Abstract
Purpose of Review
In recent years, there has been renewed interest in the use of contrast-enhanced ultrasound (CEUS) in abdominal imaging and intervention. The goal of this article is to review the practical applications of CEUS in the kidney, including renal mass characterization, treatment monitoring during and after percutaneous ablation, and biopsy guidance.
Recent Findings
Current evidence suggests that CEUS allows accurate differentiation of solid and cystic renal masses and is an acceptable alternative to either computed tomography (CT) or magnetic resonance imaging (MRI) for characterization of indeterminate renal masses. CEUS is sensitive and specific for diagnosing residual or recurrent renal cell carcinoma (RCC) following percutaneous ablation. Furthermore, given its excellent spatial and temporal resolution, CEUS is well suited to demonstrate tumoral microvascularity associated with malignant renal masses and is an effective complement to conventional grayscale ultrasound (US) for percutaneous biopsy guidance.
Summary
Currently underutilized, CEUS is an important problem-solving tool in renal imaging and intervention whose role will continue to expand in coming years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Incidental renal masses are commonly identified in cross-sectional imaging. In one recent analysis, for example, up to 14% of patients undergoing screening computed tomography colonography were found to have an incidental renal mass [1]. Distinction between benign and malignant renal masses can be challenging on the basis of imaging features alone. One systematic review of the literature showed that renal masses suspected of being renal cell carcinoma (RCC) by imaging had a significant chance of benignity after nephrectomy. The risk of the mass being benign was greatest for lesions less than 1 cm (40%) but was almost 20% for lesions measuring 2–3 cm; masses between 4 and 7 cm still had a 9% of being benign [2]. Renal mass biopsy can reliably and safely differentiate between benign and malignant lesions [3]. Approximately 15% of percutaneous, image-guided renal mass biopsies are non-diagnostic [4, 5]. Non-diagnostic rates are higher for endophytic [6], cystic, and hypoenhancing [7] lesions.
Despite ready availability and routine use in both Europe and Asia, contrast-enhanced ultrasound (CEUS) has been slow to gain traction in the USA, due in part to regulatory obstacles and reliance on alternative imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI) [8]. However, following the Food and Drug Administration’s 2016 decision to approve the use of Lumason (Bracco Diagnostics Inc., Monroe Township, NJ) for the characterization of indeterminate liver lesions, there has been renewed interest in the utility of CEUS across a range of diagnostic and interventional applications. Consisting of gas-filled microbubbles encapsulated by a supportive shell, usually composed of phospholipids [9], ultrasound contrast agents (UCAs) are safe in patients with renal insufficiency, as they are metabolized exclusively by the liver and lungs; the phospholipid shell is broken down by the liver, while the gas within the microbubbles is exhaled [10••]. Furthermore, UCAs possess an excellent safety profile, with a documented severe adverse event rate of 0.007–0.0086% [11, 12], comparable to that of gadolinium and superior to that observed with iodinated contrast agents [13, 14]. Because the microbubbles within UCAs range in size from 1.5 to 2.5 μm, they are small enough to traverse capillary beds but too large to egress the intravascular space [10••, 15]. As a result, UCAs are pure intravascular agents, providing excellent spatial and temporal evaluation of lesional vascularity [16•]. When coupled with the inherent advantages of ultrasound (US)—accessibility, affordability, portability, lack of ionizing radiation, and dynamic real-time imaging capabilities—the overall safety, absence of nephrotoxicity, and superb spatial and temporal resolution of UCAs render CEUS particularly well suited to use in image-guided renal interventions. The objective of this article is to review the potential applications of CEUS in renal imaging and intervention, including renal mass characterization, treatment monitoring during and after percutaneous ablation, and biopsy guidance.
Renal Mass Characterization
Commonly encountered in day-to-day urologic and radiologic practice, renal cysts have a reported overall prevalence of approximately 7–10% in the general population, with incidence increasing with age; it has been estimated that approximately 50% of individuals over the age of 50 years will ultimately develop renal cysts [17, 18]. Frequently, these cysts are asymptomatic and are detected incidentally during imaging examinations performed for other indications. Simple cysts pose no diagnostic dilemma, appearing on US as anechoic structures with thin walls and posterior through transmission and on CT and MRI as well-circumscribed, non-enhancing lesions with imaging characteristics identical to those of simple fluid [18, 19]. Challenges arise when cysts are complicated by infection, hemorrhage, or proteinaceous material, resulting in imaging appearances that can overlap with those of solid masses [17]. Up to 8% of renal cysts may demonstrate complex imaging features [18]. Further confounding accurate characterization of these lesions is the fact that approximately 10% of renal cell carcinomas (RCCs) appear cystic on imaging [18].
Originally developed in 1986, the Bosniak classification system aims to simplify the diagnosis of cystic renal lesions by stratifying them into one of five categories (I, II, IIF, III, IV) based on the presence or absence of worrisome imaging features, such as septations, calcification, solid mural nodules, and internal enhancement. Category I and II lesions correspond with simple and minimally complex cysts, respectively. They carry no risk of malignancy and require no further work-up. Category III and IV lesions exhibit characteristics concerning for malignancy and are typically managed surgically. Category IIF lesions are indeterminate and can be followed with repeat imaging at 6- and 12-month intervals. Stability is reassuring for benignity, while increasing size or complexity may be indicative of malignancy [18, 20]. Though the Bosniak system first described renal lesions on the basis of their CT imaging features, subsequent work demonstrated that the system could be accurately applied to lesions evaluated with MR [21].
B-mode and Doppler US permit confident assessment of simple renal cysts (Bosniak I) but are insufficiently sensitive to subtype more complex lesions [20]. The superior spatial and temporal resolution of CEUS enable exquisite evaluation of the renal microvasculature, potentially facilitating identification of blood flow within septations and nodules that may be occult even at contrast-enhanced CT or MR [20]. Recent work has focused on the utility of CEUS in the further characterization of indeterminate cystic renal lesions. An early investigation found perfect concordance between CEUS and contrast-enhanced CT (CECT) for the differentiation of surgical and non-surgical complex cystic lesions [22]. A subsequent analysis demonstrated superiority of CEUS with respect to the diagnosis of malignancy when compared to CECT, with the overall diagnostic accuracy of CEUS ranging from 80 to 83% and the accuracy of CECT ranging from 63 to 75% [23]. A study of 31 pathologically proven cystic masses by Park et al. described the overall accuracy of CEUS as 90% compared to 74% for CECT; furthermore, additional information obtained with CEUS resulted in upstaging of the Bosniak classification in 26% of lesions [24]. Similar results were obtained by Clevert et al., who used CEUS to upgrade the classification of 19% of 37 renal masses [25].
In addition to its utility in accurately categorizing complex cystic masses, CEUS has proven efficacious in differentiating solid masses from cystic lesions incidentally identified on cross-sectional imaging studies. On CT examinations, fluid typically has an attenuation value of less than 20 Hounsfield units (HUs). Accordingly, on unenhanced CTs, lesions with a mean attenuation of less than 20 HUs are consistent with simple cysts [18], while small lesions with densities of greater than 70 HUs have been shown to have a greater than 99.9% chance of benignity [26]. Lesions with HU values ranging from 20 to 70 are regarded as indeterminate and have traditionally been triaged to either CECT or MRI for definitive evaluation. On CECT, solid renal masses enhance following the administration of contrast. True enhancement is defined as an increase in HUs from the unenhanced phase to the nephrographic phase of at least 15–20. In cystic lesions, particularly those measuring less than 4 cm, a phenomenon known as pseudoenhancement has been observed; this refers to an artifactual increase in attenuation of cystic lesions following contrast administration, typically measuring less than 15–20 HUs and potentially the result of reconstruction algorithms employed by the scanner to reduce beam-hardening artifacts [27, 28]. Similar spurious enhancement has been noted on contrast-enhanced MR examinations, especially in cystic lesions that display intrinsic high signal intensity on T1-weighted images [18]. CEUS provides a cost-effective alternative to additional cross-sectional imaging studies when these indeterminate. In some instances, initial evaluation with conventional B-mode US may allow adequate differentiation of a simple cyst from a hypovascular renal mass, such as a papillary RCC. If complex features are identified, subsequent injection of UCAs can be performed, enabling immediate depiction of enhancement or internal vascularity and confirmation of malignancy [18, 20]. Because of the ability of CEUS to detect tumoral microvascularity, some authors note that it has excellent sensitivity for the detection of malignancy but that its false-positive rate is higher than that of CECT [18, 20, 23, 29]. As a result, the combined use of CEUS, with its high sensitivity, and CECT, with its superior specificity, may further decrease misdiagnosis rates and allow for the most accurate classification of renal masses [29].
Recently, literature has emerged suggesting that the increasing incidence of RCC in the last several decades is attributable to an uptick in the identification of small renal masses (defined as those measuring less than or equal to 4 cm in size); reportedly, 20–30% of these tumors have benign histology, while 70–80% of the malignant lesions are low-grade lesions with dubious metastatic potential [30]. As a result, more patients are opting for close active surveillance of small renal tumors, particularly the elderly and those with significant medical comorbidities that limit the feasibility of surgery. In these patients, intervention is deferred unless tumors exhibit demonstrable growth over time [30]. Because of its excellent sensitivity, its ability to distinguish cystic from solid masses, and its lack of ionizing radiation and nephrotoxicity, CEUS may prove valuable in the longitudinal surveillance of these masses, with CECT and MRI used as adjunctive imaging modalities as clinically necessary.
Contrast-enhanced CT in a 78-year-old male (A) shows an indeterminate mass arising from the lower pole of the right kidney, which does meet criteria for a simple cyst. Subsequently performed CEUS (B) demonstrates an anechoic simple cyst without internal enhancement. Confident diagnosis of a simple cyst by CEUS obviated the need for biopsy
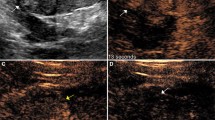
Indeterminate renal mass in a 62-year-old female. (A) Unenhanced CT shows a right renal mass with intermediate attenuation of 40 Hounsfield units (arrow). Initial biopsy (not shown) using conventional grayscale and color Doppler US was non-diagnostic. (B) A repeat biopsy was performed using CEUS, increasing the conspicuity of the endophytic mass and facilitating the diagnosis of type 2 papillary RCC. The papillary RCC lesion enhances less than the surrounding renal parenchyma (arrow). Of note, the patient had an allergy to iodinated contrast, but there was no contraindication to use of an ultrasound contrast agent
42 year old male with large right renal mass. (A) Contrast-enhanced CT shows large renal mass with areas of central necrosis (arrow). (B) Grayscale US (left panel) fails to fully depict the degree of central necrosis (arrow) within the mass. Administration of contrast (right panel) better highlights vascularized tissue separate from necrotic tissue (arrow), providing a viable biopsy target. Percutaneous CEUS-guided biopsy confirmed the diagnosis of grade 4 clear cell RCC with sarcomatoid and rhabdoid features
CEUS may also be helpful in distinguishing true renal masses from pseudolesions such as fetal lobulations, prominent columns of Bertin, and dromedary hump, which sometimes pose diagnostic challenges with conventional grayscale and Doppler US. Enhancement of the potential mass equal to that of the renal cortex on all imaging phases and the identification of medullary pyramids within the area of concern are features that allow confident diagnosis of a pseudomass (Fig. 1). [31•, 32•]. Acute focal pyelonephritis can also occasionally present a diagnostic dilemma, as infection can result in renal enlargement, effacement of the renal sinus fat, and alterations in parenchymal echogenicity that can mimic a mass [33]. Several studies [34, 35] have demonstrated the efficacy of CEUS in identifying characteristic parenchymal changes observed in cases of pyelonephritis, potentially confirming infection as the cause of the abnormal sonographic appearance of the kidney and obviating the need for further work-up.
In contrast to CECT and MRI, which capture static images of renal enhancement at discrete time points, CEUS allows monitoring of contrast wash-in and washout in real-time. This information can then be used to generate enhancement curves. Some investigators have attempted to utilize the data generated by CEUS examinations to histologically classify and subtype solid renal masses. In an early analysis of 84 pathologically proven RCCs, Xu et al. identified heterogeneous arterial-phase hyperenhancement, subsequent washout, and perilesional rim-like enhancement as features predictive of malignancy [36]. A subsequent study by King et al. found that peak enhancement greater than that of the renal cortex and rapid time to peak intensity were indicative of clear cell RCC, while hypoenhancement with respect to the renal cortex and slower time to peak enhancement were more characteristic of papillary RCC [37]. Wei et al. found similar diagnostic performance when comparing CEUS and CECT but noted that CEUS enabled allowed for better qualitative diagnosis of small papillary RCCs [38]. These early results are encouraging and suggest that the enhancement characteristics of renal masses obtained with CEUS may be beneficial in predicting histologic subtypes of malignancy, but given the significant overlap between both benign and malignant masses and the technical difficulty involved in producing time-intensity curves, further study is needed [32•].
Renal Intervention
In recent years, percutaneous ablation—including radiofrequency (RF), microwave (MW), and cryoablation technologies—has emerged as a viable alternative to either partial or complete nephrectomy for treatment of small renal masses measuring less than 3–4 cm [39, 40]. Placement of ablation probes is typically performed under sonographic guidance. However, identification of small masses, particularly those found in obese patients or located near the renal medulla, can be difficult with grayscale US. In these instances, CEUS may be useful to facilitate identification of the ablation target and to guide probe placement [10••]. Furthermore, CEUS has shown high sensitivity and specificity in the detection of residual disease in the immediate post-procedural period [41], with nodular enhancement at the periphery of the ablation cavity suggestive of incomplete treatment of tumor. It is important to differentiate this from normal post-ablation hyperemia, which usually manifests as a complete rim of uniform enhancement in all vascular phases following contrast administration [10••]. Because UCAs are intravascular agents that are metabolized by the liver and lungs, CEUS cannot be used to evaluate excretory function or depict injury to the renal collecting system following ablation [10••].
Surveillance following percutaneous ablation has customarily taken the form of either CECT or MRI. However, several studies have shown comparable performance of CEUS, with sensitivity and specificity ranging from 82.2 to 100% and 96.6 to 100% for the demonstration of residual or recurrent RCC [41]. The lack of exposure to ionizing radiation and absence of nephrotoxicity make CEUS an attractive alternative to CECT, while the relative cost-effectiveness of CEUS in comparison to MRI may make it more suitable for long-term follow-up in patients who need repeated contrast-enhanced examinations [10••].
In years past, the role of percutaneous image-guided sampling in the treatment of renal tumors was somewhat controversial, with many choosing to eschew biopsy prior to surgical resection, believing that it had little impact on management decisions [42, 43]. Recently, however, there has been something of a paradigm shift, as numerous studies have demonstrated the safety, accuracy, and utility of preoperative biopsy, particularly with regard to large or locally advanced tumors [44,45,46]. Depending on operator preference, biopsies can be performed using either CT or US guidance, though US is favored given its ability to provide real-time imaging and its lack of ionizing radiation [5]. A common cause of biopsy failure is an inability to distinguish the target from surrounding structures, a challenge more frequently encountered with small lesions and deep intraabdominal masses [10••, 47]. When difficulties with target visualization are encountered, CEUS has proven useful in increasing lesion conspicuity [10••, 48,49,50]. One study of focal liver lesions showed that performance of CEUS prior to biopsy resulted in a significant increase in diagnostic yield, with an even greater benefit described when the lesion of interest was less than 2.0 cm [51]. Conversely, when large masses are referred for biopsy, it can be challenging to identify viable tumor tissue with conventional imaging modalities. Large masses may outstrip their vascular supply, resulting in central necrosis. Administration of UCAs highlights the vascularized portions of tumor, potentially reducing the likelihood of insufficient sampling and obviating the need for repeat biopsy [16•]. CEUS has also been used effectively in patients with long-standing nephropathies and atrophic kidneys to improve visualization of the kidneys and facilitate acquisition of tissue [10••].
Currently, in our practice, we use CEUS for renal mass biopsy whenever the lesion is difficult to identify on B-mode or color Doppler US or if a prior biopsy attempt was non-diagnostic (Fig. 2). Additionally, CEUS is routinely used for large lesions measuring greater than 7 cm to ensure that the four areas targeted for biopsy are enhancing and thus more likely to contain viable tissue, reducing the likelihood of non-diagnosis and potentially providing additional information for tumor subtyping (Fig. 3). We frequently receive biopsy referrals for indeterminate masses identified on a preceding CT or MRI; in some cases, CEUS allows definitive diagnosis of benign cystic lesions, potentially eliminating the need for biopsy altogether. CEUS can also be used after a procedure to detect immediate bleeding complications.
Other Considerations
As previously described, UCAs are metabolized primarily by the liver and lungs [9, 10••], rendering them safe for use in patients in whom the nephrotoxicity of contrast media is an important consideration, such as those with renal insufficiency or end-stage renal disease (ESRD). Though several recent studies [52,53,54] have found that the link between iodinated contrast material and impaired renal function may not be as robust as previously believed, many clinicians are reluctant to recommend contrast-enhanced CT scans in patients with chronic kidney disease (CKD). Similarly, concerns about the link between gadolinium-based contrast agents and nephrogenic systemic fibrosis (NSF) impacted the use of MRI in this population in the early part of the decade [55]. These limitations may be of particular relevance in patients with polycystic kidney disease, whose disease results in deleterious effects on renal function and increased rates of RCC [56]. Though further research is needed, preliminary investigations have shown high sensitivity of CEUS in patients with CKD [57], suggesting that this may be a valid alternative to cross-sectional studies in patients with contraindications to conventional contrast media who require frequent imaging surveillance.
CEUS has also shown promise in the evaluation of renal transplants, as the uses enumerated above can also be applied to renal allografts and autografts [20]. CEUS may also play a role in the assessment of transplant complications. One recent analysis found that CEUS is superior to conventional Doppler imaging in the diagnosis of transplant renal artery stenosis and may serve as a viable alternative to CT angiography [58]. CEUS also enables diagnosis of transplant vein thrombosis and is capable of evaluating small arteries and arterioles, which are occult on traditional Doppler imaging. As such, disturbances in graft microcirculation, such as areas of subtle infarction, can be effectively interrogated with CEUS, which may allow for more rapid identification of causes of graft dysfunction and prompt implementation of appropriate therapy [59].
Conclusion
Despite its relatively recent regulatory approval in the USA, CEUS has become an important problem-solving tool in renal imaging and intervention, with proven efficacy in renal mass characterization, treatment monitoring during and after percutaneous ablation, and biopsy guidance. As experience with CEUS expands, new uses will continue to emerge. Familiarity with the roles and applications of CEUS will enable practitioners to incorporate this important modality into their day-to-day clinical practice.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
O'Connor SD, Pickhardt PJ, Kim DH, Oliva MR, Silverman SG. Incidental finding of renal masses at unenhanced CT: prevalence and analysis of features for guiding management. AJR Am J Roentgenol. 2011;197(1):139–45. https://doi.org/10.2214/AJR.10.5920.
Johnson DC, Vukina J, Smith AB, Meyer AM, Wheeler SB, Kuo TM, et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J Urol. 2015;193(1):30–5. https://doi.org/10.1016/j.juro.2014.07.102.
Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol. 2016;69(4):660–73. https://doi.org/10.1016/j.eururo.2015.07.072.
Posielski NM, Bui A, Wells SA, Best SL, Gettle LM, Ziemlewicz TJ, et al. Risk factors for complications and nondiagnostic results following 1,155 consecutive percutaneous core renal mass biopsies. J Urol. 2019. https://doi.org/10.1097/JU.0000000000000113.
Sutherland EL, Choromanska A, Al-Katib S, Coffey M. Outcomes of ultrasound guided renal mass biopsies. J Ultrasound. 2018;21(2):99–104. https://doi.org/10.1007/s40477-018-0299-0.
Richard PO, Jewett MA, Bhatt JR, Kachura JR, Evans AJ, Zlotta AR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol. 2015;68(6):1007–13. https://doi.org/10.1016/j.eururo.2015.04.004.
Prince J, Bultman E, Hinshaw L, Drewry A, Blute M, Best S, et al. Patient and tumor characteristics can predict nondiagnostic renal mass biopsy findings. J Urol. 2015;193(6):1899–904. https://doi.org/10.1016/j.juro.2014.12.021.
Wilson SR, Greenbaum LD, Goldberg BB. Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? AJR Am J Roentgenol. 2009;193(1):55–60. https://doi.org/10.2214/AJR.09.2553.
Harvey CJ, Blomley MJ, Eckersley RJ, Cosgrove DO. Developments in ultrasound contrast media. Eur Radiol. 2001;11(4):675–89. https://doi.org/10.1007/s003300000624.
•• Huang DY, Yusuf GT, Daneshi M, Ramnarine R, Deganello A, Sellars ME, et al. Contrast-enhanced ultrasound (CEUS) in abdominal intervention. Abdom Radiol (NY). 2018;43(4):960–76. https://doi.org/10.1007/s00261-018-1473-8 Provides an excellent overview of the full spectrum of uses of contrast-enhanced ultrasound, particularly with respect to abdominal interventions.
Tang C, Fang K, Guo Y, Li R, Fan X, Chen P, et al. Safety of sulfur hexafluoride microbubbles in sonography of abdominal and superficial organs: retrospective analysis of 30,222 cases. J Ultrasound Med. 2017;36(3):531–8. https://doi.org/10.7863/ultra.15.11075.
Piscaglia F, Bolondi L. Italian Society for Ultrasound in M, Biology Study Group on Ultrasound Contrast A. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32(9):1369–75. https://doi.org/10.1016/j.ultrasmedbio.2006.05.031.
Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH, et al. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology. 2012;264(2):414–22. https://doi.org/10.1148/radiol.12112025.
Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol. 2008;191(2):409–15. https://doi.org/10.2214/AJR.07.3421.
Weinstein S, Jordan E, Goldstein R, Yee J, Morgan T. How to set up a contrast-enhanced ultrasound service. Abdom Radiol (NY). 2018;43(4):808–18. https://doi.org/10.1007/s00261-017-1278-1.
• Huang DY, Yusuf GT, Daneshi M, Husainy MA, Ramnarine R, Sellars ME, et al. Contrast-enhanced US-guided interventions: improving success rate and avoiding complications using US contrast agents. Radiographics. 2017;37(2):652–64. https://doi.org/10.1148/rg.2017160123 An excellent introduction to the use of contrast-enhanced ultrasound in a variety of clinical settings.
Eknoyan G. A clinical view of simple and complex renal cysts. J Am Soc Nephrol. 2009;20(9):1874–6. https://doi.org/10.1681/ASN.2008040441.
Nicolau C, Bunesch L, Sebastia C. Renal complex cysts in adults: contrast-enhanced ultrasound. Abdom Imaging. 2011;36(6):742–52. https://doi.org/10.1007/s00261-011-9727-8.
Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, Horowitz JM, Feng C, Hammond NA, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125–41. https://doi.org/10.1148/rg.351130016.
Harvey CJ, Alsafi A, Kuzmich S, Ngo A, Papadopoulou I, Lakhani A, et al. Role of US contrast agents in the assessment of indeterminate solid and cystic lesions in native and transplant kidneys. Radiographics. 2015;35(5):1419–30. https://doi.org/10.1148/rg.2015140222.
Israel GM, Hindman N, Bosniak MA. Evaluation of cystic renal masses: comparison of CT and MR imaging by using the Bosniak classification system. Radiology. 2004;231(2):365–71. https://doi.org/10.1148/radiol.2312031025.
Ascenti G, Mazziotti S, Zimbaro G, Settineri N, Magno C, Melloni D, et al. Complex cystic renal masses: characterization with contrast-enhanced US. Radiology. 2007;243(1):158–65. https://doi.org/10.1148/radiol.2431051924.
Quaia E, Bertolotto M, Cioffi V, Rossi A, Baratella E, Pizzolato R, et al. Comparison of contrast-enhanced sonography with unenhanced sonography and contrast-enhanced CT in the diagnosis of malignancy in complex cystic renal masses. AJR Am J Roentgenol. 2008;191(4):1239–49. https://doi.org/10.2214/AJR.07.3546.
Park BK, Kim B, Kim SH, Ko K, Lee HM, Choi HY. Assessment of cystic renal masses based on Bosniak classification: comparison of CT and contrast-enhanced US. Eur J Radiol. 2007;61(2):310–4. https://doi.org/10.1016/j.ejrad.2006.10.004.
Clevert DA, Minaifar N, Weckbach S, Jung EM, Stock K, Reiser M, et al. Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clin Hemorheol Microcirc. 2008;39(1–4):171–8.
Jonisch AI, Rubinowitz AN, Mutalik PG, Israel GM. Can high-attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced CT? Radiology. 2007;243(2):445–50. https://doi.org/10.1148/radiol.2432060559.
Tappouni R, Kissane J, Sarwani N, Lehman EB. Pseudoenhancement of renal cysts: influence of lesion size, lesion location, slice thickness, and number of MDCT detectors. AJR Am J Roentgenol. 2012;198(1):133–7. https://doi.org/10.2214/AJR.10.6057.
Al Harbi F, Tabatabaeefar L, Jewett MA, Finelli A, O’Malley M, Atri M. Enhancement threshold of small (< 4 cm) solid renal masses on CT. AJR Am J Roentgenol. 2016;206(3):554–8. https://doi.org/10.2214/AJR.15.14806.
Lan D, Qu HC, Li N, Zhu XW, Liu YL, Liu CL. The value of contrast-enhanced ultrasonography and contrast-enhanced CT in the diagnosis of malignant renal cystic lesions: a meta-analysis. PLoS One. 2016;11(5):e0155857. https://doi.org/10.1371/journal.pone.0155857.
Pierorazio PM, Hyams ES, Mullins JK, Allaf ME. Active surveillance for small renal masses. Rev Urol. 2012;14(1–2):13–9.
• Kazmierski B, Deurdulian C, Tchelepi H, Grant EG. Applications of contrast-enhanced ultrasound in the kidney. Abdom Radiol (NY). 2018;43(4):880–98. https://doi.org/10.1007/s00261-017-1307-0 Provides a nice introduction to the various practical applications of contrast-enhanced ultrasound in the kidney.
• Bertolotto M, Bucci S, Valentino M, Curro F, Sachs C, Cova MA. Contrast-enhanced ultrasound for characterizing renal masses. Eur J Radiol. 2018;105:41–8. https://doi.org/10.1016/j.ejrad.2018.05.015 A cutting edge, well-written overview of the current state of contrast-enhanced ultrasound in renal imaging and intervention.
Craig WD, Wagner BJ, Travis MD. Pyelonephritis: radiologic-pathologic review. Radiographics. 2008;28(1):255–77; quiz 327-8. https://doi.org/10.1148/rg.281075171.
Mitterberger M, Pinggera GM, Colleselli D, Bartsch G, Strasser H, Steppan I, et al. Acute pyelonephritis: comparison of diagnosis with computed tomography and contrast-enhanced ultrasonography. BJU Int. 2008;101(3):341–4. https://doi.org/10.1111/j.1464-410X.2007.07280.x.
Fontanilla T, Minaya J, Cortes C, Hernando CG, Aranguena RP, Arriaga J, et al. Acute complicated pyelonephritis: contrast-enhanced ultrasound. Abdom Imaging. 2012;37(4):639–46. https://doi.org/10.1007/s00261-011-9781-2.
Xu ZF, Xu HX, Xie XY, Liu GJ, Zheng YL, Liang JY, et al. Renal cell carcinoma: real-time contrast-enhanced ultrasound findings. Abdom Imaging. 2010;35(6):750–6. https://doi.org/10.1007/s00261-009-9583-y.
King KG, Gulati M, Malhi H, Hwang D, Gill IS, Cheng PM, et al. Quantitative assessment of solid renal masses by contrast-enhanced ultrasound with time-intensity curves: how we do it. Abdom Imaging. 2015;40(7):2461–71. https://doi.org/10.1007/s00261-015-0468-y.
Wei SP, Xu CL, Zhang Q, Zhang QR, Zhao YE, Huang PF, et al. Contrast-enhanced ultrasound for differentiating benign from malignant solid small renal masses: comparison with contrast-enhanced CT. Abdom Radiol (NY). 2017;42(8):2135–45. https://doi.org/10.1007/s00261-017-1111-x.
Atwell TD, Schmit GD, Boorjian SA, Mandrekar J, Kurup AN, Weisbrod AJ, et al. Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol. 2013;200(2):461–6. https://doi.org/10.2214/AJR.12.8618.
Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34(5):1344–62. https://doi.org/10.1148/rg.345140054.
Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version). Ultraschall Med. 2018;39(2):e2–e44. https://doi.org/10.1055/a-0586-1107.
Dechet CB, Zincke H, Sebo TJ, King BF, LeRoy AJ, Farrow GM, et al. Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J Urol. 2003;169(1):71–4. https://doi.org/10.1097/01.ju.0000042211.18318.ba.
Khan AA, Shergill IS, Quereshi S, Arya M, Vandal MT, Gujral SS. Percutaneous needle biopsy for indeterminate renal masses: a national survey of UK consultant urologists. BMC Urol. 2007;7:10. https://doi.org/10.1186/1471-2490-7-10.
Maturen KE, Nghiem HV, Caoili EM, Higgins EG, Wolf JS Jr, Wood DP Jr. Renal mass core biopsy: accuracy and impact on clinical management. AJR Am J Roentgenol. 2007;188(2):563–70. https://doi.org/10.2214/AJR.06.0220.
Abel EJ, Heckman JE, Hinshaw L, Best S, Lubner M, Jarrard DF, et al. Multi-quadrant biopsy technique improves diagnostic ability in large heterogeneous renal masses. J Urol. 2015;194(4):886–91. https://doi.org/10.1016/j.juro.2015.03.106.
Wittmann TA, Abel EJ. Percutaneous biopsy in large, locally advanced or metastatic renal tumors. Urol Oncol. 2017;35(3):87–91. https://doi.org/10.1016/j.urolonc.2016.10.003.
Sainani NI, Arellano RS, Shyn PB, Gervais DA, Mueller PR, Silverman SG. The challenging image-guided abdominal mass biopsy: established and emerging techniques ‘if you can see it, you can biopsy it’. Abdom Imaging. 2013;38(4):672–96. https://doi.org/10.1007/s00261-013-9980-0.
Bang N, Bachmann Nielsen M, Vejborg I, Mellon MA. Clinical report: contrast enhancement of tumor perfusion as a guidance for biopsy. Eur J Ultrasound. 2000;12(2):159–61.
Partovi S, Lu Z, Kessner R, Yu A, Ahmed Y, Patel IJ, et al. Contrast enhanced ultrasound guided biopsies of liver lesions not visualized on standard B-mode ultrasound-preliminary experience. J Gastrointest Oncol. 2017;8(6):1056–64. https://doi.org/10.21037/jgo.2017.08.17.
Yoon SH, Lee KH, Kim SY, Kim YH, Kim JH, Lee SH, et al. Real-time contrast-enhanced ultrasound-guided biopsy of focal hepatic lesions not localised on B-mode ultrasound. Eur Radiol. 2010;20(8):2047–56. https://doi.org/10.1007/s00330-010-1757-z.
Wu W, Chen MH, Yin SS, Yan K, Fan ZH, Yang W, et al. The role of contrast-enhanced sonography of focal liver lesions before percutaneous biopsy. AJR Am J Roentgenol. 2006;187(3):752–61. https://doi.org/10.2214/AJR.05.0535.
McDonald JS, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE. Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65–73. https://doi.org/10.1148/radiol.13130775.
McDonald RJ, McDonald JS, Carter RE, Hartman RP, Katzberg RW, Kallmes DF, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology. 2014;273(3):714–25. https://doi.org/10.1148/radiol.14132418.
Hinson JS, Ehmann MR, Fine DM, Fishman EK, Toerper MF, Rothman RE, et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577–86 e4. https://doi.org/10.1016/j.annemergmed.2016.11.021.
Chang EH. An introduction to contrast-enhanced ultrasound for nephrologists. Nephron. 2018;138(3):176–85. https://doi.org/10.1159/000484635.
Hajj P, Ferlicot S, Massoud W, Awad A, Hammoudi Y, Charpentier B, et al. Prevalence of renal cell carcinoma in patients with autosomal dominant polycystic kidney disease and chronic renal failure. Urology. 2009;74(3):631–4. https://doi.org/10.1016/j.urology.2009.02.078.
Chang EH, Chong WK, Kasoji SK, Fielding JR, Altun E, Mullin LB, et al. Diagnostic accuracy of contrast-enhanced ultrasound for characterization of kidney lesions in patients with and without chronic kidney disease. BMC Nephrol. 2017;18(1):266. https://doi.org/10.1186/s12882-017-0681-8.
Pan FS, Liu M, Luo J, Tian WS, Liang JY, Xu M, et al. Transplant renal artery stenosis: evaluation with contrast-enhanced ultrasound. Eur J Radiol. 2017;90:42–9. https://doi.org/10.1016/j.ejrad.2017.02.031.
Zeisbrich M, Kihm LP, Druschler F, Zeier M, Schwenger V. When is contrast-enhanced sonography preferable over conventional ultrasound combined with Doppler imaging in renal transplantation? Clin Kidney J. 2015;8(5):606–14. https://doi.org/10.1093/ckj/sfv070.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michael C. Olson, E. Jason Abel, and Lori Mankowski Gettle each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on New Imaging Techniques
Rights and permissions
About this article
Cite this article
Olson, M.C., Abel, E.J. & Mankowski Gettle, L. Contrast-Enhanced Ultrasound in Renal Imaging and Intervention. Curr Urol Rep 20, 73 (2019). https://doi.org/10.1007/s11934-019-0936-y
Published:
DOI: https://doi.org/10.1007/s11934-019-0936-y