in the outcomes after subsequent pregnancy in women with PPCM.
Long-term outcomes
The long-term outcomes and the duration of heart failure treatment after LVEF recovery remains unclear, and women should be counseled that they may require lifelong heart failure medications. Amos et al. performed a retrospective review of 55 patients with PPCM. In this population, 51% of the patients were African-American, the baseline LVEF was 20.5%, and a high percentage was placed on guideline-directed medical therapy for heart failure (90% were on angiotensin-converting enzyme (ACE) inhibitors and 69% were on beta-blockers). At follow-up, 45% had LVEF recovery and the average LVEF was 41 ± 14%. Eleven of the patients with LVEF recovery had their ACE inhibitor or beta-blocker discontinued, and five patients had both medications discontinued. After an average of 29 months, none of these patients had a decrease in LVEF [8]. The most recent, largest registry data available about outcomes in women with PPCM is limited by only 6 months of follow-up. In their recent publication from the EuroObservational PPCM Registry, Sliwa et al. reported on 739 women with PPCM. Sixty-seven percent of the women had a LVEF of 35% or less at diagnosis. At 6 months, the all-cause mortality rate was 6%. The rate of LVEF recovery, which was defined as a LVEF of at least 50%, was 46%. At 6 months, 23% of women had persistent, severe LV systolic dysfunction with a LVEF of 35% or less [10•]. This data underscores the vast differences in outcomes and recovery among women with PPCM, which causes significant difficulty for counseling women about short- and long-term risks. These considerations are essential when women are making decisions about expanding their families with a subsequent pregnancy.
Conclusion
Understanding the methods to risk stratify women with PPCM, ways to optimize their risk when proceeding with subsequent pregnancy, and long-term maternal outcomes after subsequent pregnancy are important considerations when counseling women with PPCM about pursuing subsequent pregnancy.
Abbreviations
- PPCM:
-
Peripartum cardiomyopathy
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- IPAC:
-
Investigations of Pregnancy-Associated Cardiomyopathy
- NYHA:
-
New York Heart Association
- MRI:
-
Magnetic resonance imaging
- ESC:
-
European Society of Cardiology
- LGE:
-
Late gadolinium enhancement
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–78.
• Davis MB, Arany Z, McNamara DM, Goland S, Peripartum Cardiomyopathy EU. JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(2):207–21 This recent manuscript provides a comprehensive review of peripartum cardiomyopathy, including the diagnosis, prognosis, treatment, outcomes, and considerations for subsequent pregnancies.
Bauersachs J, König T, van der Meer P, Petrie MC, Hilfiker-Kleiner D, Mbakwem A, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2019;21(7):827–43.
Kolte D, Khera S, Aronow WS, Palaniswamy C, Mujib M, Ahn C, et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population-based study. J Am Heart Assoc. 2014;3(3):e001056.
Kao DP, Hsich E, Lindenfeld J. Characteristics, adverse events, and racial differences among delivering mothers with peripartum cardiomyopathy. JACC Heart Fail. 2013;1(5):409–16.
Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58(7):659–70.
Goland S, Modi K, Bitar F, Janmohamed M, Mirocha JM, Czer LSC, et al. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail. 2009;15(8):645–50.
Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152(3):509–13.
• McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, et al. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66(8):905–14 This is the multi-center US IPAC Study that describes the characteristics and short-term outcomes from 100 women with well-defined peripartum cardiomyopathy.
• Sliwa K, Petrie MC, van der Meer P, Mebazaa A, Hilfiker-Kleiner D, Jackson AM, et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J. 2020;41(39):3787–97 This is the most recent report from the European Sociey of Cardiology EuroObservational Peripartum Cardiomyopathy Registry. It included 739 women from 49 countries.
Irizarry OC, Levine LD, Lewey J, Boyer T, Riis V, Elovitz MA, et al. Comparison of clinical characteristics and outcomes of peripartum cardiomyopathy between African American and non-African American women. JAMA Cardiol. 2017 Nov 1;2(11):1256–60.
Fett JD. Earlier detection can help avoid many serious complications of peripartum cardiomyopathy. Futur Cardiol. 2013 Nov;9(6):809–16.
Lindley KJ, Conner SN, Cahill AG, Novak E, Mann DL. Impact of preeclampsia on clinical and functional outcomes in women with peripartum cardiomyopathy. Circ Heart Fail. 2017;10(6).
• Karaye KM, Sa’idu H, Balarabe SA, Ishaq NA, Adamu UG, Mohammed IY, et al. Clinical features and outcomes of peripartum cardiomyopathy in Nigeria. J Am Coll Cardiol. 2020;76(20):2352–64 This paper describes the characteristics and outcomes from the PEACE Registry in Nigeria and was notable for worse survival and outcomes than studies from other geographic regions.
Biteker M, Ilhan E, Biteker G, Duman D, Bozkurt B. Delayed recovery in peripartum cardiomyopathy: an indication for long-term follow-up and sustained therapy. Eur J Heart Fail. 2012;14(8):895–901.
Rosman L, Salmoirago-Blotcher E, Wuensch KL, Cahill J, Sears SF. Contraception and reproductive counseling in women with peripartum cardiomyopathy. Contraception. 2017;96(1):36–40.
Lindley KJ, Conner SN, Cahill AG, Contraception MT. Pregnancy planning in women with congenital heart disease. Curr Treat Options Cardiovasc Med. 2015;17(11):50.
Tepper NK, Paulen ME, Marchbanks PA, Curtis KM. Safety of contraceptive use among women with peripartum cardiomyopathy: a systematic review. Contraception. 2010;82(1):95–101.
Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep. 2016;65(3):1–103.
Sundaram A, Vaughan B, Kost K, Bankole A, Finer L, Singh S, et al. Contraceptive failure in the United States: estimates from the 2006-2010 National Survey of Family Growth. Perspect Sex Reprod Health. 2017;49(1):7–16.
Fett JD, Shah TP, McNamara DM. Why do some recovered peripartum cardiomyopathy mothers experience heart failure with a subsequent pregnancy? Curr Treat Options Cardiovasc Med. 2015;17(1):354.
Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344(21):1567–71.
Avila WS, de Carvalho MEC, Tschaen CK, Rossi EG, Grinberg M, Mady C, et al. Pregnancy and peripartum cardiomyopathy. A comparative and prospective study Arq Bras Cardiol. 2002;79(5):484–93.
Sliwa K, Forster O, Zhanje F, Candy G, Kachope J, Essop R. Outcome of subsequent pregnancy in patients with documented peripartum cardiomyopathy. Am J Cardiol. 2004;93(11):1441–3 A10.
Habli M, O’Brien T, Nowack E, Khoury S, Barton JR, Sibai B. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–5.
Fett JD, Fristoe KL, Welsh SN. Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2010;109(1):34–6.
Mandal D, Mandal S, Mukherjee D, Biswas SC, Maiti TK, Chattopadhaya N, et al. Pregnancy and subsequent pregnancy outcomes in peripartum cardiomyopathy. J Obstet Gynaecol Res. 2011;37(3):222–7.
Hilfiker-Kleiner D, Haghikia A, Masuko D, Nonhoff J, Held D, Libhaber E, et al. Outcome of subsequent pregnancies in patients with a history of peripartum cardiomyopathy. Eur J Heart Fail. 2017;19(12):1723–8.
• Codsi E, Rose CH, Blauwet LA. Subsequent pregnancy outcomes in patients with peripartum cardiomyopathy. Obstet Gynecol. 2018;131(2):322–7 This recent cohort study from Mayo Clinic included 25 women with 43 subsequent pregnancies and described favorable outcomes (all except one had left ventricular ejection fraction of 50% or higher).
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2015;28(1):1–39.e14.
•• Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–241 This is the 2018 European Society of Cardiology Guidelines for the management of cardiovascular diseases during pregnancy, including peripartum cardiomyopathy.
Lampert MB, Weinert L, Hibbard J, Korcarz C, Lindheimer M, Lang RM. Contractile reserve in patients with peripartum cardiomyopathy and recovered left ventricular function. Am J Obstet Gynecol. 1997;176(1 Pt 1):189–95.
Sugahara M, Kagiyama N, Hasselberg NE, Blauwet LA, Briller J, Cooper L, et al. Global left ventricular strain at presentation is associated with subsequent recovery in patients with peripartum cardiomyopathy. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2019;32(12):1565–73.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2014;27(9):911–39.
Vollema EM, Sugimoto T, Shen M, Tastet L, Ng ACT, Abou R, et al. Association of left ventricular global longitudinal strain with asymptomatic severe aortic stenosis: natural course and prognostic value. JAMA Cardiol. 2018;3(9):839–47.
Goland S, Weinstein JM, Zalik A, Kuperstein R, Zilberman L, Shimoni S, et al. Angiogenic imbalance and residual myocardial injury in recovered peripartum cardiomyopathy patients. Circ Heart Fail. 2016;9(11).
Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309(9):896–908.
Schelbert EB, Elkayam U, Cooper LT, Givertz MM, Alexis JD, Briller J, et al. Myocardial damage detected by late gadolinium enhancement cardiac magnetic resonance is uncommon in peripartum cardiomyopathy. J Am Heart Assoc. 2017;6(4).
Ersbøll AS, Bojer AS, Hauge MG, Johansen M, Damm P, Gustafsson F, et al. Long-term cardiac function after peripartum cardiomyopathy and preeclampsia: a Danish Nationwide, clinical follow-up study using maximal exercise testing and cardiac magnetic resonance imaging. J Am Heart Assoc. 2018;7(20):e008991.
Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108(4):366.
van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP, Hilfiker-Kleiner D, Bollen IAE, Sliwa K, et al. Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur Heart J. 2014;35(32):2165–73.
van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JDH, Paulus WJ, et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 2010;121(20):2169–75.
Ware JS, Seidman JG, Arany Z. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374(26):2601–2.
Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, et al. Genetic variants associated with cancer therapy-induced cardiomyopathy. Circulation. 2019;140(1):31–41.
Ware JS, Amor-Salamanca A, Tayal U, Govind R, Serrano I, Salazar-Mendiguchía J, et al. Genetic etiology for alcohol-induced cardiac toxicity. J Am Coll Cardiol. 2018;71(20):2293–302.
Haggerty CM, Damrauer SM, Levin MG, Birtwell D, Carey DJ, Golden AM, et al. Genomics-first evaluation of heart disease associated with titin-truncating variants. Circulation. 2019;140(1):42–54.
Ruys TPE, Maggioni A, Johnson MR, Sliwa K, Tavazzi L, Schwerzmann M, et al. Cardiac medication during pregnancy. data from the ROPAC Int J Cardiol. 2014;177(1):124–8.
Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128(3):589–600.
Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema J-P, Becker A, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121(13):1465–73.
Hilfiker-Kleiner D, Haghikia A, Berliner D, Vogel-Claussen J, Schwab J, Franke A, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J. 2017;38(35):2671–9.
Hopp L, Haider B, Iffy L. Myocardial infarction postpartum in patients taking bromocriptine for the prevention of breast engorgement. Int J Cardiol. 1996;57(3):227–32.
Hopp L, Weisse AB, Iffy L. Acute myocardial infarction in a healthy mother using bromocriptine for milk suppression. Can J Cardiol. 1996;12(4):415–8.
Dutt S, Wong F, Spurway JH. Fatal myocardial infarction associated with bromocriptine for postpartum lactation suppression. Aust N Z J Obstet Gynaecol. 1998;38(1):116–7.
Iffy L, Lindenthal J, Mcardle JJ, Ganesh V. Severe cerebral accidents postpartum in patients taking bromocriptine for milk suppression. Isr J Med Sci. 1996;32(5):309–12.
Drugs and Lactation Database (LactMed) [Internet]. [cited 2021 Jan 23]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK501922/
•• Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134(23):e579–646 This is the scientific statement from the American Heart Association on the current treatment strategies for dilated cardiomyopathies, including peripartum cardiomyopathy.
Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet Lond Engl. 2016;387(10017):475–90.
Sattari M, Serwint JR, Levine DM. Maternal implications of breastfeeding: a review for the internist. Am J Med. 2019;132(8):912–20.
Fett JD. Caution in the use of bromocriptine in peripartum cardiomyopathy. J Am Coll Cardiol. 2008;51(21):2083 author reply 2083-2084.
Fett JD, Murphy JG. Infant survival in Haiti after maternal death from peripartum cardiomyopathy. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2006 Aug;94(2):135–6.
• Koczo A, Marino A, Jeyabalan A, Elkayam U, Cooper LT, Fett J, et al. Breastfeeding, cellular immune activation, and myocardial recovery in peripartum cardiomyopathy. JACC Basic Transl Sci. 2019;4(3):291–300 This study from the IPAC Study Investigators showed there was no adverse association between breastfeeding and peripartum cardiomyopathy.
Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, Staniloae C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol. 2012;154(1):27–31.
Rosman L, Salmoirago-Blotcher E, Cahill J, Wuensch KL, Sears SF. Depression and health behaviors in women with peripartum cardiomyopathy. Heart Lung J Crit Care. 2017;46(5):363–8.
Rosman L, Salmoirago-Blotcher E, Cahill J, Psychosocial Adjustment SSF. Quality of life in patients with peripartum cardiomyopathy. J Cardiovasc Nurs. 2019;34(1):20–8.
Admon LK, Winkelman TNA, Zivin K, Terplan M, Mhyre JM, Racial DVK. Ethnic disparities in the incidence of severe maternal morbidity in the United States, 2012-2015. Obstet Gynecol. 2018;132(5):1158–66.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Megan S. Joseph and Melinda B. Davis declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Reproductive Health and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Joseph, M.S., Davis, M.B. Counseling Women With Peripartum Cardiomyopathy About Subsequent Pregnancies. Curr Treat Options Cardio Med 23, 41 (2021). https://doi.org/10.1007/s11936-021-00915-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-021-00915-4