Abstract
Background
Gliomas make up approximately 26.5% of all primary CNS tumors and 80.7% of malignant tumors. They are classified according to histology, location, and genetics. Grade III and IV gliomas are considered high-grade gliomas (HGGs). The cognitive signs and symptoms are attributed to mass defects depending on location, growth rapidity, and edema. Our purpose is to review the cognitive status of patients diagnosed with HGGs; the effect of treatments including surgical resection, radiotherapy, and chemotherapy; and the predictors of the cognitive status.
Methods
We utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines as a template for the methodology. A comprehensive literature search was performed from three databases (PubMed, ScienceDirect, and Cochrane Library) for clinical trials and longitudinal studies on patients diagnosed with HGGs assessing their cognitive status.
Results
Thirteen studies were selected among which 9 assessed cognitive function before and after treatment. One assessed the consistency of cognitive complaints and objective cognitive functioning. Three reported factors affecting disease progression and cognitive status. Most HGG patients have impairment in at least one cognitive domain. Treatments including surgical resection or radio-chemotherapy did not impair cognitive status.
Discussion
The cognitive status could be used to assess sub-clinical tumor progression. Factors correlated to cognitive status were tumor location, edema, and grade. Patient characteristics correlated were pre-operative epilepsy, corticosteroid use, and age at the time of diagnosis.
Conclusion
Assessment of the cognitive status of HGG patients indicates sub-clinical tumor progression and may be used to assess treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are over a hundred histologically distinct types of primary central nervous system (CNS) tumors, each with different clinical presentation, treatment, and outcome. The total incidence of primary CNS tumors is approximately 24.25 per 100,000, and 7 out of 100,000 for malignant CNS tumors globally. Malignant CNS tumors have an average annual mortality rate of 4.43. The incidence is highest among those aged above 85 years and lowest among children and adolescents 0–19 years of age [1]. Gliomas are neuroepithelial tumors that originate from the glial cells of the CNS. Gliomas make up approximately 26.5% of all primary CNS tumors and 80.7% of malignant CNS tumors [2].
The WHO classifies CNS tumors into four grades, grades I and II being low-grade, whereas grades II and III are considered high grade. Gliomas are further divided into adult-onset or pediatric-onset. For our review, we only focus on adult-type diffuse gliomas which account for the majority of primary brain tumors. In the fifth edition of the WHO Classification of Tumors of the Central Nervous System (WHO CNS-5) (2021) the adult-type, diffuse gliomas include astrocytoma, IDH-mutant; oligodendroglioma, IDH-mutant, and 1p/19q-co-deleted; and glioblastoma, IDH-wildtype [3]. In the previous WHO CNS-4 (2016), they were divided into 15 categories [4]. Earlier the classifications have been changed with the editions of WHO CNS Blue books in 1979, 1993, 2000, 2007, and 2016 [5, 6]; therefore, different studies on gliomas have taken various sub-types. The prognosis of patients with high-grade gliomas has been based on clinical factors [7]. The initial therapy in the standard management of high-grade gliomas is maximal surgical resection which can rapidly reduce the mass effect and improve neurologic symptoms, followed by radiotherapy dose of 54 to 60 Gy along with chemotherapy with temozolomide (TMZ) [7].
Cognitive functions (CFs) refer to “The mental processes involved in the acquisition of knowledge, manipulation of information, and reasoning” [8]. The components of cognitive functions may be divided into perception, memory, learning, attention, decision-making, and language abilities [8]. The cognitive effects of gliomas are dependent on location within the brain, rapidity of growth, mass effect of the tumor, and associated edema. Gliomas may damage eloquent brain areas or connectivity and cause white matter alterations secondary to glioma infiltration, which may lead to the deterioration of specific cognitive domains. Fast-growing tumors with significant cerebral edema can lead to acute onset of cognitive deficits; slower-growing tumors are more likely to produce subtle changes in behavior or cognition. This review assesses the domains of CF mostly affected by HGGs and the factors affecting the cognitive outcome in patients with HGGs.
Methodology
We utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines as a template for the methodology.
Search strategy
Systematic searches of the PubMed, Science Direct, and Cochrane Library databases were conducted for relevant evidence. The following search was done using Medical Subject Headings (MeSH) terms (Gliomas OR High-Grade Gliomas) AND (Cognitive effects OR Cognition OR Cognitive functions) in the “Title/Abstract.” Only English-language articles were considered. The eligibility of relevant articles was first assessed by screening results based on title/abstract review and removing duplicates. The full texts were then screened according to predefined inclusion and exclusion criteria.
Inclusion and exclusion criteria
Studies were included if the terms gliomas or high-grade gliomas and cognitive effects or cognition or CF were mentioned or implied in the title or the abstract.
Prospective studies on patients diagnosed with higher-grade (anaplastic astrocytoma, oligodendroglioma, anaplastic mixed glioma) or highest-grade gliomas (glioblastoma multiforme, gliosarcoma, gliomatosis cerebri) assessing their cognitive function before/after treatment were chosen. Articles assessing factors influencing the cognitive status of patients with HGGs were also included.
Review articles, trials not assessing cognitive function or impairment, articles on pediatric patients, and trials on patients with lower-grade gliomas (grade 1 or 2) or any other form of tumor were excluded. The WHO CNS classifications were limited to 2000–2021; thus, articles using classifications before these were excluded.
Data collection
Data was collected and analyzed using Zotero® software. The duplicates in the results of the initial database search were removed. The articles had titles and abstracts assessed by 2 authors MWSB and RT and articles not matching the requirement were excluded. The remaining articles were assessed for full-text by RT and MWSB. Included and excluded articles were then discussed and approved by all authors.
Data extraction and analysis
Duplicates were eliminated from the articles of the initial database searches using the Zotero software package. The titles of the articles were then reviewed independently by the authors to select articles relevant to the study. Subsequently, the abstracts of the selected articles were reviewed for eligibility within this study.
Risk of bias assessment
STROBE guidelines [9] were used to assess the quality of observational studies including case-control, cohort, and cross-sectional studies. The final included studies were assessed and approved by all authors.
Data synthesis
The demographic details including sample size, WHO classification used in the study, treatment interventions, baseline assessment, and follow-up time were tabulated in Table 1. Table 2 reports the neuro-cognitive domains assessed, the assessment tool, baseline, and follow-up outcomes. The effects of tumor characteristics and treatment modalities on CF were tabulated in Table 3.
Results
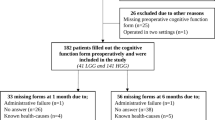
A total of 1703 articles were identified through database search and 3 through additional resources (institute libraries). After removing duplicates 991 articles were left. Nine hundred twenty out of the 991 articles were removed in the initial screening of title and abstracts for relevance. Out of the 71 remaining articles, 58 could be accessed for full text. Forty-five were excluded according to exclusion criteria and 13 were included in the systematic review. The PRISMA flowchart is illustrated in Fig. 1.
Results of individual studies
Nine articles [10–18] assessed CFs before and after treatment. Caramanna et al. assessed the consistency of cognitive complaints and objective cognitive functioning [19]. Butterbrod et al. and Dallabona et al. reported factors affecting disease progression and neuro-cognitive status, respectively [18, 20]. Zarino et al. studied neuropsychological function status predicting patient’s outcome after treatment [21].
Table 1 summarizes the populations of individual studies, the WHO-CNS classification used to select the tumors, treatment modalities, and CF assessment timings including baseline and follow-ups.
Table 2 summarizes the CF assessments of individual studies including the CF assessed, the tool used to assess the CF, and baseline and follow-up scores as compared to the healthy population.
Table 3 summarizes the effects of tumor characteristics and treatment modalities on CFs. The tumor characteristics included the location of the tumor, edema, size/volume, and recurrence. The treatment modalities included medication, surgical resection, radiotherapy, chemotherapy, age, and others.
Cognitive function before treatment
Before treatment, 79% of HGG patients showed impairment in at least one cognitive domain, while 21% were not impaired. Thirty-five percent of patients had mild, 34% moderate, and 10% severe impairment [13]. Cognitively impaired patients reported more complaints than patients without cognitive impairment [19].
Cognitive function following treatment and on follow-up
Following surgical resection, patients deteriorated in CF; however, this functional decline was not statistically significant [10]. Verbal memory, attention, and psychomotor function were the domains most frequently impaired [13].
An improvement in memory functions and in processing speed was seen after surgery, especially in patients with widespread edema [11, 12]. Awake craniotomy was reported to contribute to preserving language and decrease the risk of postoperative permanent aphasic deficits when operating in eloquent areas [12]; thus, despite the deterioration of neuropsychological performances at early follow-up, surgery is also effective for improving the cognitive performances of patients and thus their quality of life [18].
Predictors of CFs
The possible predictors and their effects on CFs have been summarized in Table 3.
Tumors in the left hemisphere had worse verbal function [11, 13, 16, 18], working memory, and attention [13]. Right-sided tumors were related to worse facial recognition [11]. Tumors in the frontal lobe had an overall worse cognition [14]. Increased tumor volume was associated with overall cognitive decline [18], and so was a greater residual tumor volume after surgery [17]. Along with tumor volume, the surrounding edema also constituted a mass effect and lead to cognitive decline [18]. Epilepsy before surgery also contributed to the decline in cognition preoperatively and was also found to cause deteriorated cognition postoperatively [13]. Corticosteroids [13, 16] and anti-epileptics [10] could also contribute to cognitive impairment. Advanced age at diagnosis may be a risk factor for a worse cognitive outcome [14, 16, 18].
Following surgical resection, there was no specific effect reported on the CF. Bodensohn et al. reported that gross-total resection (GTR) may improve some subsets of cognition [16]. Dallabona et al. reported a short-term decline in some CFs but no significant worsening in late follow-up [18].
No study reported any evidence of cognitive impairment in glioma patients who had undergone radio-chemotherapy at least within the first year [15, 16, 22]. Wang et al. reported that grade IV glioma has a higher risk of cognitive impairment after concurrent chemoradiation [17].
Tumor progression was correlated to cognitive decline [14, 20]; thus, a decline in CFs may predict sub-clinical tumor progression [21].
Discussion
We conducted a systematic review and evaluated the neuro-CF of patients with high-grade gliomas before and after treatment along with the predictors of cognitive status in HGGs. Before surgical resection HGG, patients have impairment in at least one cognitive domain. Following surgical resection and/or chemo/radiotherapy, some aspects of CF deteriorated with time; however, this functional decline was not statistically significant. The predictors of greater cognitive decline were tumor localization, the mass effect of tumor and edema, pre-operative epilepsy, medication including anti-epileptics and corticosteroids, and greater age of the patient at diagnosis. Chemo- or radiotherapy has no significant effect on CFs.
Archibald et al. studied the long-term CFs of HGG survivors and reported impaired baseline CFs of several survivors and deterioration on specific tasks of the rest within 2 years of baseline testing [23]. Archibald et al. also reported that the most impaired CFs at baseline were verbal memory and sustained attention, whereas verbal learning and flexibility in thinking were the most frequent to decline over time [23]. Weitzner and Meyers reviewed similar studies before 1996 and reported that a decline in CFs is inevitable following successful treatment of HGGs; however, contrary to our analysis, this decline is irrespective of tumor localization or grade; instead was related to the type of therapy and tumor lateralization [24]. These differences may be explained by the disparities in WHO-CNS classifications and different treatment approaches of chemo- and radiotherapy. Similar to our results, Taylor et al. reported no significant effect of radiotherapy on CFs; older age, lower baseline CFs, and subclinical tumor progression were reported to be the predictors of cognitive decline [25]. There is no significant effect of radiotherapy on CFs in low-grade gliomas either [26].
Tucha et al. studied the cognitive impairments among patients with brain tumors of the frontal or temporal lobes and reported that lesions of the left temporal lobe are often associated with disturbances of language functions similar to our findings [27]. The difference based on location is also due to the dominancy of the left cerebral hemisphere over the right cerebral hemisphere. The reason behind these deficits is the presence of Broca’s area and Wernicke’s area in the left hemisphere. Tumors that were found to be diagnosed and reported to be in the temporal lobe also significantly decreased the cognition in patients with HGGs. This also can be explained by the presence of Wernicke’s area in the posterior one-third of the temporal convolution in the left hemisphere of the brain. De Baene et al. reported that the local efficiency of the contralesional hemisphere was associated with reaction time and contralesional activity was associated with attention and cognitive flexibility [28]. Further, HGGs were associated with decreased verbal memory, worse attention, and cognitive flexibility; tumor volume was associated with visual memory; however, age at diagnosis, epilepsy, and the use of anti-epileptic medication were not associated with decline in any of the assessed CF [28]. Acevedo-Vergara et al. reviewed the effects of specific tumor localizations and also reported that language function was associated with lesion in the dominant cerebral hemisphere, memory function in the left-hemisphere, and executive functioning in prefrontal cortex of the frontal lobes [29].
Tucha et al. also reported that patients with larger lesions and those with accompanying edema displayed significantly more cognitive impairments [27]. Edema is a leading factor that causes compression of the brain matter; its removal during surgical resection helped improve the results [11, 12]. Upon eradicating associated edema, with drugs like bevacizumab and temozolomide, an improvement in processing speed and memory functions can be observed [27, 30]
Cognitive and social functioning, physical, emotional, and spiritual well-being collectively comes under the health-related quality of life (HRQOL). Since HGGs have a poor prognosis and low life expectancy, HRQOL is an important concern for doctors and caregivers. There are several determining factors including sex, tumor location, and histological classification. Rogers et al. reported lower QOL in females than in males [31]. Although tumor location and its relation to QOL is controversial, however, several studies reported that HGG patients with left-sided brain lesions were found with increased memory problems [32], poor fluency while speaking [32, 33], and more depressed state and symptoms [32] and difficulty in communication [34]. GBM patients are reported to have low HRQOL as compared to others because of its aggressively growing nature, shorter time of progression, and faster deterioration in cognition [35, 36].
Additionally, the drugs used in the treatment after resection such as dexamethasone and previously used anti-epileptic drugs [27] to treat seizures (carbamazepine, valproic acid, and phenytoin) taken alongside the radiotherapy had a correlation with the declined cognitive functions. Apart from the medicine intake, patients who presented with epileptic seizures preoperatively could have higher rates of deteriorated cognitive function. A possible explanation can be the deteriorating effects of epilepsy preoperatively which worsened postoperatively along with anti-epileptic drugs. Patients were also taking corticosteroids which was found to be associated with a decline in executive functioning, information processing, and attentive behavior preoperatively.
Moreover, the age of the patient at the time of diagnosis was also found to have an impact on the progression of the disease and the related impairment. Older patients were found to have greater cognitive impairment than the younger ones who were found to be less impaired and showed a good prognosis.
Only one study reported a molecular subgroup in relation to cognitive impairment with negative MGMT promoter methylation being a risk factors for cognitive impairment [17]. Derks et al. studied cognitive performances of HGG patients in relation to IDH-mutation status and reported that patients with IDH-with gliomas had poorer cognitive performances as compared to patients with IDH-mut gliomas [37].
Tumor progression was correlated to cognitive decline [14, 20, 21]. Klein et al. assessed the prognostic value of cognitive functioning in HGGs and reported that the measurement of CF in HGG patients may be of high clinical relevance throughout the disease [38]. Meyers et al. also reported that the assessment of CFs, HRQOL, and patient function in terms of ability to perform activities of daily living can be important in predicting the survival of patients with recurrent malignant brain tumors [39]. Taphoorn and Klein reported that cognitive deterioration may be the first indicator of progressive disease after treatment [40]. van Kessel et al. conducted a retrospective analysis of CF of HGG patients and concluded that impaired executive functions and memory were significantly correlated with survival, whereas language, psychomotor speed, and visuospatial functioning were not [41].
Specific limitations of individual studies included a risk of bias associated with the sample sizes and the number of people who showed up on all the follow-ups and participated in tests. The first limitation of our study is the heterogeneity of WHO-CNS classifications used; thus, different tumors being considered in the category of HGGs. Further limitations include non-specification in different brain tumors, variability of sample sizes, and heterogeneity of the CF tests used by individual studies.
Conclusion
HGG patients have CF impairments in at least one cognitive domain. These impairments tend to increase with time and disease progression. Factors that impact CF include tumor localization, grade, recurrence, mass impact of both tumor volume and surrounding edema, corticosteroids and antiepileptic drugs intake during radiotherapy, and patients age at diagnosis. Treatment modalities including surgical resection and radio- or chemotherapy have no significant effect on CFs. An important clinically relevant finding was that the deterioration of cognitive status may indicate subclinical tumor progression thus emphasizing the importance of CF assessment throughout the course of the disease.
References
Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2021) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro-Oncology. 23(Supplement_3):iii1–iii105. https://doi.org/10.1093/neuonc/noab200
Davis ME (2018) Epidemiology and overview of gliomas. Seminars in Oncology Nursing. 34(5):420–429. https://doi.org/10.1016/j.soncn.2018.10.001
WHO Classification of Tumours Editorial. WHO classification of tumours. Vol 6. 5th ed. . https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Central-Nervous-System-Tumours-2021
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1
Kleihues P, Burger PC, Scheithauer BW (2012) Histological typing of tumours of the central nervous system. Springer Science & Business Media
Scheithauer BW (2009) Development of the WHO classification of tumors of the central nervous system: a historical perspective. Brain Pathology. 19(4):551–564. https://doi.org/10.1111/j.1750-3639.2008.00192.x
de Groot JF (2015) High-grade gliomas. Continuum (Minneap Minn) 21(2 Neuro-oncology):332–344. https://doi.org/10.1212/01.CON.0000464173.58262.d9
Kiely KM. Cognitive function. In: Michalos AC, ed. Encyclopedia of quality of life and well-being research. Springer Netherlands; 2014:974-978. doi:https://doi.org/10.1007/978-94-007-0753-5_426
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 335(7624):806–808. https://doi.org/10.1136/bmj.39335.541782.AD
Bosma I, Vos MJ, Heimans JJ et al (2007) The course of neurocognitive functioning in high-grade glioma patients. Neuro-oncol. 9(1):53–62. https://doi.org/10.1215/15228517-2006-012
Raysi Dehcordi S, Mariano M, Mazza M, Galzio RJ (2013) Cognitive deficits in patients with low and high grade gliomas. J Neurosurg Sci. 57(3):259–266
Bonifazi S, Passamonti C, Vecchioni S et al (2020) Cognitive and linguistic outcomes after awake craniotomy in patients with high-grade gliomas. Clin Neurol Neurosurg. 198:106089. https://doi.org/10.1016/j.clineuro.2020.106089
Habets EJJ, Kloet A, Walchenbach R, Vecht CJ, Klein M, Taphoorn MJB (2014) Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir. 156(8):1451–1459. https://doi.org/10.1007/s00701-014-2115-8
Brown PD, Jensen AW, Felten SJ et al (2006) Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. JCO. 24(34):5427–5433. https://doi.org/10.1200/JCO.2006.08.5605
Bian Y, Meng L, Peng J et al (2019) Effect of radiochemotherapy on the cognitive function and diffusion tensor and perfusion weighted imaging for high-grade gliomas: a prospective study. Sci Rep. 9(1):5967. https://doi.org/10.1038/s41598-019-42321-8
Bodensohn R, Corradini S, Ganswindt U et al (2016) A prospective study on neurocognitive effects after primary radiotherapy in high-grade glioma patients. Int J Clin Oncol. 21(4):642–650. https://doi.org/10.1007/s10147-015-0941-1
Wang Q, Xiao F, Qi F, Song X, Yu Y (2020) Risk factors for cognitive impairment in high-grade glioma patients treated with postoperative radiochemotherapy. Cancer Research and Treatment : Official Journal of Korean Cancer Association. 52(2):586. https://doi.org/10.4143/crt.2019.242
Dallabona M, Sarubbo S, Merler S et al (2017) Impact of mass effect, tumor location, age, and surgery on the cognitive outcome of patients with high-grade gliomas: a longitudinal study. Neurooncol Pract. 4(4):229–240. https://doi.org/10.1093/nop/npw030
Caramanna I, Bottomley A, Drijver AJ et al (2021) Objective neurocognitive functioning and neurocognitive complaints in patients with high-grade glioma: evidence of cognitive awareness from the European Organisation for Research and Treatment of Cancer brain tumour clinical trials. Eur J Cancer B Oral Oncol. 144:162–168. https://doi.org/10.1016/j.ejca.2020.10.040
Butterbrod E, Bruijn J, Braaksma MM et al (2019) Predicting disease progression in high-grade glioma with neuropsychological parameters: the value of personalized longitudinal assessment. J Neurooncol. 144(3):511–518. https://doi.org/10.1007/s11060-019-03249-1
Zarino B, Di Cristofori A, Fornara GA et al (2020) Long-term follow-up of neuropsychological functions in patients with high grade gliomas: can cognitive status predict patient’s outcome after surgery? Acta Neurochir. 162(4):803–812. https://doi.org/10.1007/s00701-020-04230-y
Wang Q, Qi F, Song X et al (2018) A prospective longitudinal evaluation of cognition and depression in postoperative patients with high-grade glioma following radiotherapy and chemotherapy. J Cancer Res Ther. 14(Supplement):S1048–S1051. https://doi.org/10.4103/0973-1482.199431
Archibald YM, Lunn D, Ruttan LA et al (1994) Cognitive functioning in long-term survivors of high-grade glioma. J Neurosurg. 80(2):247–253. https://doi.org/10.3171/jns.1994.80.2.0247
Weitzner MA, Meyers CA (1997) Cognitive functioning and quality of life in malignant glioma patients: a review of the literature. Psycho-Oncology. 6(3):169–177. https://doi.org/10.1002/(SICI)1099-1611(199709)6:3<169::AID-PON269>3.0.CO;2-#
Taylor BV, Buckner JC, Cascino TL et al (1998) Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma. JCO. 16(6):2195–2201. https://doi.org/10.1200/JCO.1998.16.6.2195
Koutsarnakis C, Neromyliotis E, Komaitis S et al (2021) Effects of brain radiotherapy on cognitive performance in adult low-grade glioma patients: a systematic review. Radiother Oncol. 160:202–211. https://doi.org/10.1016/j.radonc.2021.04.023
Tucha O, Smely C, Preier M, Lange KW (2000) Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 47(2):333–334. https://doi.org/10.1097/00006123-200008000-00011
De Baene W, Rutten GJM, Sitskoorn MM (2019) Cognitive functioning in glioma patients is related to functional connectivity measures of the non-tumoural hemisphere. Eur J Neurosci. 50(12):3921–3933. https://doi.org/10.1111/ejn.14535
Acevedo-Vergara K, Perez-Florez M, Ramirez A et al (2022) Cognitive deficits in adult patients with high-grade glioma: a systematic review. Clin Neurol Neurosurg. 219:107296. https://doi.org/10.1016/j.clineuro.2022.107296
Wang X, Chen D, Qiu J, Li S, Zheng X (2021) The relationship between the degree of brain edema regression and changes in cognitive function in patients with recurrent glioma treated with bevacizumab and temozolomide. Quant Imaging Med Surg. 11(11):4556–4568. https://doi.org/10.21037/qims-20-1084
Rogers MP, Orav J, Black PM (2001) The use of a simple Likert scale to measure quality of life in brain tumor patients. J Neurooncol. 55(2):121–131. https://doi.org/10.1023/a:1013381816137
Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC (2003) Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 55(4):992–999. https://doi.org/10.1016/s0360-3016(02)04205-0
Hom J, Reitan RM (1984) Neuropsychological correlates of rapidly vs. slowly growing intrinsic cerebral neoplasms. J Clin Neuropsychol. 6(3):309–324. https://doi.org/10.1080/01688638408401221
Klein M, Taphoorn MJ, Heimans JJ et al (2001) Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 19(20):4037–4047. https://doi.org/10.1200/JCO.2001.19.20.4037
Osoba D, Brada M, Prados MD, Yung WK (2000) Effect of disease burden on health-related quality of life in patients with malignant gliomas. Neuro Oncol. 2(4):221–228
Giovagnoli AR, Silvani A, Colombo E, Boiardi A (2005) Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry. 76(4):562–568. https://doi.org/10.1136/jnnp.2004.036186
Derks J, Kulik S, Wesseling P et al (2019) Understanding cognitive functioning in glioma patients: the relevance of IDH-mutation status and functional connectivity. Brain Behav. 9(4):e01204. https://doi.org/10.1002/brb3.1204
Klein M, Postma TJ, Taphoorn MJB et al (2003) The prognostic value of cognitive functioning in the survival of patients with high-grade glioma. Neurology. 61(12):1796–1798. https://doi.org/10.1212/01.WNL.0000098892.33018.4C
Meyers CA, Hess KR, Yung WK, Levin VA (2000) Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 18(3):646–650. https://doi.org/10.1200/JCO.2000.18.3.646
Taphoorn MJB, Klein M (2004) Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 3(3):159–168. https://doi.org/10.1016/S1474-4422(04)00680-5
van Kessel E, Huenges Wajer IMC, Ruis C et al (2021) Cognitive impairments are independently associated with shorter survival in diffuse glioma patients. J Neurol. 268(4):1434–1442. https://doi.org/10.1007/s00415-020-10303-w
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tariq, R., Hussain, N. & Baqai, M.W.S. Factors affecting cognitive functions of patients with high-grade gliomas: a systematic review. Neurol Sci 44, 1917–1929 (2023). https://doi.org/10.1007/s10072-023-06673-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06673-4