Abstract
Bone tumor surgery is extremely challenging, particularly when tumors are located in tightly confined anatomical areas and abutting critical organs and neurovascular structures. Tumor resection requires good cutting accuracy to ensure safety, to achieve negative margins, and to preserve critical structures when possible. The purpose of this paper was to review the literature on the surgical advances for bone tumor surgery published within the last year. The majority of literature identified focused on computer-assisted surgical approaches. There is increasing evidence that 3D navigation plays an important role in the resection of bone tumors. Reconstruction materials that encourage healing and prevent infections are also in development. Optimal care includes execution of a well-developed pre-operative plan using a multidisciplinary approach led by the orthopaedic oncologist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bone tumors are a heterogeneous group of diseases [1•] with unique biologic characteristics that variably alter the appearance, architecture, and inherent stability of the skeleton. These tumors may be benign or malignant, primary or metastatic. The vast majority of bone tumors represent metastatic lesions, and palliative surgery may be indicated for local control, alleviation of symptoms, and/or stabilization of the skeleton. Benign aggressive and primary malignant tumors generally require surgery as a primary means to obtain local control. Surgery has been routinely performed for the management of bone tumors for many decades; however, in recent years, advances have allowed more sophisticated approaches to the surgical management of these complex cases. Prior to the advent of the endoprosthesis by Austin Moore in the early 1940s [2], bone tumor surgery for primary bone tumors of the appendicular skeleton was generally limited to an amputation procedure. The success of the first endoprosthesis hinged upon the use of a combination of metals, cobalt and chromium, which proved durable enough to withstand the stressors of both implantation and human activity. This groundbreaking advance supported the notion that bone tumors could be treated with limb-salvage resections and reconstructions. The potential for implantable orthopaedic devices was, therefore, realized, and an entire industry was born for the research and development of such devices. We now enjoy the routine use of implantable devices for numerous orthopaedic indications ranging from joint replacements for degenerative arthritides, to expandable prostheses for growing pediatric sarcoma patients, and custom megaprostheses for the reconstruction of large irregular bone tumor defects.
The goal of surgery for the treatment of the benign aggressive or primary malignant bone tumor is to remove all local disease and maximize postoperative function. Certain diseases, such as chondrosarcomas, are not amenable to standard chemotherapies or radiotherapies [3]; therefore, surgery is the primary treatment, and en bloc resection provides the best chance of local control and long-term survival [1•, 4–7]. Critical structures are often sacrificed in order to ensure a complete resection with negative margins. The cautious surgeon will most often err on the side of resecting extra normal tissue rather than erroneously perform an intralesional resection. Some adjuvant treatments may improve the formation and durability of the tumor pseudocapsule [8•], thereby allowing the surgeon to more easily identify the extent of the tumor intra-operatively, save nearby structures that otherwise would have been sacrificed, and decrease intralesional resection rates. While we hope to identify other novel adjuvants effective against these diseases, we also strive to make improvements in the surgical approach for bone tumors. While many advances have already been made, we still experience major challenges, including high rates of intralesional resections, wound complications, infections, and reconstruction failures. Therefore, recent advances in surgery for the management of bone tumors can be considered based on their intended aims to: 1) improve the accuracy, precision, and safety of resections; 2) improve reconstructions; and 3) decrease complications. It is our hope that current and future advances will ease the burden of the surgical intervention on both the patient and the surgeon. Here, we review the latest advances in the surgical management of bone tumors based on literature published within the last year.
Improving Resection Accuracy and Safety
Resecting bone tumors can be extremely challenging, particularly when the tumors are located in tightly confined anatomical areas and abutting critical organs and neurovascular structures. Tumor resection requires good cutting accuracy to ensure safety, to achieve negative margins, and to preserve critical structures when possible. Tumors located in complex 3-dimensional skeletal structures such as the pelvis, sacrum, and spine are uniquely challenging and often require complicated, multi-directional osteotomies for adequate resection. Unfortunately, given the inherent complexity, surgical inaccuracy of tumor resections exists, [9] and local recurrence rates within challenging areas of the skeleton such as the pelvis can be high, approximating 35 % [10].
The foundation of any surgical resection is a well thought-out, well developed pre-operative plan based on the critical evaluation of appropriate imaging. Advanced technology does not and will never supplant such an approach. Advances in technology can, however, assist the surgeon in both developing a solid pre-operative plan and accurately executing the desired plan. Various methods of improving resection accuracy and safety are being investigated. The majority of these methods incorporate some form of intra-operative imaging to improve visualization and facilitate execution of the planned tumor resection with greater accuracy, safety, and confidence.
The most common form of intra-operative imaging performed in orthopaedic surgery is fluoroscopy. Fluoroscopy is widely available, easy to use, and helps the orthopaedic oncologist identify lesions, assess potential resection planes, and facilitate complex reconstructions. Many orthopaedic oncology procedures can be performed more accurately and with greater confidence under fluoroscopic guidance; however, fluoroscopy provides only 2-dimensional information and is associated with increased radiation exposure, which has been shown to have numerous ill effects [11, 12]. Many minimally invasive orthopaedic procedures require extensive intra-operative fluoroscopy, and most orthopaedic surgeons have had little, if any, training on the use of fluoroscopy and appropriate ways to minimize exposure to the surgical team.
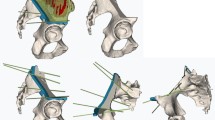
A more sophisticated form of intra-operative image guidance is CT-based navigation. CT-based navigation provides 3-dimensional representations of complex structures with the additional benefit of decreasing intra-operative radiation exposure to the surgical team. CT navigation may be accomplished using pre-operatively obtained CT scans or, more recently, intra-operative CT scans. The most common use of navigation in orthopaedic surgery is for the placement of pedicle screws during spinal instrumentation procedures. The 3D-based navigation technique provides high accuracy of pedicle screw placement, and thus, is safe for patients undergoing thoracic spine stabilization. Allam et al. evaluated the accuracy of pedicle screw placement using the freehand technique versus the 3D-based navigation technique in their cohort of 45 patients with over 200 screws placed [13•]. The placement of transpedicular screws in the thoracic spine using a 3D-based navigation technique was shown to be superior to the freehand technique. This is particularly relevant in orthopaedic oncology because unstable metastatic spine lesions often require stabilization with pedicle screw fixation. The benefit of navigation is not limited to the spine, however, and there is increasing evidence that 3D navigation plays an important role in the resection of bone tumors. Over 20 publications regarding the use of navigation were identified in the orthopaedic oncology literature within the last year [14•, 15•, 16•, 17•, 18, 19•, 20••, 21–25, 26•, 27–30, 31•, 32••, 33]. Wong et al. have published extensively on this topic, and in their largest series, evaluated 20 patients on whom navigation-assisted resections were performed [32••]. The achieved bone resections matched the planned resections with a difference of </=2 mm. The achieved positions of custom prostheses were comparable to the planned positions when merging post-operative with pre-operative CT images in five cases. Aponte-Tinao et al. published a case series of five patients who underwent multi-planar osteotomies, guided by computer-assisted navigation [14•]. A mean difference of 2.43 mm was calculated between the pre-operative plan and the actual osteotomies performed. The results show that navigation with adequate preoperative planning allows a surgeon to intra-operatively reproduce the planned resection with accuracy in complex multi-planar resections. Further proving the point, Cartiaux et al. investigated cutting accuracy during navigated and non-navigated pelvic bone tumor surgery simulations [16•]. In this study, twenty-three operators (10 senior and 13 junior surgeons) were asked to perform the resections in a simulated pelvic tumor model, initially according to a freehand procedure and later with the aid of a navigation system. The location of the osteotomy planes with respect to the target planes was significantly improved by using navigation, averaging 2.8 mm as compared to 11.2 mm for the freehand method (p < 0.001). Furthermore, there were no intralesional tumor resections when using the navigation system. Jeys et al. reviewed their clinical series of 31 patients who underwent computer-navigation-assisted surgery for pelvic and sacral tumors and also concluded that it decreased their intralesional resection rate while allowing them to preserve sacral nerve roots, resect otherwise unresectable disease, and avoid hindquarter (external hemipelvectomy) amputations [20••]. These reports clearly document the early use of navigation and its benefits in bone tumor surgery. However, the use of navigation does come with some degree of difficulty. A fundamental challenge in the use of image-guided surgical systems is the “registration,” or the alignment of the pre-operative model to the operative view of the patient [34]. This is achieved by finding corresponding structures on the pre-operative scans and the patient. Many times, however, accuracy of the registration is diminished due to difficulty in aligning the physical structure to the CT representations. Error may also arise when the patient is placed in a position during surgery that is different than the position the patient was placed in when the pre-operative CT scan was obtained. Intra-operative CT-based navigation and patient-specific instruments are two novel technologies being used to address these problems.
Intra-operative CT-based navigation is one of the latest platforms in navigation-based surgery. A high-quality CT scan is performed at the time of surgery, which automatically registers the patient’s anatomy to the navigation system, obviating the need for manual registration and removing this as a source of inaccuracy. Intra-operative CT-based navigation also decreases the potential error due to positioning because the scan is taken once the patient is already positioned for surgery. During surgery, additional scans can also be performed that allow the surgeon to evaluate the placement of instrumentation prior to the patient leaving the operating room. Despite these significant advantages, however, pre-operative CT-based navigation still has the distinct advantage in that the pre-operatively obtained CT scan required allows the surgeon to develop a robust pre-operative plan and create virtual resections and reconstructions prior to surgery.
Patient-specific instruments (PSIs) are unique cutting guides, built off pre-operative CT scans, designed to fit specifically to the patient’s anatomy and guide the use of cutting devices. Cartiaux et al. evaluated the use of PSIs in pre-clinical simulation exercises [17•]. With PSI, the location accuracy of the cut planes with respect to the target planes averaged 1 and 1.2 mm in the anterior and posterior ilium, 2 mm in the pubis, and 3.7 mm in the ischium (p < 0.0001). Compared with navigation technology, PSI technology offers similar accuracy in vitro and does not require continuous tracking and registration steps, which are both time-consuming and sources of error.
Perhaps the most sophisticated advance yet in computer-assisted tumor surgery is the integration of navigation into a minimally invasive approach. Navigation-enabled minimally invasive surgery has most commonly been performed for benign degenerative spinal tumors. However, a recent case report demonstrated the benefit of the integrated technology for musculoskeletal tumor surgery [15•]. Campos et al. reported the case of a 16-year-old girl with a symptomatic osteoid osteoma at the T9 level whose lesion was curettaged using video-assisted thoracoscopic surgery (VATS) guided by a navigation system (VATS-NAV). VATS-NAV allowed the complicated operation to be performed in less time, with less morbidity, and minimized the hazards of radiation for the surgeon, patient, and operating room staff. VATS-NAV provided real and virtual images of the targeted lesion and positioning of the instruments throughout the procedure.
Clearly, computer-assisted navigation surgery has its benefits. Unlocking the potential of novel imaging and minimally invasive surgical technologies will allow bone tumor surgery to be performed with greater safety and accuracy, while maximizing post-operative function and minimizing surgical morbidity.
Improving Reconstructions
Reconstructive options after bone tumor resection have been well described and include endoprostheses, allografts, vascularized grafts, and composites. Endoprosthetic reconstructions are generally desirable but are associated with high rates of loosening and infections. For this reason, R&D efforts have focused on developing surface coatings that promote osteointegration and prevent infections [35–42]. Much of the work on antibacterial surface coatings is still in the preclinical stages. However, Coathup et al. evaluated the use of hydroxyapatite-coated collars for the purpose of promoting osteointegration of bone with the endoprosthesis [36]. Sixty-one patients treated with a primary distal femur endoprosthesis were evaluated, with a mean follow-up of 8.2 years. Extracortical bone growth into the grooved hydroxyapatite-coated collar was quantified radiographically. Histological sections through four hydroxyapatite-coated collars and four implants with no collar, retrieved following amputation due to local recurrence or at autopsy at a mean of 3.5 years (range, 1.4 to 6.1 years) after implantation, were evaluated as well. Histological analysis showed mature lamellar bone within the grooves of the hydroxyapatite-coated collar, and bone was observed in direct contact with the hydroxyapatite coating. Extracortical bone failed to make direct contact with the surface of the implants manufactured without a collar.
Custom megaprostheses are also being used in small numbers to reconstruct large irregular defects [43–47]. Novel technologies utilizing 3D printers allow surgeons to create models of the resection, based off of the pre-operative CT scan, and build patient-specific implants, which decrease commonly observed resection/reconstruction mismatches and potentially decrease reconstruction failures.
Allograft reconstructions are commonly used by orthopaedic oncologists but are associated with numerous complications including prolonged healing rates and nonunions. Aponte et al., however, reported that, despite a greater number of complications in the short-term, allograft reconstructions have high rates of survival after 10 years and are good options for young patients with long life expectancies [48]. Vascularized grafts are ideal due to their biologic properties and very good healing rates but are associated with inherent donor site morbidity. Campanacci et al. evaluated the use of vascularized fibula grafts (VFGs) in revision surgery of a critical defect reconstruction [49]. In their series of 12 patients, seven patients received a VFG as biologic augmentation for an intercalary allograft non-union, and five patients received a combination of an allograft and VFG to replace either a cement spacer with hardware failure (four patients) or a failed intercalary prosthesis (one patient). Complete healing of the osteotomy of both allograft and VFG was observed in 10 patients at final follow-up. Two major complications were observed that required surgical revision, eventually healing in one case and leading to a poor functional outcome in one case.
Despite modern advances and improved limb salvage procedures, amputations remain a good surgical option for patients with high functional demands and should be routinely given consideration as a primary surgical option [50].
Decreasing Complications
Immunocompromised hosts, long surgical times, large surgical defects, non-biologic reconstructions, and adjuvant therapies are all factors that contribute to unacceptably high rates of wound complications and postoperative infections. Recent data suggests the important role of the plastic surgeon in preventing such high complications and averting complication-related amputations [51]. In a retrospective review, Agrawal et al. found that when a plastic surgeon was involved in the management of a patient with an extremity sarcoma, there was an associated decrease in lower-extremity amputation of approximately 20 % without any significant change in recurrence rates. The incidence of infectious complications requiring IV antibiotics was also decreased by about 20 %, and the incidence of skin graft loss decreased by 75 %. These data support the notion that plastic surgeons should be routinely included in the multidisciplinary surgical team when feasible. When the expertise of a plastic surgeon is not available, all effort should be made to obtain excellent hemostasis and provide healthy soft tissue coverage of the critical defect. Additionally, negative pressure wound therapy (NWPT) has been shown to clearly benefit complex infected wounds [52]. Traditionally, NWPT has been used in open wounds; however, the use of negative pressure wound therapy on closed surgical wounds for the prevention of wound healing complications is on the rise. A recent review of the literature identified 26 papers published in the last three years on the use of NWPT on closed surgical wounds [53]. The investigations suggest reduced incidence of wound healing complications via reductions in hematoma and seroma formation, accelerated wound healing, and increased clearance of local edema. Currently there are no randomized studies on the use of NWPT in the orthopaedic oncology literature, but given the demonstrated benefit of NWPT on closed surgical incisions in the management of other complex wounds and given the high rates of wound complications and infections in the orthopaedic oncology patient population, its use in this population should be evaluated.
Future Advances
The refinement of existing imaging modalities and the development of new technologies such as image fusion [54] for computer-assisted bone tumor surgery will help surgeons produce a detailed and reliable pre-operative plan that can be achieved with greater safety and accuracy. This promises to be the future of complex musculoskeletal tumor resections, especially in challenging sites such as the pelvis, spine, and sacrum. As these modalities improve, one can imagine a future that allows the surgeon to prepare for surgery in a virtual setting with patient-specific simulation data [55]. And as technologies improve and enable the surgeon to perform resections with greater ease, our hope is that similar advances will improve our implantable devices, making them more apt to promote healing and prevent infections.
Conclusion
Bone tumor surgery is extremely complex and requires a solid pre-operative plan and the expertise of a multidisciplinary team led by the orthopaedic oncologist. Many of the recent surgical advances involve the use of 3-dimensional, real-time image guidance for improving the accuracy and safety of the resection. Additional research efforts should focus on improving reconstructions using materials designed to better promote tissue integration and prevent infection.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fletcher, C.D.M., World Health Organization, and International Agency for Research on Cancer., WHO classification of tumours of soft tissue and bone. 4th ed. World Health Organization classification of tumours. 2013, Lyon: IARC Press. 468 p. Update on the comprehensive classification of tumours of soft tissue and by the world health organization.
Moore AT, Bohlman HR. Metal hip joint: a case report. 1942. Clin Orthop Relat Res. 2006;453:22–4.
Italiano A et al. Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol. 2013;24(11):2916–22.
Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40(2):818–31.
Sheth DS et al. Chondrosarcoma of the pelvis. Prognostic factors for 67 patients treated with definitive surgery. Cancer. 1996;78(4):745–50.
Pring ME et al. Chondrosarcoma of the pelvis. A review of sixty-four cases. J Bone Joint Surg Am. 2001;83-A(11):1630–42.
Weber KL, Pring ME, Sim FH. Treatment and outcome of recurrent pelvic chondrosarcoma. Clin Orthop Relat Res. 2002;397:19–28.
O’Donnell PW et al. Chemotherapy influences the pseudocapsule composition in soft tissue sarcomas. Clin Orthop Relat Res. 2014;472(3):849–55. Neoadjuvant chemotherapy contributed to the development of a pseudocapsule and decreased the number of tumors with malignant cells identified within and beyond the pseudocapsule.
Cartiaux O et al. Surgical inaccuracy of tumor resection and reconstruction within the pelvis: an experimental study. Acta Orthop. 2008;79(5):695–702.
Delloye C et al. Pelvic reconstruction with a structural pelvic allograft after resection of a malignant bone tumor. J Bone Joint Surg Am. 2007;89(3):579–87.
Ding GX, Munro P. Radiation exposure to patients from image guidance procedures and techniques to reduce the imaging dose. Radiother Oncol. 2013;108(1):91–8.
Schils F, Schoojans W, Struelens L. The surgeon's real dose exposure during balloon kyphoplasty procedure and evaluation of the cement delivery system: a prospective study. Eur Spine J. 2013;22(8):1758–64.
Allam Y et al. Computer tomography assessment of pedicle screw placement in thoracic spine: comparison between freehand and generic 3D-based navigation techniques. Eur Spine J. 2013;22(3):648–53. In conclusion, 3D navigation-assisted pedicle screw placement proved superior to the freehand technique in the thoracic spine.
Aponte-Tinao LA et al. Multiplanar osteotomies guided by navigation in chondrosarcoma of the knee. Orthopedics. 2013;36(3):e325–30. Concluded that navigation with adequate preoperative planning allows surgeons to intraoperatively reproduce the planned resection with accuracy in complex multiplanary resections.
Campos WK, Gasbarrini A, Boriani S. Case report: curetting osteoid osteoma of the spine using combined video-assisted thoracoscopic surgery and navigation. Clin Orthop Relat Res. 2013;471(2):680–5. Reports the intergration of navigation and minimally invasive surgical techniques for the resection of a spinal tumor.
Cartiaux O et al. Computer-assisted planning and navigation improves cutting accuracy during simulated bone tumor surgery of the pelvis. Comput Aided Surg. 2013;18(1–2):19–26. Cutting accuracy during simulated bone cuts of the pelvis was improved by using a navigation system.
Cartiaux O et al. Improved accuracy with 3D planning and patient-specific instruments during simulated pelvic bone tumor surgery. Ann Biomed Eng. 2014;42(1):205–13. PSI technology demonstrated an equivalent value-added for bone cutting accuracy compared to navigation and requires no intraoperative registration and tracking.
Cengic T et al. Intraoperative gamma hand-held probe navigation in resection of osteoid osteoma tumor–report of two cases. Acta Clin Croat. 2013;52(2):261–5.
Gerbers JG, Jutte PC. Hip-sparing approach using computer navigation in periacetabular chondrosarcoma. Comput Aided Surg. 2013;18(1–2):27–32. In this case report, the authors document the safe use of navigation, which allowed the resection to be performed while saving the hip joint.
Jeys L et al. Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Joint J. 2013;95-B(10):1417–24. The authors present their experience using navigation-assisted surgery in 31 patients and found it reduced intralesional surgeries for the pelvis and sacrum.
Kang HG, Cho CN, Kim KG. Percutaneous navigation surgery of osteoid osteoma of the femur neck. Minim Invasive Ther Allied Technol. 2014;23(1):58–62.
Khan F et al. Haptic robot-assisted surgery improves accuracy of wide resection of bone tumors: a pilot study. Clin Orthop Relat Res. 2013;471(3):851–9.
Mezger U, Jendrewski C, Bartels M. Navigation in surgery. Langenbecks Arch Surg. 2013;398(4):501–14.
Miyazaki T et al. Chondroblastoma of the distal femur resected through a small fenestra via computed tomography navigation and endoscopy: a case report. J Med Case Rep. 2013;7(1):164.
Ritacco LE et al. Accuracy of 3-D planning and navigation in bone tumor resection. Orthopedics. 2013;36(7):e942–50.
Ritacco LE et al. Bone tumor resection: analysis about 3D preoperative planning and navigation method using a virtual specimen. Stud Health Technol Inf. 2013;192:1162. Report on a reliable method of evaluating accuracy of the resection using CT reconstructions of the surgical specimen.
Satcher Jr RL. How intraoperative navigation is changing musculoskeletal tumor surgery. Orthop Clin North Am. 2013;44(4):645–56.
So TY, Lam YL, Mak KL. Computer-assisted navigation in bone tumor surgery: seamless workflow model and evolution of technique. Clin Orthop Relat Res. 2010;468(11):2985–91.
Stubig T et al. 3D-navigated implantation of the glenoid component in reversed shoulder arthroplasty. Feasibility and results in an anatomic study. Int J Med Robot. 2013;9(4):480–5.
Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1–9.
Wong KC, Kumta SM. Joint-preserving tumor resection and reconstruction using image-guided computer navigation. Clin Orthop Relat Res. 2013;471(3):762–73. Report on 6 cases demonstrating the benefit of navigation in performing joint-preserving tumor resections.
Wong KC, Kumta SM. Computer-assisted tumor surgery in malignant bone tumors. Clin Orthop Relat Res. 2013;471(3):750–61. Reports outcomes in 20 patients who underwent navigation-assisted surgery.
Wong KC, Kumta SM. Use of computer navigation in orthopedic oncology. Curr Surg Rep. 2014;2:47.
Gao Q et al. Modeling of the bony pelvis from MRI using a multi-atlas AE-SDM for registration and tracking in image-guided robotic prostatectomy. Comput Med Imaging Graph. 2013;37(2):183–94.
Akiyama T et al. Silver oxide-containing hydroxyapatite coating has in vivo antibacterial activity in the rat tibia. J Orthop Res. 2013;31(8):1195–200.
Coathup MJ et al. Long-term survival of cemented distal femoral endoprostheses with a hydroxyapatite-coated collar: a histological study and a radiographic follow-up. J Bone Joint Surg Am. 2013;95(17):1569–75.
De Giglio E et al. An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: development and analytical characterization. Anal Bioanal Chem. 2013;405(2–3):805–16.
Della Valle C et al. A novel antibacterial modification treatment of titanium capable to improve osseointegration. Int J Artif Organs. 2012;35(10):864–75.
Gulati K et al. Biocompatible polymer coating of titanium nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 2012;8(1):449–56.
He P et al. Enhanced osteoinductivity and osteoconductivity through hydroxyapatite coating of silk-based tissue-engineered ligament scaffold. J Biomed Mater Res A. 2013;101(2):555–66.
Jennison T, McNally M, Pandit H. Prevention of infection in external fixator pin sites. Acta Biomater. 2014;10(2):595–603.
Kose N et al. A silver ion-doped calcium phosphate-based ceramic nanopowder-coated prosthesis increased infection resistance. Clin Orthop Relat Res. 2013;471(8):2532–9.
Biau D et al. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88(6):1285–93.
Bruns J et al. Cementless fixation of megaprostheses using a conical fluted stem in the treatment of bone tumours. J Bone Joint Surg (Br). 2007;89(8):1084–7.
Muller PE et al. Internal hemipelvectomy and reconstruction with a megaprosthesis. Int Orthop. 2002;26(2):76–9.
Natarajan MV, Mohanlal P, Bose JC. The role of limb salvage surgery and custom megaprosthesis in multiple myeloma. Acta Orthop Belg. 2007;73(4):462–7.
Rudert M et al. Partial pelvic resection (internal hemipelvectomy) and endoprosthetic replacement in periacetabular tumors. Oper Orthop Traumatol. 2012;24(3):196–214.
Aponte-Tinao LA et al. The principles and applications of fresh frozen Allografts to bone and joint reconstruction. Orthop Clin North Am. 2014;45(2):257–69.
Campanacci DA et al. Vascularised fibular grafts as a salvage procedure in failed intercalary reconstructions after bone tumour resection of the femur. Injury. 2014;45(2):399–404.
Harris JD et al. Exceptional functional recovery and return to high-impact sports after Van Nes rotationplasty. Orthopedics. 2013;36(1):e126–31.
Agrawal N et al. Outcomes analysis of the role of plastic surgery in extremity sarcoma treatment. J Reconstr Microsurg. 2013;29(2):107–11.
Falagas ME et al. Impact of vacuum-assisted closure (VAC) therapy on clinical outcomes of patients with sternal wound infections: a meta-analysis of non-randomized studies. PLoS One. 2013;8(5):e64741.
Karlakki S et al. Negative pressure wound therapy for management of the surgical incision in orthopaedic surgery: a review of evidence and mechanisms for an emerging indication. Bone Joint Res. 2013;2(12):276–84.
Hacihaliloglu I et al. Non-iterative partial-view 3D ultrasound to CT registration in ultrasound-guided computer-assisted orthopedic surgery. Int J Comput Assist Radiol Surg. 2013;8(2):157–68.
Bullock P et al. Integration of image guidance and rapid prototyping technology in craniofacial surgery. Int J Oral Maxillofac Surg. 2013;42(8):970–3.
Compliance with Ethics Guidelines
Conflict of Interest
Justin E. Bird declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Sarcomas
Rights and permissions
About this article
Cite this article
Bird, J.E. “Advances in the Surgical Management of Bone Tumors”. Curr Oncol Rep 16, 392 (2014). https://doi.org/10.1007/s11912-014-0392-2
Published:
DOI: https://doi.org/10.1007/s11912-014-0392-2