Abstract
Purpose of Review
Isocitrate dehydrogenase (IDH) mutation status has important prognostic implications in glioma patients, with IDH wild-type (IDH-WT) gliomas being associated with worse prognosis and shorter survival when compared with IDH mutant (IDH-mut) gliomas. Optimization of quality of life is a priority in the management of glioma patients. The goal of this systematic review was to identify studies that explored the association of IDH mutation status with patient-reported outcomes (PROs) and cognitive functioning of glioma patients.
Recent Findings
Studies that evaluated the association of IDH mutation status with PROs and/or cognitive functioning of glioma patients were identified from the Pubmed/MEDLINE, Clarivate analytics, and Google Scholar databases. Eight studies (7 journal articles and 2 conference abstracts) with a total of 658 low-grade glioma and high-grade glioma patients investigated the association of cognitive functioning and/or QoL with IDH status. IDH-WT status was associated with greater cognitive impairment relative to IDH-Mut status in three studies, while one study did not find the association between IDH status and perioperative cognitive functioning. One study reported worse postoperative cognitive functioning patients with IDH-WT vs. IDH-mut gliomas. In one study, IDH-WT status was linked to greater impairment on physical and communication functioning after surgery.
Summary
IDH-WT gliomas are associated with greater cognitive burden than IDH-Mut tumors. The association of IDH status with QoL remains less clear. Assessment of IDH status should be considered when evaluating QoL and cognitive complaints of glioma patients. Further studies linking glioma molecular phenotypes with PROs and cognitive functioning are encouraged.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma is the most common malignant intracranial tumor and is a significant source of cancer-related morbidity and mortality worldwide [1, 2]. The prognosis for World Health Organization (WHO) grade III and IV (high-grade) remains dismal, with limited treatment options, progressive clinical course, and median survival under 2 years for the most aggressive tumors, glioblastoma (WHO grade IV) [3]. Lower-grade gliomas are associated with a more indolent clinical course and longer survival than high-grade; however, lower-grade gliomas often progress into high-grade gliomas resulting in expedited clinical deterioration and death [3, 4].
Mutation in the enzymes isocitrate dehydrogenase 1 and 2 (IDH1/2) has recently emerged as an important prognostic biomarker in glioma [5, 6]. IDH1/2 plays numerous key roles in cellular metabolism and also protects cells from oxidative damage [7]. IDH mutations occur in the majority of WHO grade II and III gliomas, as well as in glioblastomas evolved from lower-grade glioma [5, 7]. It is now well established that IDH wild-type (WT) gliomas, when compared with IDH-mutant (mut) gliomas, carry poorer clinical prognosis and are associated with shortened survival [5, 6, 8]. IDH-WT low-grade gliomas were shown to be more similar to primary glioblastomas in terms of genomic aberrations and clinical behavior [9,10,11]. Hence, IDH mutation status has been included in the most recent iteration of the WHO diagnostic classification of brain tumors and is increasingly being considered in treatment planning for glioma patients [12].
In addition to the differences in survival, it is becoming increasingly clear that distinct underlying tumorigenic processes drive IDH-mut and IDH-WT [9, 13, 14] highlighting the idea that these two glioma types are best considered different diseases. Despite the divergent biology, genomic alterations, and clinical course, there continues to be a tendency within the community to treat these two patient populations similarly. To some extent, this tendency is appropriate, as both IDH-mut and IDH-WT tumors are characterized by an infiltrative growth pattern through the brain, as opposed to brain metastases, which tend to be more well-encapsulated. Consequently, the two glioma types disrupt normal brain function in similar ways. On the other hand, recent studies suggest IDH-Mut and IDH-WT gliomas affect the neuronal and immune microenvironments in different ways [15, 16] which very likely impact symptomatology, cognitive outcomes, and response to certain therapies. The significant differences in biology require incorporation of IDH mutation status into outcome analyses to better understand the impact of this important disease variable.
Progressive decline in cognitive functioning and increasing symptom burden are common in both IDH-mut and IDH-WT glioma patients and can be attributed to both disease progression and side effects from standard glioma treatments (surgery, radiotherapy, and chemotherapy) [17]. Patient-reported outcomes (PROs) are valuable metrics for evaluation of the experience, symptoms, and quality of life (QoL) of glioma patients [18]. They are usually obtained using self-report measures and reflect patient perception of the burden imposed by a disease. Cognitive symptoms can be evaluated by both PROs, in the form of self-report of cognitive dysfunction or by performance-based neuropsychological tests. Because brief cognitive screens have been shown to be insensitive to important cognitive symptoms in brain tumor patients [19], neuropsychological tests with adequate sensitivity have been increasingly added to clinical trials as outcome measures [20] and are increasingly being incorporated into the clinical management of glioma patients [21, 22].
PROs and performance-based measures have shown that progressive deterioration of QoL and cognitive function are common and potentially inevitable complications of gliomas that carry independent prognostic significance for high-grade glioma patients [23]. Long-term survivors of low-grade gliomas also often experience decline in QoL [24, 25] and cognitive function. Preservation of optimal cognitive functioning and QoL are increasingly becoming priorities in the management of glioma patients [18, 26, 27].
The association between clinically relevant biomarkers, such as IDH status, and survival has been well established, but the impact of these biomarkers on important outcomes such as cognitive functioning and QoL has only recently become the target of research. The relevance of IDH mutational status to cognitive function and QoL may be important for treatment guidance in glioma patients. Individual, clinically relevant molecular profiles might be considered in treatment decision-making in order to optimize patient quality of life. Although literature linking PROs and cognitive function with IDH status is growing, a systematic review of the state of this literature is needed to assess the maturity of knowledge in this area, determine whether there are implications for clinical decision making, and guide future research. The goal of this review was to systematically examine the association of IDH mutation status with cognitive function and QoL of glioma patients.
Methods
The review was implemented in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [28].
Data Sources and Search Strategy
This systematic review of the literature was conducted on May 15, 2020, with the goal to identify available published studies or conference abstracts that evaluated the association of IDH1/2 mutation status (IDH-Mut or IDH-WT) with cognitive functioning and QoL of patients with gliomas. Articles were identified from Pubmed/Medline, Clarivate analytics, and Google Scholar databases using relevant keywords (mesh vocabulary or free text terms): “IDH,” “isocitrate dehydrogenase,” “cognitive,” and “quality of life”. There were no restrictions on country of origin and publication date. Only original research papers or conference proceedings of studies performed in humans and with their abstracts or full-texts available in English were considered for this review. Review articles, case reports, commentaries, and editorials were not included in the analyses. References of identified papers were reviewed for other relevant publications.
Study Selection and Data Extraction
Initial analysis of the identified publications was performed by reviewing titles and abstracts of all identified papers and conference abstracts. Case-control and prospective cohort studies were included in the analyses if they compared PRO measures (cognitive functioning and/or QoL) in patients diagnosed with gliomas (any grade) as a function of IDH status (IDH-Mut vs. IDH-WT). There were no restrictions with regard to PRO measures used, method used for IDH status assessment, and glioma histological grade.
Relevant articles were extracted and subjected to full-text analyses. In cases of conference proceedings, only relevant data provided in the abstract was considered for the analysis. Full-text of selected articles was reviewed, and the following variables were extracted from the full-text and/or abstracts of each paper: year and country of publication, glioma histological grade, proportion of patients with IDH-WT gliomas, investigated PROs (instruments/questionnaires and scoring with thresholds), and differences of investigated PROs as a function of IDH status (IDH-Mut vs. IDH-WT).
Results
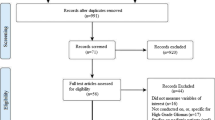
We identified 8 studies (7 journal articles and 2 conference abstracts) that included a total of 658 low-grade glioma and high-grade glioma patients that investigated the association of cognitive functioning and/or QoL as a function of IDF-WT or IDH-Mut status (Fig. 1 and Table 1). The results of one study with overlapping samples of glioma patients were published in two peer-review journal publications [33, 34]. Published study sample sizes ranged from 49 [32] to 168 [33, 34] patients.
Cognitive Functioning
The association of IDH status with cognitive functioning as measured by performance-based testing was investigated in seven studies [29•, 30••, 31••, 32,33,34,35,36]. Five studies found that baseline preoperative cognitive functioning was worse in IDH-WT glioma patients when compared with patients harboring IDH-Mut gliomas [29••, 30••, 34, 35, 38]. Specifically, Wefel and colleagues employed a comprehensive battery of 14 neurocognitive tests in 119 malignant glioma patients before surgery and found that patients with IDH1-WT tumors performed significantly worse on verbal learning and memory, processing speed, executive function, auditory comprehension, and manual dexterity relative to patients with IDH1-mut gliomas [29•] prior to any intervention. Furthermore, the proportion of patients with impairment on 3 or more and 5 or more cognitive domains was greater in patients with IDH1-WT gliomas, indicating greater cognitive burden associated with IDH1-WT status. Although this study did not include PRO measures of QoL or self-reported cognitive symptoms, there were indications of slightly poorer Karnofsky performance status (KPS) in the IDH-WT group. Another study by Kessler and colleagues employed a flexible battery of cognitive measures based on clinical needs prior to initial surgery and found that incidence of cognitive impairment defined as z-score of ≤ − 1.5 on two tests or ≤ −2.0 on one test was significantly higher in patients with IDH1-WT gliomas when compared with that in patients with IDH1-mut gliomas (84% vs. 65%, respectively) [30••]. Derks and colleagues used a battery of cognitive tests measuring verbal memory, executive functioning and psychomotor speed, working memory, information processing speed and psychomotor speed, attention functioning, and executive functioning in 54 diffuse grade II–IV glioma patients prior to initial surgery [38]. They found that IDH1-WT glioma patients performed worse on cognitive testing relative to patients with IDH-Mut gliomas; however, only difference in the summary score of a verbal memory test reached statistical significance after adjusting for age, presence of epilepsy, and education. Van Kessel and colleagues examined predictors of overall and domain-specific neurocognitive functioning in 168 (age range: 19 to 82 years) consecutive diffuse WHO grade II (n = 64), grade III (n = 28), and grade IV (n = 76) glioma patients before and 3–6 after awake glioma surgery [33, 34]. Patients were evaluated for attention and executive functioning (four tests), memory (four tests), language (two tests), visuospatial functioning (two tests), and psychomotor speed (three tests). Seventy-five (48.3%) gliomas were IDH-mut. IDH mutation status correlated with impairment of memory, psychomotor speed, and visuospatial domains at baseline independent of tumor volume on T2/FLAIR-weighted MRI [34]. IDH wild-type status was also associated with a further decline in overall neurocognitive functioning and executive functioning after surgery, independent of other clinical and demographic variables [33]. Another study of 20 (n = 8 IDH-wt) WHO grade I (n = 1), II (n = 6), III (n = 7), and IV (n = 5) glioma patients found that patients harboring IDH-wt gliomas had worse verbal recall and verbal recognition than patients with IDH-mut gliomas [35].
Lee and colleagues evaluated health-related quality of life and cognitive functions (digit span tests, verbal fluency, and the Trail Making Tests parts A) in 61 high-grade glioma patients before and after tumor resection surgery [36]. They reported that cognitive function scores were similar in patients with IDH1-WT gliomas when compared with those in patients with IDH-Mut gliomas. However, at postoperative assessment, Trail Making Test and verbal fluency scores were significantly better in patients with IDH1-mut gliomas compared with that in patients IDH1-WT gliomas, and this association was independent of patient age, performance status, tumor size and location, and extent of resection. Furthermore, these authors found that other molecular biomarkers of gliomas, including 1p/19q deletion, MGMT promoter methylation, epidermal growth factor receptor (EGFR) amplification, phosphatase and tensin homolog (PTEN) loss, and c-Met expression, were not associated with cognitive functioning and PROs either before or after surgery.
Barzilai and colleagues tested memory, language, attention/working memory, and visuomotor organization in a sample of 49 low-grade glioma patients before and after glioma resection surgery [32]. They noted improvement of cognitive functioning after resection of glioma; however, IDH1 mutation status was not associated with the extent of improvement in the overall sample of patients. The authors comment that within non-dominant hemisphere patients (n = 23), IDH-mut (13/23 patients) status was associated with larger residual tumor volume at follow-up, but was not related to cognitive performance. It is not clear if the authors specifically tested the association between IDH status and cognitive function at baseline or the change in cognitive function after surgery.
Quality of Life
Two studies explored the association of IDH status with QoL of glioma patients and provided mixed findings [36, 37]. Specifically, a study in 61 high-grade glioma patients did not find an association of scores on the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) and EORTC brain cancer module (BN20 brain cancer module) with IDH1 mutation status before glioma surgery [36]. After surgery, however, scores on the EORTC BN20, physical functioning, and communication deficits dimensions were better in the IDH1-mut gliomas when compared with IDH1s-WT gliomas. This association was independent from other tumor and non-tumor factors. A conference abstract from the Adelphi’s Disease-Specific Programme in Canada, France, Germany, Italy, Spain, and the UK that included 26 IDH-Mut and 109 IDH-WT glioblastoma patients found no difference in EuroQol EQ-5D index score and EORTC Global health status score as a function of IDH status [37].
Discussion
Our review of this small but growing literature demonstrates that IDH status is critical to consider in understanding the impact of glioma on cognitive functioning and QoL. At this time, the literature is fairly consistent in demonstrating that IDH-WT gliomas are associated with worse cognitive functioning on objective, performance-based neuropsychological tests when compared with IDH-Mut gliomas prior to any intervention. Furthermore, there are some indications that IDH-WT tumors confer a greater risk of cognitive decline after surgery. The association of IDH status with self-reported cognitive symptoms was not the focus of any studies we identified. Patients with IDH-WT glioma should be considered at increased risk for cognitive impairment in addition to their poorer overall survival and prognosis.
The reviewed studies were cross-sectional or included two evaluation time-points (before and after surgery). Longitudinal studies exploring whether IDH status is associated with cognitive trajectories of glioma patients are strongly encouraged to understand the impact of IDH status on cognitive sequelae in long-term glioma survivors. Such data may improve risk stratification and monitoring of glioma patients for cognitive decline. This information could be used to guide monitoring strategies for cognitive progression with self-report and/or performance-based cognitive assessments.
Only two studies presented as conference abstracts explored the association of IDH status with QoL and provided mixed findings, suggesting an association with better QoL post-surgically for IDH-Mut patients [36, 39]. The small sample size and lack of peer-reviewed literature in this area suggest that the data are too preliminary to draw any meaningful conclusions about these relationships. Given that QoL assessment is increasingly becoming an important endpoint in neuro-oncology clinical trials and preservation of QoL is considered a priority in the management of glioma patients, further studies in larger samples of glioma patients should attempt to elucidate the association of IDH status with QoL.
Potential Mechanisms Underlying the Association of IDH Status with Cognitive Functioning
Mechanisms underlying the association of IDH status with cognitive functioning remain to be fully understood, but it has been demonstrated that structural and functional neural network disruption, differences in tumor micro-environment, and cellular level interactions can be an important mechanism of IDH mutation status associated impaired cognition of glioma patients (Table 2). The relationship between tumor growth rate and cognitive dysfunction has been well demonstrated [43], and the Wefel et al. (2016) study detailed here extended that logic to the IDH-Mut vs. WT situation [29•]. There is growing evidence to suggest that the more rapid growth rate of IDH-WT compared with IDH-Mut gliomas disrupts structural and functional brain connectivity and could be an underlying mechanism of cognitive dysfunction [30••, 31••]. Specifically, Derks and colleagues explored the association between cognitive status and global functional connectivity measured using magnetoencephalography (MEG) in 54 diffuse glioma patients (31 IDH1-mut and 23 IDH1-WT) [31••]. They found that patients with IDH1-WT gliomas had lower functional connectivity in the alpha band than patients with IDH-Mut gliomas when controlling for age and presence of epilepsy. Furthermore, in the total sample of glioma patients, lower alpha band functional connectivity was associated with poorer cognitive performance after adjusting for patient age, education, and presence of epilepsy. Similar results were reported by Stoecklein and colleagues using resting-state functional MRI in 34 (20 IDH-wt and 14 IDH-mut) newly diagnosed WHO grade II–IV glioma patients [41]. The authors calculated an abnormality index (ABI), which quantifies the strength of functional connectivity in each voxel with a reference cohort of 1000 healthy subjects. ABI in lesional and non-lesional brain hemispheres was elevated (indicating greater functional connectivity damage) in patients with IDH-wt vs. IDH-mut gliomas irrespective of the WHO grade. Higher ABI in both ipsilateral and contralateral brain hemispheres was associated with poorer cognitive function on a screening test (Montreal Cognitive Assessment test).
Along similar lines, structural imaging modalities have demonstrated important impacts of IDH mutational status on metrics of connectively. Kesler et al. examined the association of cognitive functioning with brain network organization evaluated using voxel-based morphometry in 35 IDH1-mut and 32 IDH1-WT malignant astrocytomas [30••]. They found that IDH1-WT patients had lower network efficiency in several medial frontal, posterior parietal, and subcortical regions, and network efficiency was inversely related to cognitive impairment (independent of IDH1 status). Price and colleagues investigated invasiveness of 9 IDH1-mut and 61 IDH1-WT glioblastomas and found that all of the patients with IDH-1 mutation had a minimally invasive DT imaging phenotype, while among the IDH-1 wild-type tumors, 69% were diffusively invasive, 23% were locally invasive, and 8% were minimally invasive [40]. Jütten and colleagues examined microstructural characteristics of normal-appearing white matter using DT imaging in 20 (12 IDH-mut and 8 IDH-wt) patients with different glioma before surgery and 20 matched controls [35]. Study participants were also subjected to neurocognitive assessment. Patients with IDH-mut gliomas had better preserved microstructural integrity of normal-appearing white matter compared with patients with IDH-wt gliomas, as indicated by higher fractional anisotropy (FA), and lower mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) values, suggesting more infiltrative growth pattern of IDH-wt gliomas. Brain microstructural characteristics were associated with neurocognitive functioning. Specifically, higher FA values were associated with better performances in verbal learning, attention, and task switching, whereas higher MD and RD predicted poorer attentional performance. These findings imply the importance of IDH dependent brain microstructural brain changes for cognitive/behavioral functioning of glioma patients. The combination of the functional and structural brain imaging literature demonstrates that IDH-WT gliomas disrupt local brain connectivity and distributed network efficiency, which is related to cognitive dysfunction in these patients.
IDH status has an important influence on the tumor microenvironment, which may also impair normal brain functioning. In a study of 43 grade II–III gliomas and 14 glioblastoma, IDH WT tumors were found to have more prominent tumor lymphocyte infiltration and higher programmed death-ligand 1 expression compared with IDH-Mut gliomas [42]. Additional work revealed that the IDH mutant–associated metabolite R − 2-hydroxyglutarate suppresses T cell activity [44], offering a potential explanation for the immune microenvironment differences between IDH WT and Mut tumors. Exciting new work by Monje and colleagues has demonstrated complex interactions between glioma cells and surrounding neurons [15]. Their findings suggest that glioma growth rate influences neuronal excitability and that integration of glioma cells into neural networks may be an important factor in glioma progression. Further studies of interactions between glioma and neural structures are needed to understand the extent to which IDH mutation may play a role at the cellular level. Furthermore, exploring biological mechanisms underlying impairment of brain functioning in IDH-WT gliomas may provide candidate therapeutic targets that could be used to optimize cognitive functioning of glioma patients.
Neuropsychiatric comorbidities are common and associated with impaired QoL and worse prognosis in glioma patients [45,46,47]. Given the absence of literature regarding IDH mutational status impact on QoL, considerations of mechanistic factors are necessarily speculative. IDH-WT gliomas might be predicted to have a more negative impact on QoL, given the poorer prognosis and higher rates of cognitive impairment. However, previous literature has demonstrated that younger (compared with older) glioma patients with lower-grade tumors (compared with higher grade) experience more anxiety [48]. Given that young age and lower grade are characteristics of IDH-Mut tumors, perhaps QoL is more affected in these patients. Some neuropsychiatric comorbidities, such as mood and anxiety symptoms and fatigue, can respond to pharmacologic and non-pharmacologic treatments and contribute to improved QoL of glioma patients. Hence, further studies should investigate the association between clinically relevant molecular biomarkers, such as IDH, and neuropsychiatric complications of gliomas. These findings can be used to better risk-stratify glioma patients for neuropsychiatric complications and highlight the population of patients that would most benefit from recommendations for pharmacological and non-pharmacological therapies.
There are several limitations of this review. First, the studies involved heterogeneous cohorts of patients with varying clinical characteristics, glioma grades, and molecular features. Tumor laterality and location, which can impair neuro-cognitive functioning and QoL, were not reported by all studies. Furthermore, there is a possibility that some patient cohorts overlapped between two of the studies, indicating that the relationships between IDH mutation status and cognition may not represent an independent replication [29•, 30••]. Furthermore, instruments used for neuro-cognitive and QoL assessment varied between studies. Individual patient data meta-analysis may allow a more reliable evaluation of the association of IDH status with cognitive status/QoL while controlling for possible clinical confounders.
Conclusions
IDH-WT gliomas are associated with greater cognitive burden when compared with IDH-Mut gliomas; a finding replicated over several studies. The association of IDH status with QoL measures remains unclear. Patients with IDH-WT gliomas should be considered at greater risk for unfavorable cognitive outcome in addition to their poorer clinical status and shorter survival. Impaired brain connectivity associated with IDH-WT gliomas and/or tumor micro-environment factors may be an important mechanism underlying neurocognitive dysfunction in these patients. Studies exploring the association of IDH status with QoL and other PROs are encouraged.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncology. 2018;20:iv1–86.
Leece R, Xu J, Ostrom QT, Chen Y, Kruchko C, Barnholtz-Sloan JS. Global incidence of malignant brain and other central nervous system tumors by histology, 2003-2007. Neuro-Oncology. 2017;19:1553–64.
Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4:1254.
Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, Schildkraut JM, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus. NIH Public Access. 2015;38:E6.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73.
Parsons DW, Jones S, Zhang X, Lin JC-H, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12.
Waitkus MS, Diplas BH, Yan H. Isocitrate dehydrogenase mutations in gliomas. Neuro-Oncology. Oxford University Press. 2016;18:16–26.
Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–6.
Cancer Genome Atlas Research Network, Brat DJ, Verhaak RGW, Aldape KD, Yung WKA, Salama SR, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372:2481–98.
Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707–18.
Olar A, Wani KM, Alfaro-Munoz KD, Heathcock LE, van Thuijl HF, Gilbert MR, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol. 2015;129:585–96.
Louis DN, Perry A, Guido R, Von Deimling A, Figarella-Branger D, Webster, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131:803–20.
Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–63.
Unruh D, Zewde M, Buss A, Drumm MR, Tran AN, Scholtens DM, et al. Methylation and transcription patterns are distinct in IDH mutant gliomas compared to other IDH mutant cancers. Scientific Reports. Nat Publ Group. 2019;9:8946.
Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. Nature Publishing Group. 2019;573:539–45.
Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. Nature Publishing Group. 2020;20:12–25.
Taphoorn MJB. Neurocognitive sequelae in the treatment of low-grade gliomas. Semin Oncol. 2003;30:45–8.
Dirven L, Armstrong TS, Blakeley JO, Brown PD, Grant R, Jalali R, et al. Working plan for the use of patient-reported outcome measures in adults with brain tumours: a response assessment in neuro-oncology (RANO) initiative. Lancet Oncol. Elsevier. 2018;19:e173–80.
Meyers CA, Wefel JS. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol. 2003;21:3557–8.
Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. Neuro-oncology. 2003;5:89–95.
Noll KR, Bradshaw ME, Parsons MW, Dawson EL, Rexer J, Wefel JS. Monitoring of neurocognitive function in the care of patients with brain tumors. Curr Treat Options Neurol. 2019;21:33.
Noll KR, Bradshaw ME, Rexer J, Wefel JS. Neuropsychological practice in the oncology setting. Arch Clin Neuropsychol. 2018;33:344–53.
Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 2000;18:646–50.
Boele FW, Douw L, Reijneveld JC, Robben R, Taphoorn MJB, Aaronson NK, et al. Health-related quality of life in stable, long-term survivors of low-grade glioma. J Clin Oncol. 2015;33:1023–9.
Aaronson NK, Taphoorn MJB, Heimans JJ, Postma TJ, Gundy CM, Beute GN, et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29:4430–5.
Renovanz M, Hechtner M, Kohlmann K, Janko M, Nadji-Ohl M, Singer S, et al. Compliance with patient-reported outcome assessment in glioma patients: predictors for drop out. Neurooncol Pract. Oxford University Press. 2018;5:129–38.
Blakeley JO, Coons SJ, Corboy JR, Leidy NK, Mendoza TR, Wefel JS. Clinical outcome assessment in malignant glioma trials: measuring signs, symptoms, and functional limitations. Neuro-Oncology. Oxford University Press. 2016;18:ii13–20.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6:e1000097.
• Wefel JS, Noll KR, Rao G, Cahill DP. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro-Oncology. 2016;18:1656–63 This study examined the association of IDH status with neurocognitive status, evaluated using a comprehensive battery neurocognitive tests, in 106 patients with malignant gliomas. The authors documented greater incidence of cognitive deterioration and greater cognitive burden in patients harboring IDH-WT gliomas when compared to IDH-Mut tumors.
•• Kesler SR, Noll K, Cahill DP, Rao G, Wefel JS. The effect of IDH1 mutation on the structural connectome in malignant astrocytoma. J Neuro-Oncol. 2017;131:565–74 This study examined the association of IDH status with neurocognitive status, evaluated using a comprehensive battery neurocognitive tests, in 106 patients with malignant gliomas. The authors documented greater incidence of cognitive deterioration and greater cognitive burden in patients harboring IDH-WT gliomas when compared with IDH-Mut tumors.
•• Derks J, Kulik S, Wesseling P, Numan T, Hillebrand A, van Dellen E, et al. Understanding cognitive functioning in glioma patients: the relevance of IDH-mutation status and functional connectivity. Brain Behav. 2019:e01204 This study in 54 diffuse glioma patients reported that IDH1-WT glioma patients had worse verbal memory after adjusting for age, presence of epilepsy, and education status. Patients with IDH1-WT gliomas had lower functional connectivity (evaluated with magnetoencephalography) in the alpha band than patients with IDH-Mut gliomas when controlling for age and presence of epilepsy.
Barzilai O, Ben Moshe S, Sitt R, Sela G, Shofty B, Ram Z. Improvement in cognitive function after surgery for low-grade glioma. J Neurosurg. 2018:1–9. https://doi.org/10.3171/2017.9.JNS17658.
van Kessel E, Snijders TJ, Baumfalk AE, Ruis C, van Baarsen KM, Broekman ML, et al. Neurocognitive changes after awake surgery in glioma patients: a retrospective cohort study. J Neuro-Oncol. 2020;146:97–109.
van Kessel E, Emons MAC, Wajer IH, van Baarsen KM, Broekman ML, Robe PA, et al. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a retrospective cohort study prior to antitumor treatment. Neurooncol Pract. 2019;6:463–72.
Jütten K, Mainz V, Gauggel S, Patel HJ, Binkofski F, Wiesmann M, et al. Diffusion tensor imaging reveals microstructural heterogeneity of normal-appearing white matter and related cognitive dysfunction in Glioma patients. Front Oncol. 2019;9:536.
Lee S, Jang Y. P18.04 IDH-1 mutation determines health-related quality of life and cognitive deficit after surgery in high grade glioma. Neuro-Oncology. Oxford University Press. 2017;19:iii121.
Waller J, Atkinson T, Byrne K. Impact of isocitrate dehydrogenase (IDH) status on the performance status and quality of life (QOL) of glioblastoma multiforme (GBM) patients. Value Health. Elsevier. 2017;20:A453.
Derks J, Kulik S, Wesseling P, Numan T, Hillebrand A, de Witt Hamer PC, et al. P01.075 Understanding cognitive functioning in diffuse glioma patients: the relevance of IDH mutation status and functional connectivity. Neuro-Oncology. Oxford University Press. 2018;20:iii247.
Weller M, Wick W, Aldape K, Brada M, Berger M, Pfister SM, et al. Glioma. Nat Rev Dis Primers. 2015;1:15017.
Price SJ, Allinson K, Liu H, Boonzaier NR, Yan J-L, Lupson VC, et al. Less invasive phenotype found in Isocitrate dehydrogenase–mutated glioblastomas than in isocitrate dehydrogenase wild-type glioblastomas: a diffusion-tensor imaging study. Radiology. 2017;283:215–21.
Stoecklein VM, Stoecklein S, Galiè F, Ren J, Schmutzer M, Unterrainer M, et al. Resting-state fMRI detects alterations in whole brain connectivity related to tumor biology in glioma patients. Neuro-oncology. 2020. https://doi.org/10.1093/neuonc/noaa044.
Berghoff AS, Kiesel B, Widhalm G, Wilhelm D, Rajky O, Kurscheid S, et al. Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro-Oncology. 2017;19:1460–8.
Noll KR, Sullaway C, Ziu M, Weinberg JS, Wefel JS. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro-oncology. 2015;17:580–7.
Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018;24:1192–203.
van Coevorden-van Loon EMP, Coomans MB, Heijenbrok-Kal MH, Ribbers GM, van den Bent MJ. Fatigue in patients with low grade glioma: systematic evaluation of assessment and prevalence. J Neuro-Oncol. 2017;133:237–46.
Rooney AG, Brown PD, Reijneveld JC, Grant R. Depression in glioma: a primer for clinicians and researchers. J Neurol Neurosurg Psychiatry. 2014;85:230–5.
Boele FW, Rooney AG, Grant R, Klein M. Psychiatric symptoms in glioma patients: from diagnosis to management. Neuropsychiatr Dis Treat. 2015;11:1413–20.
Arnold SD, Forman LM, Brigidi BD, Carter KE, Schweitzer HA, Quinn HE, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro-Oncology. 2008;10:171–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Adomas Bunevicius and Julie Miller declare no conflict of interest. Michael Parsons has received consulting fees from Agios Pharmaceuticals for participation in research design.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Bunevicius, A., Miller, J. & Parsons, M. Isocitrate Dehydrogenase, Patient-Reported Outcomes, and Cognitive Functioning of Glioma Patients: a Systematic Review. Curr Oncol Rep 22, 120 (2020). https://doi.org/10.1007/s11912-020-00978-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-020-00978-9