Abstract
In orthopaedic surgery, resection of pelvic bone tumors can be inaccurate due to complex geometry, limited visibility and restricted working space of the pelvis. The present study investigated accuracy of patient-specific instrumentation (PSI) for bone-cutting during simulated tumor surgery within the pelvis. A synthetic pelvic bone model was imaged using a CT-scanner. The set of images was reconstructed in 3D and resection of a simulated periacetabular tumor was defined with four target planes (ischium, pubis, anterior ilium, and posterior ilium) with a 10-mm desired safe margin. Patient-specific instruments for bone-cutting were designed and manufactured using rapid-prototyping technology. Twenty-four surgeons (10 senior and 14 junior) were asked to perform tumor resection. After cutting, ISO1101 location and flatness parameters, achieved surgical margins and the time were measured. With PSI, the location accuracy of the cut planes with respect to the target planes averaged 1 and 1.2 mm in the anterior and posterior ilium, 2 mm in the pubis and 3.7 mm in the ischium (p < 0.0001). Results in terms of the location of the cut planes and the achieved surgical margins did not reveal any significant difference between senior and junior surgeons (p = 0.2214 and 0.8449, respectively). The maximum differences between the achieved margins and the 10-mm desired safe margin were found in the pubis (3.1 and 5.1 mm for senior and junior surgeons respectively). Of the 24 simulated resection, there was no intralesional tumor cutting. This study demonstrates that using PSI technology during simulated bone cuts of the pelvis can provide good cutting accuracy. Compared to a previous report on computer assistance for pelvic bone cutting, PSI technology clearly demonstrates an equivalent value-added for bone cutting accuracy than navigation technology. When in vivo validated, PSI technology may improve pelvic bone tumor surgery by providing clinically acceptable margins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Resecting bone tumors within the pelvis is challenging due to the geometrical complexity of the pelvic bone and the proximity of delicate organs and structures such as the bladder, rectum, sciatic nerve and numerous vessels. Tumor resection requires good cutting accuracy to achieve satisfactory margins.15,17,23,27,33 Local recurrence rates within the pelvis can be high, ranging from 28 to 35%.10
A previous study whereby four senior surgeons simulated three different tumor resections on a pelvic bone model emphasized a significant lack of accuracy with the conventional (solely freehand) bone-cutting procedure.4 Under ideal working conditions on an experimental test bed (non-aseptic condition, cutting of a synthetic bone, immobilization of the bone, complete visualization and accessibility to the bony surface), the errors in the 10-mm desired safe margin averaged 5.3 mm. In addition, two (17%) of the twelve resections were intralesional with a 5-mm negative margin. The complex three-dimensional (3D) geometry of the pelvic bone aggravated the hand-controlled cutting errors.
Computer-assisted technologies have been developed for pelvic bone tumor surgeries to improve cutting accuracy. Preoperative planning and intraoperative navigation systems are available for the positioning of surgical tools (chisels, burrs, saws…),1,6,14 and clinical studies have already demonstrated the feasibility of achieving clinically adequate (tumor-free) resection margins within the pelvis with the aid of these assistance technologies.9,12,21,34,38 A previous report5 has simulated bone-cutting on a pelvic bone model and assessed quantitatively the value-added of the navigation technology. Results indicated that navigated bone-cutting errors were twice smaller when compared with a solely freehand process.
Patient-specific instrumentation (PSI) technology has been developed as an alternative to intraoperative navigation. PSI exists for total knee arthroplasty,16,24,25,36 hip resurfacing,22 pedicle screw insertion,32 pelvic osteotomy,35 and long-bones corrective osteotomy.11,26 To date, only few studies have reported quantitative data of PSI accuracy for the aforementioned applications. Recently, PSI technology has been adapted for bone tumor surgery, allowing the surgeon to perform the resection with a patient-specific cutting guide that indicates the trajectories of the cutting tool around the tumor.20,37 However, no studies have reported quantitative data on cutting accuracy during bone tumor resection within the pelvis with PSI.
This experimental study aimed at investigating cutting accuracy during simulated bone tumor cutting of the pelvis with PSI technology, and comparing with a previous report on navigation-technology.5
Materials and Methods
Experimental Setup
The experimentations were conducted using synthetic bones made from solid rigid polyurethane closed foam18 (Sawbones, Vashon, WA). The simulated bones consisted of right hemipelvic models (57-mm diameter acetabulum) with a dimensional tolerance of 0.5 mm, according to the manufacturer (Sawbones, Vashon, WA). The test bed was composed of a clamping device to rigidly fix the bones in unique position for planning and evaluation purposes. The test bed was also composed of a reference block (size 40 × 40 × 40 mm) with a dimensional tolerance of 0.05 mm. The reference block defined a fixed global reference frame (R 0) considering three faces of the block as the XY, YZ and ZX planes (Fig. 1a).
Experimental setup. (a) The simulated bone is a right hemipelvic model made of polyurethane foam. The fragments simulating the patient’s bone after the tumor resection are fixed by means of template clamping supports and screw fixation for evaluation purposes. The reference block is a plastic POM-C resin block and is used to define the global reference frame R 0. (b) The cut planes are digitized using a coordinate measuring machine with a spherical sensor
The test bed was scanned using a CT-scanner (Somatom Definition AS, Siemens, Germany) with 2D slices of 1.5-mm thickness and 1.5-mm step. A virtual 3D CT model of the test bed was reconstructed using in-house validated software for the planning of bone tumor resection.28 In the 3D CT model, a simple bone tumor involving Enneking’s zone II13 was simulated by a 75-mm sphere centered on the acetabulum. Finally, the frame R 0 was defined in the 3D CT model by computationally identifying the XY, YZ and ZX planes of the reference block.
Twenty-four operators composed of 10 experienced surgeons (senior) and 14 in-training residents (junior) performed the simulated bone cuts. The cuts were performed using a pneumatic oscillating saw (Compact Air Drive II, Synthes, Solothurn, Switzerland) equipped with a 70-mm long, 18-mm wide, and 1.2-mm thick blade.
Planning of the Resection Strategy
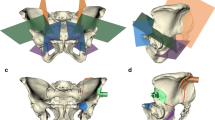
The software provided 2D and 3D visualization of the 3D CT pelvic model and enabled to position target planes close to the boundary of the tumor with a 10-mm safe margin. The resection strategy consisted of four target planes defining the desired bone cutting, including a first plane in the ischium, a second plane in the pubis, and the third and fourth planes forming two angular cuts in the anterior and posterior ilium respectively (Fig. 2). In practice, the resection strategy was recorded by storing the coordinates of each target plane expressed in the reference frame R 0 as described by:
Preoperative planning of the tumor cutting on the 3D CT model of the hemipelvic bone. The tumor is simulated by a 75-mm sphere centered on the acetabulum. The resection strategy consists of four target planes defining the desired cuts around the simulated tumor, including the first cut in the ischium, the second cut in the pubis and the third and fourth angular cuts in the ilium. The plane coordinates are expressed in the reference frame R 0
where \( \vec{n}\;(n_{1}^{0} ;n_{2}^{0} ;n_{3}^{0} ) \) is the normal vector to the plane expressed in R 0 and \( A\;(a_{1}^{0} ;a_{2}^{0} ;a_{3}^{0} ) \) is a point in the plane expressed in R 0.
Patient-Specific Instrumentation
The software enabled to design patient-specific instruments according to the desired resection strategy expressed in R 0. Three instruments were designed including a first guide for the ischial cut, a second guide for the pubic cut, and the third guide for the two angular iliac cuts. Each instrument has a bone-specific surface that fitted in unique position on the bony surface of the pelvic model, and was equipped with a flat surface materializing the target plane (Fig. 3). The instruments were manufactured using rapid-prototyping technology with a dimensional tolerance of 0.2 mm and using polyamide material. The instruments were equipped with 2 mm-diameter holes to be pinned on the pelvic bone using Kirschner wires (Fig. 4).
Computer-assisted design of the patient-specific instruments, including a first guide for the ischial cut, a second guide for the pubic cut, and the third guide for the two angular iliac cuts. Each instrument has a position of best fit on the bony surface of the pelvic model, and is equipped with a flat surface materializing the desired bone cut
Bone Cuts
The 24 surgeons individually performed four cuts using PSI, one cut in each of the four target planes. Before the cuts, each surgeon was asked to position freehand the three patient-specific instruments and fix them on the pelvic model using the K-wires. After the cuts, each surgeon was asked to take off the K-wires. To simulate clinically relevant positioning of the patient on the operating table, the pelvic bone model was fixed using a steel clamp with a 360° rotating base (Fig. 4). Instruction was given to respect target planes as accurately as possible to avoid intralesional tumor cutting.
Evaluation of Bone Cuts
A dataset of 96 cut planes were available for evaluating the cutting accuracy with the PSI technology (Fig. 5).
Three parameters were used to evaluate the cutting accuracy. The location accuracy (L) and the flatness (F) were used in accordance with the ISO1101 standard to evaluate the geometrical accuracy of the cut planes.19 L evaluated the position of the cut plane with respect to the target plane and was defined as the maximum distance (mm) between the cut plane and the target plane (Fig. 6).7 F evaluated the form of the cut plane and was defined as the minimum distance (mm) between two parallel planes that included the cut plane. Finally, the surgical margin (SM) evaluated the accuracy of the bone cut relative to the simulated bone tumor. SM was defined as the minimum distance (mm) between the cut plane and the boundary of the simulated tumor (Fig. 6). Consequently, the errors in the 10-mm desired safe margin were defined as the difference (mm) between SM and 10 mm.
Illustration of location L (mm) and surgical margin SM (mm) parameters for the evaluation of each cut plane of a tumor cutting. The target plane is the dashed line. The cut plane is the solid line. aThe cut plane is represented here by a line instead of a curve for better clarity in the definition of L and SM. See text for details
Each of the 96 cut planes was digitized using a coordinate measuring machine (Signum® SL, Mycrona, Elgin IL, 1 μm resolution) (Fig. 1b), following the guidelines for ISO-based assessment of L and F.2,8 Using a 2-mm spherical sensor enabled to ignore microscopic properties such as roughness. The minimum cut-plane dataset was comprised of 20 measurement points.
For each cut plane, the measurement points were fitted to a least square plane, a common procedure in checking ISO parameters, as described by:
and
where (a, b, c) are the parameters to determine, n is the number of measurement points, and \( P(x_{i}^{0} ,y_{i}^{0} ,z_{i}^{0} ) \) is a measurement point expressed in R 0. The parameters L, F and SM were calculated using numerical computation software (Matlab®, The MathWorks, Natick, MA).
Finally, the operative time required by each surgeon for tumor resection, including fixing the instruments with K-wires, bone-cutting with the oscillating saw, and taking off the K-wires, was reported.
Statistical Analysis
Performances of PSI Technology
A mixed model3 was performed to evaluate the effect of the categorical variables “Target plane” (ischium, pubis, anterior ilium, and posterior ilium) and “Group of operator” (senior and junior). These variables were considered fixed effects (the variable “Operator” was considered as a random effect). The location accuracy, the flatness of the cut planes and the achieved surgical margins were considered as the three (numerical) response variables of the mixed model. Statistical differences between mean values of the response variables were determined by Fisher’s tests. In practice, both location and flatness parameters are semi-positive: the statistical differences have then been investigated using their logarithms to base 10. p values less than 0.05 were considered statistically significant. When the effect of the variable “Target plane” was significant (p value < 0.05), post hoc Tukey’s tests were performed to determine which target planes have a significantly different mean in terms of the response variables.
Comparison with Navigation Technology and No Assistance
The study presented here is the continuation of previous laboratory experiments5 that assessed quantitatively the value-added of the navigation technology when compared with a solely freehand cutting process. Both the present and previous studies have been designed using the same experimental setup (i.e., a pelvic bone-cutting simulator) and include a common set of seven senior surgeons. For these surgeons in common (dataset of 28 cut planes), a new mixed model was performed to evaluate the effect of the variable “Cutting process” (PSI-assisted from the present study, and navigation-assisted and freehand from the previous study). This variable was considered fixed effect. Again, statistical differences between mean values of the response variables, including the location accuracy of the cut planes and the achieved surgical margins, were determined by Fisher’s tests combined with post hoc Tukey’s tests. p values less than 0.05 were considered statistically significant.
Results
Performances of PSI Technology
96 cut planes (four for each of the 24 surgeons) were available for the evaluation of the cutting performances with PSI technology.
The location accuracy of the cut planes and the achieved surgical margins were subject to significant variations in terms of mean and 95% confidence interval (CI) among the four target planes. The location accuracy achieved in the anterior and posterior ilium (average 1.0 mm (CI 0.8–1.3 mm) and 1.2 mm (CI 0.9–1.6 mm) respectively) was significantly different from that achieved in the pubis and ischium (average 2.0 mm (CI 1.5–2.7 mm) and 3.7 mm (CI 2.8–4.9 mm) respectively, p < 0.0001). The surgical margins achieved in the pubis (average 11.8 mm (CI 11.3–12.3 mm)) were significantly higher than those achieved in the ischium and anterior and posterior ilium (average 9.2 mm (CI 8.6–9.7 mm), 10.0 mm (CI 9.5–10.6 mm) and 9.7 mm (CI 9.2–10.3 mm) respectively, p < 0.0001). The flatness of the cut planes was subject to slight variations among the target planes. The flatness achieved in the pubis (average 0.5 mm (CI 0.4–0.7 mm)) was slightly different from that achieved in the posterior ilium (average 0.8 mm (CI 0.6–1.0 mm), p = 0.0245).
There was no difference between the senior and junior surgeons with respect to the location accuracy (p = 0.2114) and the achieved surgical margins (p = 0.8449). The difference between the senior and junior surgeons was slightly significant with respect to the flatness of the cut planes (p = 0.0271).
The median value of the achieved surgical margins was 10.1 mm. The maximum differences between the achieved margins and the 10-mm desired safe margin were found in the pubis (3.1 and 5.1 mm for senior and junior surgeons respectively). Of the 24 simulated resections, there was no intralesional tumor cutting.
The operative time required for performing the cutting of the simulated bone tumor averaged 7.6 min (range, 5.3–12.4 min) for all surgeons. The time averaged 6.9 min (range, 5.6–7.9 min) for senior surgeons, compared to 8.1 min (range, 5.3–12.4 min) for junior surgeons.
Comparison with Navigation Technology and No Assistance
Twenty-eight cut planes (four for each of the seven senior surgeons in common with the previous report5) were available for the comparison between PSI-assisted, navigation-assisted and freehand cutting performances. The location of the cut planes with respect to the target planes was highly improved by PSI and navigation technologies, in terms of mean and 95% CI, especially in the ilium, compared to no assistance (Fig. 7a). The achieved surgical margins were subject to slight variations in terms of mean values (Fig. 7b). However, the standard deviation of the surgical margins was decreased by the PSI technology, compared to navigation technology and no assistance.
Comparison of location accuracy (L) and surgical margin (SM) achieved by the seven surgeons in common with the previous report5 among the three cutting processes. Mean values are shown with the lower and upper limits of the 95% confidence interval. The 10-mm desired safe margin is represented by the solid line “target” on subfigure b. a = p < 0.05 compared with navigation; b = p < 0.05 compared with PSI
Discussion
This experimental study reported good accuracy when using PSI technology during simulated bone tumor cutting of the pelvis. The results in terms of the ISO1101 location parameter demonstrated how PSI is a promising intraoperative technology to replicate a preoperative resection strategy on a pelvic structure with a clinically relevant accuracy. These results are consistent with the findings of Khan et al. 20 who assessed a location accuracy of 2 mm (also according to ISO1101 standard) during a PSI-assisted bone tumor resection within the knee.
The results in terms of the mean and median values of the surgical margins as well as the maximum differences between the planned and the achieved surgical margins reflected that PSI technology was capable of providing tumor-free (extralesional) resections within the pelvis. This is consistent with the findings of Wong et al. 37 who assessed a millimetric resection accuracy during an extremity bone tumor surgery.
The results presented here clearly showed that the PSI cutting errors in terms of the location and the surgical margins are much lower in the ilium than in the ischium and pubis. This could be a consequence of one of the main pre-experimentation design choices that consisted in performing the four cuts around the tumor with three cutting guides instead of one. It appears that the surgical margins achieved in both the ischium and pubis are systematically below and above the 10-mm desired safe margin. This could be induced by a bias in the design, the fabrication, or the positioning of the ischial and pubic guides. Khan et al. 20 investigated a three-cut tumor resection within the knee with a unique cutting guide and found similar cutting errors, but they did not emphasize any bias in the design, fabrication and positioning of their cutting guide. The data presented here could be useful for further quantitative comparison of several PSI design processes for bone-cutting within the pelvis.
The results observed here did not reveal any significant difference between senior and junior surgeons with PSI technology in terms of the location accuracy and the achieved surgical margins. It appears that PSI could be an easy-to-handle technology for the experienced surgeons as well as the younger “digital-age” surgeons. However, these results need to be further validated through complementary experiments including statistical power analysis. A slight statistical significance was found between senior and junior surgeons in terms of the flatness of the cut planes, but the actual difference in terms of the mean values was not considered clinically relevant (0.6 mm for senior surgeons vs. 0.8 mm for junior surgeons).
The results in terms of the ISO1101 flatness parameter reflected the fact that PSI technology was capable of providing a clinically relevant smoothness during bone-cutting. These results are consistent with the study of Hafez et al. 16 who reported gaps between implant and bone of about 1 mm during total knee arthroplasty with PSI.
The study presented here is the continuation of a previous report that assessed the performances of the navigation technology when compared with a solely freehand cutting process.5 Both the present and previous studies have common criteria for comparison because their designs were based on the same experimental setup and pelvic bone-cutting simulator, including the same synthetic pelvic bone model, the same simple 75-mm periacetabular tumor model, the CT images acquired using the same protocol, the 3D reconstruction and segmentation of the simulated tumor using the same computer algorithms, the planning of the resection using the same in-house validated software, as well as the digitization of the cut-planes using the same 3D measuring machine and the measurement of the same quantitative parameters (location, flatness and surgical margins) following the guidelines of the ISO1101 standard. Compared to the previous report, the performances in terms of accuracy and repeatability of bone-cutting with the PSI technology developed in the present study are clearly demonstrated. The results in terms of the location accuracy showed how PSI and navigation are intraoperative technologies allowing the surgeon to replicate a preoperative resection planning with a good accuracy (Fig. 7a). In other words, the value-added of the PSI technology to improve bone-cutting accuracy is equivalent than that of the navigation technology. The results in terms of the achieved surgical margins (Fig. 7b) suggest that the PSI technology has a tendency to provide smaller standard deviation than navigation and no assistance. In other words, the by-hand cutting errors appear to be more controllable with the PSI technology than with navigation technology and with no assistance. Further studies should be performed to fully validate the results observed here.
The operative times that have been measured in the present study were consistent with the findings of Hafez et al. 16 who reported bone-cutting times of about 10 min during knee surgeries with PSI. Moreover, compared to the previous report on navigation technology,5 the results of the present study reflected that the bone-cutting process can be performed much faster with PSI than with navigation: less than 10 min with PSI vs. more than 40 min with navigation. This could be explained by the predefined best-fit position of the PSI on the bony surface that plays the role of direct and automatic planning transfer, compared to the time-consuming semi-automatic image-to-patient registration step of the navigated procedure. Further studies on animals, cadavers or in vivo should be performed to validate the results observed here when taking into account factors that could be time-consuming including the presence of muscles and nerves, bleeding, movements of the patient, etc.
Clinical integration of the PSI technology that has been developed in the present study for pelvic bone tumor surgery is consistent with current PSI technologies whose feasibility and clinical applicability have already been shown in spine, hip, and knee surgery.16,30 First, the process requires using CT images and getting a personal computer with the adequate software for planning tumor resection and designing PSI. In case of bone tumor surgeries, preoperative CT images of the patient (and even MRI) are usually available from the diagnostic phase for tumor delineation by the surgeon and the radiologist.12 The PSI technology developed in the present study enables the use of these available preoperative images and does not require any additional image acquisition that would be costly and time-consuming. Then, manufacturing of PSI with rapid-prototyping technology (3D printing) can be performed outside the hospital: dimensional and geometrical specifications of the desired PSI were transferred to the 3D printing machines via e-mail (in stl format, for example), and the manufactured instruments were sent back by post. The patient-specific instruments that have been developed for the present study are autoclaved and can be used with the conventional tools for pelvic bone tumor surgery, obviating the need to acquire additional costly equipment. Compared with navigation technology and surgical robotics, PSI technology does not require continuous tracking and registration (planning transfer) steps that are sources of errors and time-consuming.
This study has some limitations. First, bone cutting was performed on a hemipelvic model made of homogenous, solid polyurethane foam. Cutting of this simulated bone was easier compared to actual bones with cortical and trabecular substructures. Secondly, by using only CT images with a simple spherical tumor model, this study hypothesized an ideal delineation of the tumor boundaries, without taking into account the range of complex geometries that bone tumors may have. Real surgeries involve the use of CT images usually combined with MRI images to delineate actual bone tumors presenting irregular boundaries and extent in surrounding bones and/or connective soft tissues. Preoperative tumor delineation is a difficult step performed by the surgeon and the radiologist, potentially inducing errors in the tumoral volume to be resected. Thirdly, all the operators worked under ideal conditions including non aseptic environment, immobilization of the synthetic bone, complete visualization and accessibility to the bony surface, etc. Moreover all the operators worked on the resection of a unique bone tumor located on the acetabulum. Pelvic tumor resection is a challenging intervention because of the complex 3D geometry, the limited visibility and the restricted working space of the pelvic structure, rendering it a good case study for initial development and assessment of a new PSI technology. However, bone tumors can affect other locations (such as long bones, sacrum, spine, etc.), and it is commonly accepted that the tumor location is influencing the resection strategy, accounting for the geometry of the affected bone, the connective tissues in presence, as well as the available contact surfaces in the case of PSI-assisted cutting. Consequently, further studies should be performed to account for major clinical and surgical factors that may influence the resection strategy (and thus the design of PSI) as well as being time-consuming and additional sources of inaccuracy. These factors are tumor location, bone geometry, tumor delineation accounting for neighboring bone and connective tissues, surgical exposure, presence of muscles and nerves, bleeding, movements of the patient, as well as patient anesthesia, patient positioning and draping, etc.
This experimental study has been designed according to the recent concept of quantitative orthopedic surgery.29,31 This is also the first study to quantitatively assess the accuracy of PSI-assisted pelvic bone tumor resection by using ISO1101 location and flatness parameters. Like the current works of Khan et al.,20 the results presented here suggested that ISO standards are promising tools for evaluating the quality of orthopaedic surgical procedures.
Conclusion
This study demonstrates that using PSI during simulated bone cuts of the pelvis can provide good cutting accuracy. This study also assessed quantitatively an equivalent value-added of both PSI and navigation technologies in terms of location accuracy and achieved surgical margins during simulated bone cuts of the pelvis. Complementary animal and in vivo studies could be performed to fully validate the results observed here in terms of accuracy, repeatability, time and ergonomics. When completed, the PSI technology may improve bone-cutting accuracy during pelvic tumor resection by providing clinically acceptable margins.
References
Abraham, J. A. Recent advances in navigation-assisted musculoskeletal tumor resection. Curr. Orthop. Pract. 22(4):297–302, 2011.
Anselmetti, B. Tolérancement—Cotation de fabrication et métrologie, Vol. 3. Paris: Lavoisier, p. 334, 2003.
Brown, H., and R. Prescott. Applied Mixed Models in Medicine. 1st ed. New York: John Wiley & Sons, 1999.
Cartiaux, O., P. L. Docquier, L. Paul, B. G. Francq, O. Cornu, C. Delloye, B. Raucent, B. Dehez, and X. Banse. Surgical inaccuracy of tumor resection and reconstruction within the pelvis: an experimental study. Acta Orthop. 79:695–702, 2008.
Cartiaux, O., X. Banse, L. Paul, B. G. Francq, C.-E. Aubin, and P.-L. Docquier. Computer-assisted planning and navigation improves cutting accuracy during simulated bone tumor surgery of the pelvis. Comput. Aided Surg. 2012 Nov 26. [Epub ahead of print].
Cartiaux, O., L. Paul, P. L. Docquier, and X. Banse. Computer- and robot-assisted resection and reconstruction of pelvic bone tumours—a review. Eur. Musc. Rev. 6(2):125–130, 2011.
Cartiaux, O., L. Paul, P. L. Docquier, B. G. Francq, B. Raucent, E. Dombre, and X. Banse. Accuracy in planar cutting of bones: an ISO-based evaluation. Int. J. Med. Robot. 5:77–84, 2009.
Cartiaux, O., L. Paul, P. L. Docquier, B. Raucent, E. Dombre, and X. Banse. Computer-assisted and robot-assisted technologies to improve bone-cutting accuracy when integrated with a freehand process using an oscillating saw. J. Bone Joint Surg. Am. 92(11):2076–2082, 2010.
Cho, H. S., H. G. Kang, H. S. Kim, and I. Han. Computer-assisted sacral tumor resection. A case report. J. Bone Joint Surg. Am. 90:1561–1566, 2008.
Delloye, C., X. Banse, B. Brichard, P. L. Docquier, and O. Cornu. Pelvic reconstruction with a structural pelvic allograft after resection of a malignant bone tumor. J. Bone Joint Surg. Am. 89:579–587, 2007.
Dobbe, J. G., K. J. Pré, P. Kloen, L. Blankevoort, and G. J. Streekstra. Computer-assisted and patient-specific 3-D planning and evaluation of a single-cut rotational osteotomy for complex long-bone deformities. Med. Biol. Eng. Comput. 49(12):1363–1370, 2011.
Docquier, P. L., L. Paul, O. Cartiaux, C. Delloye, and X. Banse. Computer-assisted resection and reconstruction of pelvic tumor sarcoma. Sarcoma 125162, 2010. Epub 2010 Nov 28.
Enneking, W. F., and W. K. Dunham. Resection and reconstruction for primary neoplasms involving the innominate bone. J. Bone Joint Surg. Am. 60:731–746, 1978.
Fehlberg, S., S. Eulenstein, T. Lange, D. Andreou, and P. U. Tunn. Computer-assisted pelvic tumor resection: fields of application, limits, and perspectives. Recent Results Cancer Res. 179:169–182, 2009.
Fuchs, B., N. Hoekzema, D. R. Larson, C. Y. Inwards, and F. H. Sim. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin. Orthop. Relat. Res. 467:510–518, 2009.
Hafez, M. A., K. L. Chelule, B. B. Seedhom, and K. P. Sherman. Computer-assisted total knee arthroplasty using patient-specific templating. Clin. Orthop. Relat. Res. 444:184–192, 2006.
Han, I., Y. M. Lee, H. S. Cho, J. H. Oh, S. H. Lee, and H. S. Kim. Outcome after surgical treatment of pelvic sarcomas. Clin. Orthop. Surg. 2(3):160–166, 2010.
International, A. S. T. M. Standard F1839-08e1: Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopedic Devices and Instruments. West Conshohocken, PA: ASTM International, 2008.
International Organization for Standardization. Standard 1101:2004: Geometrical Product Specifications (GPS)—Geometrical Tolerancing—Tolerances of Form, Orientation, Location and Run-Out (2nd ed.). Geneva, Switzerland: International Organization for Standardization, 2004.
Khan, F. A., J. D. Lipman, A. D. Pearle, P. J. Boland, and J. H. Healey. Surgical technique: computer-generated custom jigs improve accuracy of wide resection of bone tumors. Clin. Orthop. Relat. Res. 2013 Jan 5. [Epub ahead of print].
Krettek C., J. Geerling, L. Bastian, M. Citak, F. Rücker, D. Kendoff, and T. Hüfner. Computer aided tumor resection in the pelvis. Injury 35(Suppl 1):S-A79-83, 2004.
Kunz, M., J. F. Rudan, G. C. Wood, and R. E. Ellis. Registration stability of physical templates in hip surgery. Stud. Health Technol. Inform. 163:283–289, 2011.
Lewis, V. O. What’s new in musculoskeletal oncology. J. Bone Joint Surg. Am. 91:1546–1556, 2009.
Ng, V. Y., J. H. DeClaire, K. R. Berend, B. C. Gulick, and A. V. Lombardi, Jr. Improved accuracy of alignment with patient-specific positioning guides compared with manual instrumentation in TKA. Clin. Orthop. Relat. Res. 470(1):99–107, 2012.
Nunley, R. M., B. S. Ellison, J. Zhu, E. L. Ruh, S. M. Howell, and R. L. Barrack. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin. Orthop. Relat. Res. 470(3):895–902, 2012.
Oka, K., T. Murase, H. Moritomo, and H. Yoshikawa. Corrective osteotomy for malunited both bones fractures of the forearm with radial head dislocations using a custom-made surgical guide: two case reports. J. Shoulder Elbow Surg. 21(10):e1–e8, 2012.
Ozaki, T., S. Flege, M. Kevric, N. Lindner, R. Maas, G. Delling, R. Schwarz, A. R. von Hochstetter, M. Salzer-Kuntschik, W. E. Berdel, H. Jürgens, G. U. Exner, P. Reichardt, R. Mayer-Steinacker, V. Ewerbeck, R. Kotz, W. Winkelmann, and S. S. Bielack. Osteosarcoma of the pelvis: experience of the cooperative osteosarcoma study group. J. Clin. Oncol. 21:334–341, 2003.
Paul, L., O. Cartiaux, P. L. Docquier, and X. Banse. Ergonomic evaluation of 3D plane positioning using a mouse and a haptic device. Int. J. Med. Robot. 5:435–443, 2009.
Pearle, A. D., D. Kendoff, and V. Musahl. Perspectives on computer-assisted orthopaedic surgery: movement toward quantitative orthopaedic surgery. J. Bone Joint Surg. Am. 91(Suppl.1):7–12, 2009.
Radermacher, K., F. Portheine, M. Anton, A. Zimolong, G. Kaspers, G. Rau, and H. W. Staudte. Computer assisted orthopaedic surgery with image based individual templates. Clin. Orthop. Relat. Res. 354:28–38, 1998.
Rivkin, G., and M. Liebergall. Challenges of technology integration and computer-assisted surgery. J. Bone Joint Surg. Am. 91:13–16, 2009.
Schkommodau, E., N. Decker, U. Klapper, K. Birnbaum, H. W. Staudte, and K. Radermacher. Pedicle Screw Implantation Using the DISOS Template System. In: Navigation and Robotics in Total Joint and Spine Surgery, edited by J. B. Stiehl, W. H. Konermann, and R. G. Haaker. Berlin: Springer-Verlag, 2003, pp. 501–505.
Sherman, C. E., M. I. O’Connor, and F. H. Sim. Survival, local recurrence, and function after pelvic limb salvage at 23 to 38 years of followup. Clin. Orthop. Relat. Res. 470(3):712–727, 2012.
So, T. Y., Y. L. Lam, and K. L. Mak. Computer-assisted navigation in bone tumor surgery: seamless workflow model and evolution of technique. Clin. Orthop. Relat. Res. 468(11):2985–2991, 2010.
Staudte, H. W., E. Schkommodau, F. Portheine, and K. Radermacher. Pelvic Osteotomy with Template Navigation. In: Navigation and Robotics in Total Joint and Spine Surgery, edited by J. B. Stiehl, W. H. Konermann, and R. G. Haaker. Berlin: Springer-Verlag, 2003, pp. 455–463.
White, D., K. L. Chelule, and B. B. Seedhom. Accuracy of MRI vs CT imaging with particular reference to patient specific templates for total knee replacement surgery. Int. J. Med. Robot. 4(3):224–231, 2008.
Wong, K. C., S. M. Kumta, K. Y. Sze, and C. M. Wong. Use of patient-specific CAD/CAM surgical jig in extremity bone tumor resection and custom prosthetic reconstruction. Comput. Aided Surg. 1–10, 2012, Early Online.
Wong, K. C., S. M. Kumta, K. H. Chiu, K. W. Cheung, K. S. Leung, P. Unwin, and M. C. Wong. Computer assisted pelvic tumor resection and reconstruction with a custom-made prosthesis using an innovative adaptation and its validation. Comput. Aided Surg. 12:225–232, 2007.
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. Grants from the Brussels-Capital Region (“Brains Back to Brussels” grant BB2B 2012-1-05) and Fondation Belge contre le Cancer (grant SCIE 2006/20) were used to pay for the salaries of laboratory personnel and for purchase of the bone models and surgical supplies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Mona Kamal Marei oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Cartiaux, O., Paul, L., Francq, B.G. et al. Improved Accuracy with 3D Planning and Patient-Specific Instruments During Simulated Pelvic Bone Tumor Surgery. Ann Biomed Eng 42, 205–213 (2014). https://doi.org/10.1007/s10439-013-0890-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0890-7