Abstract
Purpose of Review
We aimed to synthesize the recent work on the intra-vertebral heterogeneity in density, trabecular architecture and mechanical properties, its implications for fracture risk, its association with degeneration of the intervertebral discs, and its implications for implant design.
Recent Findings
As compared to the peripheral regions of the centrum, the central region of the vertebral body exhibits lower density and more sparse microstructure. As compared to the anterior region, the posterior region shows higher density. These variations are more pronounced in vertebrae from older persons and in those adjacent to degenerated discs. Mixed results have been reported in regard to variation along the superior-inferior axis and to relationships between the heterogeneity in density and vertebral strength and fracture risk. These discrepancies highlight that, first, despite the large amount of study of the intra-vertebral heterogeneity in microstructure, direct study of that in mechanical properties has lagged, and second, more measurements of vertebral loading are needed to understand how the heterogeneity affects distributions of stress and strain in the vertebra. These future areas of study are relevant not only to the question of spine fractures but also to the design and selection of implants for spine fusion and disc replacement.
Summary
The intra-vertebral heterogeneity in microstructure and mechanical properties may be a product of mechanical adaptation as well as a key determinant of the ability of the vertebral body to withstand a given type of loading.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vertebral body is one of the most important structural elements in the body. Through its shape and composition, and its role in anchoring neighboring elements—the ribs, vertebral arch, intervertebral discs, and spinal ligaments and tendons—the vertebral body exemplifies the mechanical functions of the skeleton: protecting vital organs, supporting load, and facilitating movement (Fig. 1). The vertebral body is also a main site of hematopoiesis and mineral storage, the two other main functions of the skeleton, owing to the large volume of marrow and high ratio of bone surface to bone volume in the trabecular compartment [1, 2]. It is worth noting that these last two characteristics also mean that the porosity of the vertebral body is high, which limits its strength and stiffness [3,4,5]. Hence, the vertebral body illustrates well the tension that exists between mechanical and metabolic demands in the skeleton.
Components of the human vertebral body and adjacent tissues. The individual vertebra is split into the vertebral arch and vertebral body, with the arch to the posterior side. The vertebral body is made up of the trabecular centrum (containing trabecular bone) surrounded by a cortical shell. At the superior and inferior ends of this body is the endplate region. This area contains the bony vertebral endplate adjacent to the cartilage endplate. The intervertebral disc is located between the vertebrae, and consists of the gel-like nucleus pulposus surrounded by the comparatively more fibrous annulus fibrosus
The vertebral body is a common location of major complications associated with aging and disease. Vertebral fractures, a hallmark of osteoporosis and source of excess mortality and morbidity, most frequently occur in the vertebral body [6,7,8]. The progression and treatment of disc degeneration also involve the vertebral body. Marked changes, such as development of osteophytes, occur in vertebral bodies adjacent to degenerated discs [9]. Surgical treatment for severe disc degeneration often involves using screws, cages, and other hardware placed into or against the vertebrae to restore stability to the spine. The success of these interventions depends on the ability of the vertebral body to support these implants.
The porosity and trabecular architecture (the 3D arrangement of individual trabeculae) are spatially non-uniform throughout the vertebral body. This observation is not new; for example, Georg Schmorl frequently noted spatial heterogeneity in bone microstructure in the vertebral column in his comprehensive notes on spine anatomy and pathology [10]. However, the ubiquity of 3D imaging and computer modeling in the present day has intensified interest in quantifying the magnitude of the heterogeneity and understanding the extents to which it reflects physiological demands, influences risk of fracture, and affects success of surgical interventions. In this review, we attempt to synthesize recent work in the area. We start by summarizing the nature of the heterogeneity and then focus on its implications for fracture risk, its association with disc degeneration, and its implications for implant design. We do not focus explicitly on methods of quantifying the spatial heterogeneity and instead refer the reader to the references cited herein for those methods.
Nature of Heterogeneity in Microstructure in the Vertebra
Trabecular bone in human vertebrae displays substantial heterogeneity in density and architecture throughout the vertebral body. As compared to the peripheral regions of the centrum, the central region exhibits lower bone mineral density (BMD) [11•], bone volume fraction (BV/TV), and trabecular thickness (Tb.Th) and higher trabecular number (Tb.N) [12]. As compared to the anterior region, the posterior region has higher BMD [11•, 12], BV/TV [12, 13•, 14], Tb.N [12, 13•, 14], and connectivity density (Conn.D) [12, 14] and lower trabecular separation (Tb.Sp) [12], degree of anisotropy (DA), and structure model index (SMI) [14]. With respect to variations along the superior-inferior axis, the data are more mixed. Studies of differences among the superior, mid-transverse, and inferior thirds of the centrum have produced conflicting results for BV/TV, Tb.Th, and Conn.D. An HR pQCT study on T12 vertebrae from young (31.6 ± 6.2 years) and aged (71.6 ± 4.8 years) women reported that Tb.Th is highest mid-horizontally [13•], yet a μCT study on thoracolumbar vertebrae from both sexes (70 ± 12 years) found no differences among the three sections [15]. The latter study also found that Conn.D is lowest in the mid-transverse plane, whereas another μCT study with slightly older donors (80.5 ± 10.4 years), though on only L1 vertebrae, found the opposite [16•]. BV/TV has been reported to be lowest in the superior [13•], mid-transverse [15], and inferior [16•] planes, depending on the study, although these studies have all found that Tb.N and SMI are lowest in the mid-transverse third of the vertebral body as compared to the superior and inferior thirds [13•, 15, 16•]. These mixed results may reflect the effect of aging and age-associated conditions on the nature of the heterogeneity, as suggested previously [17] and discussed in later sections. Despite the discrepancies, however, the areas of consensus in these recent studies are both consistent with earlier reports [18,19,20,21,22,23] that typically used coarser sampling schemes and paint an overall picture of lower density and more sparse microstructure centrally vs. peripherally and highest density posteriorly.
In addition to the heterogeneity of trabecular microstructure throughout a single vertebra, differences among vertebral levels have been reported. In the lumbar spine, BV/TV is relatively high in both the L1 and L5 levels, and the L1 also exhibits high values of trabecular number and trabecular thickness) [12, 24]. Comparisons between the half of the vertebra superior (cranial) to the disc vs. that inferior (caudal) found higher density (BMD and BV/TV), Conn.D, and Tb.N, and lower Tb.Sp, in the former, though only in the peripheral regions, and not the central regions [25•]. These differences may simply be due to the heterogeneity along the superior-inferior axis noted above, though it is noteworthy that these differences in superior vs. inferior halves were found in the upper lumbar spine but not the lower lumbar spine and that the study included only male donors.
Data indicate that the heterogeneity of trabecular microstructure within the vertebra changes with age. In a cross-sectional study that analyzed QCT scans of the L3 vertebra (n = 377) from both sexes (181 males, 196 females, 61.69 ± 9.05 years), the ratio of BMD in the central to peripheral regions of the centrum decreases with age [11•] (Fig. 2). Similar results were found for the superior:mid-transverse BMD ratio, while no associated with age was found for the anterior:posterior or inferior:mid-transverse ratios. Consistent with these findings, a μCT study of lumbar vertebrae (n = 150, L1-L5) from slightly younger male donors (21–64 years, mean = 50 years, N = 48 donors) found that aging is associated with an increasing bias toward more robust trabecular architecture peripherally vs. centrally, but no change in the bias toward higher density posteriorly vs. anteriorly [12]. However, the HR-pQCT data from the T12 vertebra in young (31.6 ± 6.2 years, n = 11) vs. aged (71.6 ± 4.8 years, n = 18) women suggests an increasing posterior:anterior bias in BV/TV with age [13•]. Although the QCT study in both sexes did not find any age*sex interaction in the BMD ratios [11•], this study did not include individuals as young as the donors in the younger group used in the HR-pQCT study. As such, the discrepant results could be due to an age effect and/or to differences between sexes, vertebral levels, and image resolution.
a Four different ratios of BMD between two different regions of the L3 vertebral body (defined in the schematic shown at the bottom), plotted against age for n = 377 men and women. b BMD of the entire vertebral body (“integral BMD”) and of just the trabecular centrum (“trabecular BMD”) for the same dataset [11•]
Disc degeneration may also be associated with intra-vertebral heterogeneity in microstructure. Recent studies in this area follow early reports, often using 2D imaging, of an inverse association between bone density and degenerative changes in the disc [26,27,28,29,30,31,32]. As defined by disc space narrowing (also known as disc height loss) and structural disruptions to the disc observed from discography, disc degeneration has been found to be associated with increased peripheral:central Tb.Th ratio [12]. The anterior:posterior Tb.Th ratio also increased with disc space narrowing [12], though no effect on the differences in density or architecture superior vs. inferior to the disc has been found [25•]. These results agree with a more recent study that found that disc height loss is associated not only with increased BMD of the entire centrum but also increased anterior:posterior, superior:mid-transverse, and peripheral:central BMD ratios [11•]. These data spark questions as to whether the changes in heterogeneity are a mechanical adaptation to changes in stress (or strain or strain energy density) that occur within the vertebral body as the disc degenerates. It is also possible that the changes in bone microstructural heterogeneity, if accompanied by changes in the distribution of mechanical properties such as stiffness and permeability that might then change the distribution of stresses and nutrient supply in the disc, influence the course of disc degeneration. These questions are explored further later in this article.
Implications of Microstructural Heterogeneity for Osteoporotic Fractures
Much of the study of intra-vertebral heterogeneity in trabecular density and architecture has focused on use of measures of microstructural heterogeneity to predict vertebral strength and fracture risk. The underlying rationale is that, owing to the heavy dependence of the strength and stiffness of trabecular bone on microstructural properties such as BV/TV, the microstructural heterogeneity is indicative of mechanical heterogeneity. It is well known that the average density of the vertebra (or vertebral body or its trabecular compartment) explains only 40–70% of the variance in vertebral strength. Studies have also consistently found moderate improvements in prediction of strength when average values of architectural parameters throughout the trabecular compartment are used in combination with an average measure of density or bone mass [33, 34]. On the other hand, adding simple quantitative measures of the intra-vertebral variance in density or architecture into the prediction model has produced mixed results. Conflicting reports exist as to whether greater heterogeneity is positively or negatively associated with bone strength [35,36,37,38] and stiffness [35, 37]. Variations in experimental protocols, including differences in how the sampling of local measures of density was performed, loading mode, spinal level, and use of isolated vertebrae vs spine segments, might have contributed to this discrepancy. These factors may also explain why the ratio of anterior to posterior density was found to be inversely associated with prevalent fracture in a case-control study [39•], and yet laboratory biomechanical tests have found that this ratio is inversely associated with vertebral strength [38].
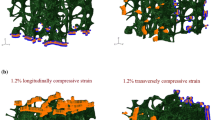
In parallel with these statistical approaches, researchers have examined how the spatial distribution of density and trabecular architecture may relate to the process of failure in the vertebra. A common approach now is to use time-lapse imaging to visualize, whether qualitatively or quantitatively, how the vertebra collapses in relation to the local microstructure. In a recent study on rat vertebrae subjected to axial compression, failure was qualitatively observed to occur at the vascular apertures in the dorsal aspect of the cortical shell, suggesting that these elliptical pores may acts as stress concentrations, and in regions of the trabecular compartment with low BV/TV, Tb.Sp, and Tb.N compared to the entire compartment [40]. These latter findings are consistent with earlier studies that have shown that when cylindrical cores of trabecular bone are compressed, the regions that are first to collapse are those with low BV/TV [41].
However, studies on human vertebrae have reported more complicated failure phenomena. In thoracolumbar vertebrae loaded in either axial compression or axial compression with anterior flexion, the highest strains within the vertebral body as it reaches its yield and then ultimate points are observed at or near the vertebral endplates, even though these are not the locations of lowest density or least robust architecture [16•, 42] (Fig. 3). Consistent with these findings, regression models that use the density of the endplate region as well as the average density of the entire vertebral body to predict vertebral strength outperform those that use only the average density [44]. In slight contrast, a separate study on thoracolumbar vertebrae found that permanent deformations (assessed by measuring changes in trabecular spacing) induced by a compressive overload and subsequent fatigue loading were most pronounced in the mid-transverse third of the vertebral body followed by the superior third [15]. This apparent discrepancy could suggest differences in failure mechanisms between monotonic and fatigue loading and could also be due to differences in the period of observation. Whereas the fatigue study examined deformations after induction of a fracture severe enough to be observed in radiographs, the studies using monotonic loading quantified displacement and strain fields at or before the ultimate point, which is earlier in the failure process. As the failure process proceeds, the regions of largest deformation at a given point in time can change [16•].
a Strains incurred on the surface of the L1 vertebral body during compression to yield, as measured by digital image correlation [42]. Each row shows a different vertebra. (Used with permission from Wolters Kluwer). b Strains incurred in 27 different regions of the L1 trabecular centrum during compression to yield, as measured by digital volume correlation [16•]. The color of each region corresponds to the median value over n = 26 vertebrae, while the number that labels each region is the interquartile range over all vertebrae with the same units and on the same scale as the median values. *Difference between transverse planes. c Displacements incurred throughout the T8 vertebral body (representative specimen) during compression to just past the ultimate point, as measured by digital volume correlation (left column: top panel shows the microCT rendering before (gray) and upon (blue) loading to just past the ultimate point; bottom panel shows displacements) and as predicted by QCT-based FE simulations for four different types of boundary conditions (middle and right columns) [43]: using displacements measured by digital volume correlation across the endplates (“Experimentally Matched FE”); using uniform displacement boundary conditions (“Idealized FE”); using force boundary conditions calculated from distributions of intradiscal pressure averaged over intervertebral discs (IVDs) at different stages of degeneration (“IVD-Generic FE”); and using force boundary conditions calculated from distributions of intradiscal pressure averaged over intervertebral discs at the stage of degeneration exhibited by the given specimen (“IVD-Specific FE”). Positive values are downward displacements
The evidence that the failure phenomena in the vertebral body may be more complicated than in test coupons of trabecular bone may simply reflect the complexity of the way vertebral trabecular bone is loaded in situ. In a uniaxial test of a specimen of trabecular bone, each cross section nominally experiences the same stress, and therefore, failure is controlled by the weakest cross section. The heavy dependence of trabecular bone strength on density, particularly when considering uniaxial loading along the principal trabecular orientation, means that the first cross section to fail would be expected to be the one with the lowest density. In a mechanical test on an entire vertebra or vertebral body, on the other hand, the distribution of stress (and strain) in the trabecular compartment of the centrum is more complex, due to the shape of the vertebral body, the presence of the cortical shell, and the spatial heterogeneity in mechanical behavior. The applied loading is also more complicated if the adjacent intervertebral discs are present: even when the spine segment is nominally loaded in axial compression, the vertebral body is not compressed uniformly [16•, 43], and the trabecular bone in the centrum experiences multiaxial loading [45]. Hence, while the first region to fail in the vertebral body is by definition the one that is most overloaded, this region is not necessarily the weakest region.
Finite element analysis is a natural choice for study of such complex failure phenomena. Models of the vertebra that include the spatial heterogeneity in density and in some cases architecture can be built on a specimen-specific basis from micro-computed tomography (μCT) and on a patient-specific basis from quantitative computed tomography (QCT). However, the utility of these models to study the effects of the heterogeneity hinges on the accuracy of the model inputs, namely the boundary conditions and material properties. Errors in the displacement fields predicted by finite element models of human thoracic vertebrae loaded to failure are nearly two-fold higher for boundary conditions corresponding to uniform axial compression rather than the non-uniform loading actually supplied by the intervertebral disc [43] (Fig. 3C). Yet, the errors for the latter are still high (> 50% for some specimens), suggesting the presence of other sources of error. In regard to material properties, the question of how to use knowledge of the spatial variations in microstructure that are obtained from the CT images to infer distributions of elastic and strength properties is not fully resolved. Commonly used relationships between material properties and microstructure were developed from studies of trabecular bone specimens pooled across anatomic sites or from only the central region of the vertebral trabecular centrum [2, 46, 47]. These relationships may be inaccurate for trabecular bone in the periphery of the centrum [45, 48], on account of the distinctive architecture in this region. It is also possible that the material properties assigned in the FE models may need to account for spatial variations in the mechanical behavior of trabecular tissue. Although evidence to date suggests no differences in elastic modulus of the mineralized tissue in the centrum, vertebral endplate, and shell [49], further examination of variations throughout the centrum is needed, in light of the large errors that exist in FE predictions of failure patterns in the human vertebra. These errors highlight that despite how much is known about the spatial heterogeneity in density and architecture, there are still gaps in understanding of the mechanical heterogeneity.
Potential Correspondence with Disc Degeneration
As noted above, the changes in spatial variations in microstructure throughout the vertebral body with degeneration of the adjacent discs may suggest a biomechanical interplay between these two main elements of the spinal column. In degenerated discs, the distribution of internal pressure when the spine is in an erect posture is highest in regions overlying the periphery of the vertebral body [50, 51]. In contrast, for healthy discs, the pressure is more evenly distributed, with the highest values distributed in a plateau shape over the central region. This shift mirrors that in the bone density in the central vs. peripheral regions of the trabecular centrum [11•], suggesting that this change in the heterogeneity in bone density may be a mechanical adaptation.
However, direct extrapolation of the pressure distribution in the disc to the force distribution over the vertebral endplate imposes a simplistic view of both the internal structure of the disc and its integration with the vertebral body. Multiaxial strain states within the disc differ between the nucleus pulposus and annulus fibrosus [52, 53]. Moreover, the fibers of the annulus fibrosus insert into the bony vertebral endplate and may create high tensile transverse stresses superimposed on axial compressive stresses within the vertebral endplate [54]. Load transfer between the cartilage endplate and vertebral endplate has not been studied in detail, but the interface between these two layers is highly susceptible to damage [55•] and may affect stresses within the vertebral body. These stresses likely also vary with posture [56], in the presence of osteophytes [57, 58], with degenerative changes in the facet joints, and along the spinal column, due to the differences in natural curvature between, for example, the thoracic and lumbar regions. All of these factors may explain the large amount of variability among individuals in the correspondence between microstructural heterogeneity and disc degeneration, and they emphasize the need for quantitative definition of vertebral loading at various stages of disc degeneration.
Even less studied is the effect of the microstructural and mechanical heterogeneity within the vertebral body on the course of disc degeneration. Due to the low density of the trabecular centrum, the trabecular bone of the vertebral body is some of the most compliant bone in the skeleton [4] and undergoes non-negligible deformations, most notably in the central endplate region, during physiological loading [59, 60]. How these deformations change with alterations in bone microstructural and mechanical heterogeneity and how these changes might affect stresses within the intervertebral are incompletely understood. Similarly, the effects of changes in the spatial heterogeneity of bone microstructure in the endplate region on transport have not been resolved [61]. It is important to note that questions of cause-effect relationships between disc degeneration and bone remodeling are difficult to address with cross-sectional studies.
Implications for Spinal Implant Design and Selection
How the microstructural and mechanical heterogeneity within the vertebral body affects the ability of the vertebral to withstand a given set of mechanical demands is also centrally relevant to the success of spinal implants such as interbody fusion cages and total disc replacements. In both cases, the implants occupy the disc space and require support from the adjacent vertebral bodies for good outcomes. Stresses that develop in the vertebral body in the presence of these implants depend on design variables such as implant material, size and shape, and procedural choices such as the where on the vertebral endplate, the cages are placed and how much of the endplate is removed in preparation for cage placement. Interbody fusion cages typically use titanium, titanium alloys, or polyether ether ketone (PEEK), all of which are orders of magnitude stiffer than the vertebral endplate and subchondral bone [62, 63•, 64, 65]. Although the high stiffness and strength abide by current design standards that focus on minimizing failure of the implant over the surrounding bone [66], the high stiffness also means increased stress in the supporting bone. Implants for total disc replacement tend to have a higher contact area with the supporting bone, but like fusion cages, they feature teeth and other protrusions that facilitate precise placement of the implant while also elevating local stresses in the bone tissue [67]. Current vertebra implant systems pose high risk of postoperative complications: 15–18% of lumbar surgery cases require reoperation as early as within 1 year of the initial operation due to subsidence or other failures [68].
While some newer implant systems have explored the use of less stiff materials such as poly-L-actic acid or polycarbonate-urethane [69,70,71], there is also an opportunity to leverage information on the intra-vertebral heterogeneity in bone microstructure to improve implant performance. Indentation studies have mapped spatial variations in the mechanical behavior of the endplate region [62, 63•, 64, 65] in order to develop recommendations for implant placement. Spatial variations in microstructure, as a surrogate for mechanical properties, may be possible to obtain on a patient-specific basis for preoperative planning [72]. This information, combined with ongoing advancements in biomaterials, modeling, and manufacturing [70, 73], could bring to fruition design, selection, and deployment of spinal implants that are tailored to the local bone structure in ways that provide the greatest chance of long-term success.
Summary
Research over the past several years has advanced knowledge on the nature and consequences of spatial variations in bone microstructure throughout the vertebral body. This work has solidified understanding of general trends in, first, how the density and trabecular architecture vary among different anatomic regions of the vertebral body, and second, the association of these variations with aging. This work has also illustrated how the heterogeneity in microstructure influences the mechanisms of failure during vertebral fracture, albeit in ways that are more complicated than in test coupons of bone. Beyond these general trends, however, the data also reveal a sizable amount of variability among individuals in the spatial variations in microstructure and have identified other potential explanatory factors, such as degeneration in the disc and facet joints, sex, and posture. Given that many of these factors relate to vertebral loading, these findings suggest that the microstructural heterogeneity may be a product of mechanical adaptation as well as a key determinant of the ability of the vertebra to withstand a given type of loading.
Although the weight of the evidence supports the use microstructural heterogeneity as an indicator of mechanical heterogeneity throughout the vertebra, it is important to note that gaps in knowledge regarding the latter remain. Recent studies raise questions as to whether the available constitutive models for trabecular bone suffice across the diversity of trabecular microstructures exhibited throughout the human vertebral centrum. The extent of spatial heterogeneity in the mechanical properties of the mineralized tissue—whether in the trabeculae, cortical shell, or vertebral endplate—is also comparatively underexplored. As such, further work on measurement of bone mechanical properties at these length scales will shed more light on how to use imaging assessments of heterogeneity in bone microstructure to infer spatial distributions of bone mechanical properties. The results can be readily incorporated into patient-specific and parametric models of the vertebra and spine and will facilitate longitudinal studies of the origins and ramifications of spatial variations in bone microstructure during spine development, aging, and disease progression.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Mosekilde L. The effect of modelling and remodelling on human vertebral body architecture. Technol Health Care. 1998;6:287–97.
Ulrich D, van Rietbergen B, Laib A, Rüegsegger P. The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone. 1999;25:55–60.
Carter DR, Hayes WC. Bone compressive strength: the influence of density and strain rate. Science (80-). 1976;194:1174–6.
Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. J Biomech. 2003;36:897–904. https://doi.org/10.1016/S0021-9290(03)00071-X.
Morgan EF, Keaveny TM. Dependence of yield strain of human trabecular bone on anatomic site. J Biomech. 2001;34:569–77. https://doi.org/10.1016/S0021-9290(01)00011-2.
Burger H, Van Daele PL, Grashuis K, et al. Vertebral deformities and functional impairment in men and women. J Bone Miner Res. 1997;12:152–7.
Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–61.
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–75. https://doi.org/10.1359/jbmr.061113.
Galbusera F, Van Rijsbergen M, Ito K, Huyghe JM, Brayda-Bruno M, Wilke HJ. Ageing and degenerative changes of the intervertebral disc and their impact on spinal flexibility. Eur Spine J. 2014;23:324–32.
Junghanns H, Schmorl G. The human spine in health and disease. 2nd ed America. New York: Grune & Stratton; 1971.
• Kaiser JM, Allaire B, Fein PM, et al. Correspondence between bone mineral density and intervertebral disc degeneration across age and sex. Arch Osteoporos. 2018;13:123. The regional distribution of bone mineral density throughout the centrum of the lumbar vertebra (L3) is associated with age and disc degeneration, in a population of men and women ages 41-83 years.
Wang Y, Owoc JS, Boyd SK, Videman T, Battié MC. Regional variations in trabecular architecture of the lumbar vertebra: associations with age, disc degeneration and disc space narrowing. Bone. 2013;56:249–54.
• vom Scheidt A, Grisolia Seifert EF, Pokrant C, et al. Subregional areal bone mineral density (aBMD) is a better predictor of heterogeneity in trabecular microstructure of vertebrae in young and aged women than subregional trabecular bone score (TBS). Bone. 2019;122:156–65. https://doi.org/10.1016/j.bone.2019.02.014. Trabecular number, but not trabecular thickness, is lower in the anterior vs. region of the lower thoracic (T12) centrum in older (71.6±4.8 years) but not younger (31.6±6.2 years) women.
Hulme PA, Boyd SK, Ferguson SJ. Regional variation in vertebral bone morphology and its contribution to vertebral fracture strength. Bone. 2007;41:946–57.
Li S, Wang C, Shan Z, Liu J, Yu T, Zhang X, et al. Trabecular microstructure and damage affect cement leakage from the Basivertebral foramen during vertebral augmentation. Spine (Phila Pa 1976). 2017;42:E939–48. https://doi.org/10.1097/BRS.0000000000002073.
• Hussein AI, Louzeiro DT, Unnikrishnan GU, Morgan EF. Differences in trabecular microarchitecture and simplified boundary conditions limit the accuracy of quantitative computed tomography-based finite element models of vertebral failure. J Biomech Eng. 2018;140. https://doi.org/10.1115/1.4038609. During compression of lumbar vertebrae (L1) to yield, the largest strains develop progressively in the superior third of the vertebral body and, concomitantly, in the mid-transverse plane, in a manner associated with spatial variations in microstructural parameters such as connectivity density.
Thomsen JS, Ebbesen EN, Mosekilde L. Zone-dependent changes in human vertebral trabecular bone: clinical implications. Bone. 2002;30:664–9.
Nepper-Rasmussen J, Mosekilde L. Local differences in mineral content in vertebral trabecular bone measured by dual-energy computed tomography. Acta Radiol. 1989;30:369–71.
Sandor T, Felsenberg D, Kalendar WA, Brown E. Global and regional variations in the spinal trabecular bone: single and double energy examinations. J Clin Endocrinol Metab. 1991;72:1157–68.
Keller TS, Moeljanto E, Main JA, Spengler DM. Distribution and orientation of bone in the human lumbar vertebral centrum. J Spinal Disord. 1992;5:60–74.
Antonacci MD, Hanson DS, LeBlanc A, Heggeness MH. Regional variation in vertebral bone density and trabecular architecture are influenced by osteoarthritic change and osteoporosis. Spine (Phila Pa 1976). 1997;22:2392–3.
Banse X, Devogelaer JP, Munting E, Delloye C, Cornu O, Grynpas M. Inhomogeneity of human vertebral cancellous bone: systematic density and structure patterns inside the vertebral body. Bone. 2001;28:563–71.
Gong H, Zhang M, Yeung HY, Qin L. Regional variations in microstructural properties of vertebral trabeculae with aging. J Bone Miner Metab. 2005;23:174–80.
Palepu V, Rayaprolu SD, Nagaraja S. Differences in trabecular bone, cortical shell, and endplate microstructure across the lumbar spine. Int J Spine Surg. 2019;13:361–70. https://doi.org/10.14444/6049.
• Yang G, Battié MC, Boyd SK, Videman T, Wang Y. Cranio-caudal asymmetries in trabecular architecture reflect vertebral fracture patterns. Bone. 2017;95:102–7. https://doi.org/10.1016/j.bone.2016.11.018. This study on male vertebrae (ages 21-63), trabecular bone in the peripheral but not central region of the inferior half of the centrum exhibits higher BMD, bone volume fractions, trabecular number and connectivity density than that in the superior half of the centrum.
Verstraeten A, Van Ermen H, Haghebaert G, Nijs J, Geusens P, Dequeker J. Osteoarthrosis retards the development of osteoporosis. Observation of the coexistence of osteoarthrosis and osteoporosis. Clin Orthop Relat Res. 1991;264:169–77. https://doi.org/10.1007/s00586-014-3203-4.
Arden NK, Griffiths GO, Hart DJ, et al. The association between osteoarthritis and osteoporotic fracture: the Chingford study. Br J Rheumatol. 1996;35:1299–304.
Roux C, Fechtenbaum J, Briot K, Cropet C, Liu-Léage S, Marcelli C. Inverse relationship between vertebral fractures and spine osteoarthritis in postmenopausal women with osteoporosis. Ann Rheum Dis. 2008;67:224–8. https://doi.org/10.1136/ard.2007.069369.
Livshits G, Ermakov S, Popham M, MacGregor AJ, Sambrook PN, Spector TD, et al. Evidence that bone mineral density plays a role in degenerative disc disease: the UK twin spine study. Ann Rheum Dis. 2010;69:2102–6.
Wang Y, Boyd SK, Battié MC, Yasui Y, Videman T. Is greater lumbar vertebral BMD associated with more disk degeneration? A study using μCT and discography. J Bone Miner Res. 2011;26:2785–91.
Pye SR, Reid DM, Adams JE, Silman AJ, O'Neill TW. Radiographic features of lumbar disc degeneration and bone mineral density in men and women. Ann Rheum Dis. 2006;65:234–8. https://doi.org/10.1136/ard.2005.038224.
Castaño-Betancourt MC, Oei L, Rivadeneira F, de Schepper EIT, Hofman A, Bierma-Zeinstra S, et al. Association of lumbar disc degeneration with osteoporotic fractures; the Rotterdam study and meta-analysis from systematic review. Bone. 2013;57:284–9.
Guenoun D, Fouré A, Pithioux M, Guis S, le Corroller T, Mattei JP, et al. Correlative analysis of vertebral trabecular bone microarchitecture and mechanical properties: a combined ultra-high field (7 Tesla) MRI and biomechanical investigation. Spine (Phila Pa 1976). 2017;42:E1165–72. https://doi.org/10.1097/BRS.0000000000002163.
Fields AJ, Keaveny TM. Trabecular architecture and vertebral fragility in osteoporosis. Curr Osteoporos Rep. 2012;10:132–40. https://doi.org/10.1007/s11914-012-0097-0.
Yerramshetty J, Kim D-G, Yeni YN. Increased microstructural variability is associated with decreased structural strength but with increased measures of structural ductility in human vertebrae. J Biomech Eng. 2009;131:094501. https://doi.org/10.1115/1.3148473.
Wegrzyn J, Roux J-P, Arlot ME, Boutroy S, Vilayphiou N, Guyen O, et al. Role of trabecular microarchitecture and its heterogeneity parameters in the mechanical behavior of ex vivo human L3 vertebrae. J Bone Miner Res. 2010;25:2324–31.
Kim D-G, Hunt CA, Zauel R, Fyhrie DP, Yeni YN. The effect of regional variations of the trabecular bone properties on the compressive strength of human vertebral bodies. Ann Biomed Eng. 2007;35:1907–13.
Hussein AI, Jackman TM, Morgan SR, Barest GD, Morgan EF. The intravertebral distribution of bone density: correspondence to intervertebral disc health and implications for vertebral strength. Osteoporos Int. 2013;24:3021–30. https://doi.org/10.1007/s00198-013-2417-3.
• Kaiser J, Allaire B, Fein PM, et al. Heterogeneity and spatial distribution of intravertebral trabecular bone mineral density in the lumbar spine is associated with prevalent vertebral fracture. J Bone Miner Res. 2020;35:641–8. https://doi.org/10.1002/jbmr.3946. In a case-control study of prevalent vertebral fracture, increased trabecular BMD in the anterior versus posterior region of centrum, was associated with decreased risk of prevalent fracture independent of average BMD.
Morton JJ, Bennison M, Lievers WB, Waldman SD, Pilkey AK. Failure behaviour of rat vertebrae determined through simultaneous compression testing and micro-CT imaging. J Mech Behav Biomed Mater. 2018;79:73–82. https://doi.org/10.1016/j.jmbbm.2017.11.021.
Stauber M, Nazarian A, Müller R. Limitations of global morphometry in predicting trabecular bone failure. J Bone Miner Res. 2014;29:134–41. https://doi.org/10.1002/jbmr.2006.
Gustafson HM, Melnyk AD, Siegmund GP, Cripton PA. Damage identification on vertebral bodies during compressive loading using digital image correlation. Spine (Phila Pa 1976). 2017;42:E1289–96. https://doi.org/10.1097/BRS.0000000000002156.
Jackman TM, DelMonaco AM, Morgan EF. Accuracy of finite element analyses of CT scans in predictions of vertebral failure patterns under axial compression and anterior flexion. J Biomech. 2016;49:267–75. https://doi.org/10.1016/j.jbiomech.2015.12.004.
McKay ML, Jackman TM, Hussein AI, Guermazi A, Liu J, Morgan EF. Association of vertebral endplate microstructure with bone strength in men and women. Bone. 2020;131:115147. https://doi.org/10.1016/j.bone.2019.115147.
Wu Y, Morgan EF. Effect of fabric on the accuracy of computed tomography-based finite element analyses of the vertebra. Biomech Model Mechanobiol. 2020;19:505–17. https://doi.org/10.1007/s10237-019-01225-2.
Kopperdahl DL, Morgan EF, Keaveny TM. Quantitative computed tomography estimates of the mechanical properties of human vertebral trabecular bone. J Orthop Res. 2002;20:801–5. https://doi.org/10.1016/S0736-0266(01)00185-1.
Kabel J, van Rietbergen B, Odgaard A, Huiskes R. Constitutive relationships of fabric, density, and elastic properties in cancellous bone architecture. Bone. 1999;25:481–6.
Xavier F, Jauregui JJ, Cornish N, et al. Regional variations in shear strength and density of the human thoracic vertebral endplate and trabecular bone. Int J Spine Surg. 2017;11:7. https://doi.org/10.14444/4007.
Dall’Ara E, Karl C, Mazza G, et al. Tissue properties of the human vertebral body sub-structures evaluated by means of microindentation. J Mech Behav Biomed Mater. 2013;25:23–32. https://doi.org/10.1016/j.jmbbm.2013.04.020.
Adams MA, McNally DS, Dolan P. “Stress” distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg (Br). 1996;78:965–72.
McNally DS, Adams MA. Internal intervertebral disc mechanics as revealed by stress profilometry. Spine (Phila Pa 1976). 1992;17:66–73.
O’Connell GD, Vresilovic EJ, Elliott DM. Human intervertebral disc internal strain in compression: the effect of disc region, loading position, and degeneration. J Orthop Res. 2011;29:547–55. https://doi.org/10.1002/jor.21232.
Jacobs NT, Cortes DH, Peloquin JM, Vresilovic EJ, Elliott DM. Validation and application of an intervertebral disc finite element model utilizing independently constructed tissue-level constitutive formulations that are nonlinear, anisotropic, and time-dependent. J Biomech. 2014;47:2540–6. https://doi.org/10.1016/j.jbiomech.2014.06.008.
Fields AJ, Lee GL, Keaveny TM. Mechanisms of initial endplate failure in the human vertebral body. J Biomech. 2010;43:3126–31.
• Berg-Johansen B, Fields AJ, Liebenberg EC, Li A, Lotz JC. Structure-function relationships at the human spinal disc-vertebra interface. J Orthop Res. 2018. https://doi.org/10.1002/jor.23627. In tension tests of parasagittal slabs of portions of motion segments containing the annulus fibrosus and either anterior or posterior portions of the vertebral body, 71% of specimens failed initially at the cartilage endplate-bone interface of the inner annulus region. The interface failure strength was increased in samples with higher trabecular bone volume fraction in the vertebral endplates.
Pollintine P, Dolan P, Tobias JH, Adams MA. Intervertebral disc degeneration can lead to “stress-shielding” of the anterior vertebral body: a cause of osteoporotic vertebral fracture? Spine (Phila Pa 1976). 2004;29:774–82.
Al-Rawahi M, Luo J, Pollintine P, et al. Mechanical function of vertebral body osteophytes, as revealed by experiments on cadaveric spines. Spine (Phila Pa 1976). 2011;36:770–7. https://doi.org/10.1097/BRS.0b013e3181df1a70.
Wagnac E, Aubin CÉ, Chaumoître K, Mac-Thiong JM, Ménard AL, Petit Y, et al. Substantial vertebral body osteophytes protect against severe vertebral fractures in compression. PLoS One. 2017;12:e0186779. https://doi.org/10.1371/journal.pone.0186779.
Brinckmann P, Frobin W, Hierholzer E, Horst M. Deformation of the vertebral endplate under axial loading of the spine. Spine (Phila Pa 1976). 1983;8:851–6.
Hulme PA, Ferguson SJ, Boyd SK. Determination of vertebral endplate deformation under load using micro-computed tomography. J Biomech. 2008;41:78–85.
Rodriguez AG, Slichter CK, Acosta FL, Rodriguez-Soto AE, Burghardt AJ, Majumdar S, et al. Human disc nucleus properties and vertebral endplate permeability. Spine (Phila Pa 1976). 2011;36:512–20.
Noshchenko A, Plaseied A, Patel VV, Burger E, Baldini T, Yun L. Correlation of vertebral strength topography with 3-dimensional computed tomographic structure. Spine (Phila Pa 1976). 2013;38:339–49. https://doi.org/10.1097/BRS.0b013e31826c670d.
• Patel RR, Noshchenko A, Dana Carpenter R, et al. Evaluation and prediction of human lumbar vertebrae endplate mechanical properties using indentation and computed tomography. J Biomech Eng. 2018;140:101011. https://doi.org/10.1115/1.4040252. The indentation stiffness of the vertebral endplate region was moderately dependent on mineral density of the vertebral endplate, the presence of defects in the vertebral endplate, and donor body weight.
Hou Y, Yuan W. Influences of disc degeneration and bone mineral density on the structural properties of lumbar end plates. Spine J. 2012;12:249–56. https://doi.org/10.1016/j.spinee.2012.01.021.
Grant JP, Oxland TR, Dvorak MF, Fisher CG. The effects of bone density and disc degeneration on the structural property distributions in the lower lumbar vertebral endplates. J Orthop Res. 2002;20:1115–20. https://doi.org/10.1016/S0736-0266(02)00039-6.
Stevens T. Class II special controls guidance document: intervertebral body fusion device. 2007. https://www.fda.gov/medical-devices/guidance-documents-medical-devices-andradiation-emitting-products/intervertebral-body-fusion-device-class-ii-special-controls-guidanceindustry-and-fda-staff.
Bonnheim NB, Keaveny TM. Load-transfer in the human vertebral body following lumbar total disc arthroplasty: effects of implant size and stiffness in axial compression and forward flexion. JOR Spine. 2020;3:e1078. https://doi.org/10.1002/jsp2.1078.
Deyo RA, Martin BI, Kreuter W, Jarvik JG, Angier H, Mirza SK. Revision surgery following operations for lumbar stenosis. J Bone Joint Surg Am. 2011;93:1979–86. https://doi.org/10.2106/JBJS.J.01292.
Vadapalli S, Sairyo K, Goel VK, Robon M, Biyani A, Khandha A, et al. Biomechanical rationale for using polyetheretherketone (PEEK) spacers for lumbar interbody fusion - a finite element study. Spine (Phila Pa 1976). 2006;31:E992–8. https://doi.org/10.1097/01.brs.0000250177.84168.ba.
Warburton A, Girdler SJ, Mikhail CM, Ahn A, Cho SK. Biomaterials in spinal implants: a review. Neurospine. 2020;17:101–10.
Jacobs E, Roth AK, Arts JJ, van Rhijn LW, Willems PC. Reduction of intradiscal pressure by the use of polycarbonate-urethane rods as compared to titanium rods in posterior thoracolumbar spinal fixation. J Mater Sci Mater Med. 2017;28:148. https://doi.org/10.1007/s10856-017-5953-0.
Mei K, Kopp FK, Bippus R, Köhler T, Schwaiger BJ, Gersing AS, et al. Is multidetector CT-based bone mineral density and quantitative bone microstructure assessment at the spine still feasible using ultra-low tube current and sparse sampling? Eur Radiol. 2017;27:5261–71. https://doi.org/10.1007/s00330-017-4904-y.
Izatt MT, Thorpe PLPJ, Thompson RG, D’Urso PS, Adam CJ, Earwaker JWS, et al. The use of physical biomodelling in complex spinal surgery. Eur Spine J. 2007;16:1507–18. https://doi.org/10.1007/s00586-006-0289-3.
Funding
NIH AR054620
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomechanics
Rights and permissions
About this article
Cite this article
Auger, J.D., Frings, N., Wu, Y. et al. Trabecular Architecture and Mechanical Heterogeneity Effects on Vertebral Body Strength. Curr Osteoporos Rep 18, 716–726 (2020). https://doi.org/10.1007/s11914-020-00640-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00640-0