Abstract
Purpose of Review
Degeneration of the maculopapillary bundle (MPB) is a prominent feature in a spectrum of optic neuropathies. MPB-selective degeneration is seen in specific conditions, such as nutritional and toxic optic neuropathies, Leber hereditary optic neuropathy (LHON), and dominant optic atrophy (DOA). Despite their distinct etiologies and clinical presentations, which encompass variations in age of incidence and monocular or binocular onset, these disorders share a core molecular mechanism: compromised mitochondrial homeostasis. This disruption is characterized by dysfunctions in mitochondrial metabolism, biogenesis, and protein synthesis. This article provides a comprehensive understanding of the MPB’s role in optic neuropathies, emphasizing the importance of mitochondrial mechanisms in the pathogenesis of these conditions.
Recent Findings
Optical coherence tomography studies have characterized the retinal nerve fiber layer changes accompanying mitochondrial-affiliated optic neuropathies. Selective thinning of the temporal optic nerve head is preceded by thickening in early stages of these disorders which correlates with reductions in macular ganglion cell layer thinning and vascular atrophy. A recently proposed mechanism underpinning the selective atrophy of the MPB involves the positive feedback of reactive oxygen species generation as a common consequence of mitochondrial dysfunction. Additionally, new research has revealed that the MPB can undergo degeneration in the early stages of glaucoma, challenging the historically held belief that this area was not involved in this common optic neuropathy. A variety of anatomical risk factors influence the propensity of glaucomatous MPB degeneration, and cases present distinct patterns of ganglion cell degeneration that are distinct from those observed in mitochondria-associated diseases.
Summary
This review synthesizes clinical and molecular research on primary MPB disorders, highlighting the commonalities and differences in their pathogenesis.
Key Points (Box)
1. Temporal degeneration of optic nerve fibers accompanied by cecocentral scotoma is a hallmark of maculopapillary bundle (MPB) degeneration.
2. Mechanisms of MPB degeneration commonly implicate mitochondrial dysfunction.
3. Recent research challenges the traditional belief that the MPB is uninvolved in glaucoma by showing degeneration in the early stages of this common optic neuropathy, yet with features distinct from other MPB-selective neuropathies.
4. Reactive oxygen species generation is a mechanism linking mitochondrial mechanisms of MPB-selective optic neuropathies, but in-vivo and in-vitro studies are needed to validate this hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and Anatomy
The papillomacular bundle, more accurately the maculopapillary bundle (MPB), is the name given to the dense collection of retinal ganglion cell (RGC) axons projecting from the retinal macula to the optic nerve. This axonal collection accounts for over 90% of the total optic nerve axons [1]. Notably, the MPB is characterized by the highest axon density in the retinal nerve fiber layer (RNFL), and, like all RGC axons anterior to the lamina cribrosa (LC), the fibers in retinal layer are unmyelinated [2]. The high axonal density in this region limits vascularization, which consequently affects oxygen and nutrient supply to this area [2].
Centrally located within the macula, the anatomical fovea features a sparse ganglion cell layer (GCL) and a sparse RNFL at the periphery. The central 350 μm of the fovea, referred to as the foveola, lacks both a GCL and RNFL [3,4,5]. This arrangement maximizes photoreceptor photon absorption by reducing light scattering from the inner retinal layers. Consequently, RNFL fibers circumnavigate the fovea to form the MPB (Fig. 1). Axons from the nasal macula, both in the superior and inferior foveal periphery, directly and compactly traverse to the optic nerve head (ONH), also referred to as the optic disc. In contrast, RGC fibers temporal to the fovea curve around it to reach the optic nerve head, forming structures known as arcuate bundles (Fig. 1) [1]. Upon reaching the disc, the MPB fibers collect and turn 90 degrees at the superficial nerve fiber layer of the disc to form the prelaminar optic nerve [6].
Configuration of the RNFL. Schematic depiction of the retinal ganglion cell fibers (RGC) in the retinal nerve fiber layer projecting to the optic nerve head (ONH). In the maculopapillary bundle there is a greater density of retinal ganglion cell axons than in the peripheral retina, leading to a greater density of RGC fibers in the temporal ONH compared to the nasal ONH
Adjacent to the fovea, the thickness of the GCL and RNFL in the macula increases sharply, exhibiting the highest ganglion cell density in the entire retina [4, 5]. RGC density then rapidly decreases from the foveal perimeter to the retinal periphery by over 1000-fold [4]. Early studies overestimated the ganglion cell concentration outside the macula along the horizontal meridian because of confusion with amacrine cell bodies [4, 5]. The pattern of amacrine cell density mirrors that of RGCs and peaking between 2 and 6 mm from the center of the fovea [7]. Amacrine cell concentration declines at a slower rate than RGCs following increasing retinal eccentricities. At the peak ganglion cell density, the proportion of displaced amacrine cells in the GCL has been measured as low as 3%, rising to approximately 70% at the ora serrata [4, 7].
Primates are the closest animal model for the study of human neuroretinal diseases due to their retina’s close homology to the human organ. Studies in macaques have characterized 18 distinct classes of RGC cells by electrophysiological response properties and gene expression [8]. More recent transcriptomic analysis of human postmortem retinas has identified 12 types of RGCs. The overall transcriptome similarity between macaque and human retinal cells validates the macaque as a suitable model [9]. ON-midget and OFF-midget RGCs (P-cells) make up over 80% of all RGCs in the retina, followed by ON-parasol and OFF-parasol RGCs (M-cells), which comprise approximately 10% [8, 9]. Midget RGCs are 20 times more abundant than parasol RGCs in the GCL of the maculopapillary area [10]. P-cells predominantly establish synaptic contact with amacrine cells and single contact with midget bipolar cells, while M-cells have diffuse contact with several parasol bipolar cells [1].
In accordance with their high prevalence in the macula, P-cells are smaller than M-cells in terms of both cell body size and axonal diameter. Additionally, P-cells comprise most of the MPB [11,12,13]. However, in spite of the general difference in average size between the two cell types, both midget and parasol cells in the peripheral retina tend to have larger receptive fields than those in the fovea, serving to facilitate converging input in retinal areas corresponding to lower acuity vision [12, 13].
Foveal and macular RGCs tend to have smaller diameter axons than those emanating from the peripheral retina [14]. Similarly, axons that terminate on the temporal side of the ONH tend to have lower diameters than those on the nasal side [15•]. Both facts are consistent with the high ratio of P-cell to M-cell fibers present in the MPB [15•]. The grouping of dense small-diameter axonal fibers emanating from the macula creates the characteristic dense parallel fibers of the MPB, especially in the region temporal to the optic disc and nasal to the macula.
Different segments of the optic disc can be distinguished based on the origin of their axonal fibers in the retina. Specifically, fibers originating from the macula, which constitute the MPB, are primarily found in the central and medial temporal portions of the disc [16,17,18]. Subsequent research corroborated these findings by showing that partial vision loss in specific visual field areas aligns with RNFL thinning in regions of the ONH. These findings were later incorporated into what is now known as the Garway-Heath mapping [19,20,21].
Optical coherence tomography (OCT) is a noninvasive imaging technology that employs laser pulses to measure the thickness of the tissue based on the tissue’s optical reflectivity [22]. OCT was first employed to measure in-vivo retinal thickness followed shortly after its development [23]. Segmentation of the various retinal layers imaged by OCT allows for the measurement of individual retinal layer thicknesses and comparison to age-adjusted norms. Over the years, OCT has been employed to identify RNFL changes in multiple optic neuropathies [24,25,26,27]. Later, OCT’s application was expanded as a tool to measure the thickness of the GCL and inner plexiform layer (IPL) in many optic neuropathies [28]. Since then, OCT has developed into a powerful tool for detecting neural degradation in the retina.
OCT has been instrumental in identifying thinning of the MPB in several optic neuropathies, including Leber hereditary optic neuropathy (LHON), dominant optic atrophy (DOA) nutritional optic neuropathies, and toxic optic neuropathies [15•, 29, 30]. Conditions associated with classical degeneration of the MPB are distinguished by cecocentral scotomas, a diminishing of central visual acuity, a reduction in color sensitivity, and are concurrent with atrophy of the ganglion cells within the MPB. Cecocentral scotomas are predominantly associated with a characteristic atrophy in the macular GCL and temporal peripapillary RNFL, and are often present in the early stages of inherited, toxic, and nutritional optic neuropathies [15•, 29, 31,32,33].
The various MPB disorders have different etiologies, many of which implicate mitochondrial mechanisms. For example, LHON and DOA are both associated with the existence of specific pathogenic mutations in genes encoding mitochondrial proteins [34]. The current state of the literature has yet to provide a consensus on why MPB degeneration emerges as a shared consequence across these distinct causative factors. Beyond these conditions, non-selective MPB degradation is also observed in other optic neuropathies, including glaucoma. We review the current literature on the subject, both clinical and experimental, and suggest possible mechanisms that could trigger optic neuropathies involving this highly vulnerable area.
Pathophysiology and Disease
Hereditary Optic Neuropathies
Leber Hereditary Optic Neuropathy
Leber hereditary optic neuropathy (LHON) and dominant optic atrophy (DOA) are optic neuropathies with a genetic basis and which are characterized by cecocentral scotomas leading to bilateral blindness (Fig. 2) [35,36,37]. Despite similar characteristics, each disease has a distinct etiology and disease progression.
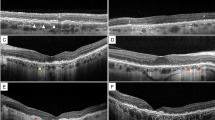
Example of structural and functional deficits in LHON.. A) Fundus images from a patient with LHON. B) Optical coherence tomography (OCT) of the ganglion cell complex (ganglion cell and inner plexiform layers) in the maculae. There is significantly more thinning in the right eye than the left. C) OCT scan of the retinal nerve fiber layer (RNFL) of the right optic nerve of the OD demonstrated thinning with a temporal peripapillary predominance. D) A 24–2 visual field analysis of the OD shows profound visual sensitivity loss in the superior paracentral visual field, consistent with dysfunction corresponding to the inferior macular retinal ganglion cells and inferotemporal optic nerve head
LHON is a rare condition whose onset requires the existence of one of three primary point mutations in the mitochondrial genome: G3460A, G11778A, and T14484C (Fig. 3) [38]. Recent studies have estimated the prevalence of cases for the G3460A and T14484C mutations to 1:66,000 in India [38], and 1:65,000 in Europe [39]. Males are eight times more likely to be affected than females [38, 39]. However, it is crucial to note that being a carrier of the mutations does not guarantee the development of visual loss, with LHON penetrance being 50% in males and 10% in females [35, 38, 40].
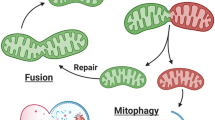
Current summary of implicated proteins in LHON and DOA. LHON mutations all affect complex I (NADH dehydrogenase) in the electron transport chain. These mutations have been shown to increase \({\text{O}}_{2}^{-}\), which is converted to H2O2 by superoxide dismutase 2 (SOD2) in mitochondria. H2O2 is converted to water by catalase (Cat) or glutathione peroxidase (GPX). Reactive oxygen species are direct drivers of apoptosis. Mutations in KIF5A also increase the amount of apoptosis in LHON cybrid cells. DOA has several driving mutations, the most common implicating the pathways for mitochondrial fission and fusion. DRP1 stimulates proteins on the outer mitochondrial membrane to initiate fission mediated through several outer membrane proteins: FIS1, MFF, MiD49, and MiD51. MFN1/2 is responsible for homomeric adhesion between mitochondrial outer membranes to initiate outer membrane fusion. OPA1 has two forms: a membrane-bound long isoform (OPA1-L) and a short (OPA1-S) isoform, cleaved by YMEL1 and OMA1. The long isoform is responsible for triggering inner mitochondrial membrane fusion. AMPK-stimulated autophagy increases in OPA1 mutant animals and cell lines, and its inhibition prevents neurodegeneration, suggesting a mechanism in the pathophysiology of the disease. PNPT1, a protein responsible for mtRNA transport, and SSBI, responsible for mtDNA replication, are also genes that can be mutated in a DOA disease context
LHON is characterized by an initial thinning of the peripapillary RNFL starting in the inferotemporal optic disc, which then extended centrally, sparing the superonasal quadrant, an area devoid of MPB (Fig. 2) [15•]. Recent research has shed light on the pattern of macular ganglion cell layer (GCL) degeneration, noting concurrent changes in vascularization throughout disease progression. Castillo et. Al (2022) found that even in LHON mutation carriers, there is a significant reduction in macular GCL thickness unaccompanied by visual loss or MPB RNFL thinning [41•]. These carriers also had decreased capillary vessel density in the superficial capillary plexus of the nasal macula without an effect on the ONH. In contrast, advanced patients had more pronounced vessel density changes in the temporal disc than in the macula [41•].
Castillo et al. (2022) noted minimal differences in GCL between short-term and long-term LHON patients. However, peripapillary RNFL thinning, especially in the temporal quadrant, was progressive and related to the duration of the illness [41•]. These results suggest that changes in macular GCL parameters are linked to, but not necessary for, subsequent ONH and peripapillary RNFL thinning. This affirms that RNFL thinning is a progressive hallmark of the disease.
Carbonelli et al. (2022) tracked the changes in OCT parameters in four patients with LHON mutations over an interval spanning two months before and five months after the onset of visual loss. They observed that preceding visual loss the superior and inferior peripapillary RNFL (pRNFL) increased in thickness, which then persisted up to 40 days following the onset of visual impairment, [42••]. This regional pRNFL thickening was followed by rapid thinning and decline in visual acuity. In contrast, there were no thickness changes in macular GCL prior to visual loss onset. However, within 30 days of the conversion to affective LHON, there was a decline of up to 40% in macular GCL thickness, most prominently in the nasal region [42••].
Similar observations were made by Wang et al. (2021), who demonstrated pRNFL thickening before temporal pRNFL thinning, accompanied by central visual field defects [43••]. Approximately 3–6 months post visual loss, the average pRNFL thickness was observed to be lower than its original baseline reflecting a transition from MPB deterioration to broader disc atrophy. This trend persisted over the 60-month monitoring period, with the nasal quadrant being the last region to experience pRNFL thinning [43••].
The findings of Carbonelli et al. (2022) and Castillo et al. (2022) provide two perspectives on MPB vulnerability. The first demonstrates that RNFL changes precede the subsequent reduction in VA, and the second suggests that changes in macular vascularization could be an inciting factor for progressive optic nerve damage [41•, 42••].
Mitochondrial features other than the principal genetic mutations can affect the penetrance of LHON. Mitochondrial haplogroups (collections of other correlated single nucleotide polymorphisms in the mitochondrial genome) have been linked to LHON penetrance in LHON carriers [44,45,46,47]. Mitochondrial motility is a factor influencing the vulnerability of RGCs. In vitro studies using human induced pluripotent stem cells (hiPSCs) carrying the G11778A mutation have demonstrated an increase in reactive oxygen species (ROS) within induced RGCs, both in affected patients and unaffected mutation carriers (Fig. 3).
Variations in mitochondrial motility were observed between cells that were likely to undergo apoptosis and unaffected cells. This divergence was correlated with reduced levels of the protein kinesin-1 family member KIF5A, a motor protein involved in microtubule-associated ATP-dependent transport and responsible in part for mitochondrial motility, in cells likely to undergo apoptosis (Fig. 3) [48•]. These results indicate mitochondrial dynamics are crucial in determining RGC axonal vulnerability. In addition to this, older studies have hinted that higher levels of mitochondrial biogenesis in carriers is a protective factor against the development of disease [49••].
Hereditary Optic Neuropathies
Dominant Optic Atrophy
Dominant optic atrophy (DOA) exhibits several clinically similar features to LHON, particularly in terms of the pattern of neuroretinal damage and pathophysiological mechanisms. Early characterizations of the disorder noted the simultaneous onset of a gradually expanding binocular cecocentral scotoma that spares peripheral vision [50, 51]. DOA is triggered by a set of nuclear genetic mutations that affect encoding of mitochondrial proteins (Table 1). The onset of DOA can begin within the first two decades of life and progress into adulthood, leading to legal blindness [50,51,52,53]. The speed of visual deterioration is typically much slower than LHON [52, 54].
The visual impairment in DOA has been linked to a loss of ganglion cell density in the segments of the retina associated with the MPB. OCT scans reveal both macular GCL and peripapillary RNFL thinning in DOA patients. This thinning is in all quadrants of the ONH and macula in the late stages of DOA [55]. Yet, the most rapid and pronounced DOA-associated ganglion cell atrophy is noted in the MPB-associated areas, especially the peripapillary temporal RNFL and nasal sections of the macular RNFL, GCL, and IPL [56, 57]. The specificity of early DOA atrophy to the MPB is underscored by the relative preservation of ganglion cells in the nasal peripapillary region of the optic disc [55, 56].
In advanced stages of DOA, diminished vascularization is evident in the temporal radial peripapillary plexus [58, 59•]. Both the deep and superficial macular capillary plexuses exhibit thinning in patients with advanced DOA, and the degeneration of this vascular network has been shown to correlate strongly with macular GCL thinning [59•]. Studies consistently show that visual acuity loss correlates most strongly with temporal peripapillary RNFL, nasal macular GCL, and the macular deep capillary plexus [56, 57, 59•]. Interestingly, a comparative study comparing acute and chronic LHON patients to DOA patients found no significant differences in the macular GCL thickness between these groups [60••].
In contrast to LHON, where pathological mutations are in the mitochondrial genome, DOA-causing mutations are in nuclear DNA yet code for mitochondrial proteins [61]. Optic atrophy 1 (OPA1) is a nuclear gene located on chromosome 3q28 and is the predominant gene implicated in DOA, accounting for the majority of cases [52, 62]. OPA1 encodes a mitochondrial inner membrane-bound GTPase membrane that is involved in dynamin-associated mitochondrial internal membrane fusion and fission (Fig. 3) [63, 64]. Mutations in OPA1 that cause DOA can be missense, deletion, or inversions [55, 65]. While less prevalent, other DOA-linked mutations encompass genes integral to mitochondrial fusion, fission, and apoptosis, such as OPA3, MFN1, MFN2, and DRP1 [63, 64, 66,67,68,69]. The breadth of recognized DOA mutations has expanded to include those involved in mitochondrial RNA transport (PNTP1), mitochondrial DNA replication (SSB1), and mitochondrial protease activity (AFG3L2, SPG7) (Fig. 3) [70,71,72]. Mitochondrial homeostasis emerges as a common theme among various DOA-related gene functions (Table 1). The process of mitochondrial biogenesis has been commonly implicated in both LHON and DOA pathophysiology [49••, 64]. A notable biochemical difference between the two diseases is the specific mutation effects on mitochondrial oxygen consumption and total mitochondrial respiration rate. In LHON cybrids, the total oxygen consumption rate and ATP production in cells are unaffected by the disease-associated mutations despite a demonstrated reduction of complex I respiration [73, 74••]. In contrast, OPA1 has been shown to decrease oxygen consumption and oxidative respiration in induced pluripotent stem cell lines [75•, 76].
Another proposed cellular mechanism for DOA involves mitochondrial autophagy. A study of OPA1 mutations in purified RGC cultures demonstrated increased calcium-dependent mitophagy through the calcineurin/AMPK (AMP-dependent protein kinase) signaling pathway (Fig. 3) [77]. In mouse models, it was shown that the neuronal effects of OPA1 can be curtailed via the inhibition of AMPK, which results in a decrease in neuron autophagy and an elevation in the mitochondrial content [78].
Despite the differences in possible mechanisms of LHON and DOA, both conditions possibly respond to idebenone treatment. Idebenone, a synthetic coenzyme Q that passes the blood–brain barrier, was initially formulated to address complex I deficiencies in LHON [79, 80]. Subsequent studies showed that idebenone also is a superoxide scavenger [81]. A randomized control trial of idebenone in LHON failed to meet its primary outcome measure [80], but there were suggestive effects in analysis of other outcome measures in a nonrandomized study [79]. A subsequent nonrandomized trial of OPA1-associated DOA visual loss showed better vision compared to historical controls [82]. Although the clinical evidence is not strong for idebenone in these diseases, it does point to a potential role in ROS scavenging as a treatment.
Nutritional and Toxic Optic Neuropathies
Nutritional and toxic optic neuropathies are distinct yet related conditions which manifest with characteristic clinical features, including optic disc pallor, reduced color vision, and the presence of unilateral or bilateral cecocentral scotomas. In the initial stages of these disorders there is a thickening of the temporal RNFL and GCL, which subsequently progresses to thinning [83,84,85,86,87,88,89,90,91,92,93,94].
The primary etiological difference between the two types of metabolic neuropathies lies in their underlying causes: nutritional neuropathies stem from deficiencies in specific nutrients, whereas toxic neuropathies result from exposure to harmful toxins or medications.
Nutritional optic neuropathies primarily stem from deficiencies that facilitate oxidative metabolism (Table 1). The most prevalent nutritional optic neuropathy results from vitamin B12 deficiency, although deficits of folate, thiamine, pyridoxine (vitamin B6), or copper have also been implicated [29, 94]. Vitamin B12 is essential to protein synthesis, fatty acid metabolism, and the conversion of homocysteine to methionine [95]. It also can scavenge ROS, including superoxide [96,97,98]. Folate acts as a coenzyme in nucleotide synthesis for both purines and pyrimidines [99, 100]. Deficiencies in folate have been hypothesized to alter mitochondrial metabolism through low levels of ADP synthesis [101]. Thiamine or vitamin B1 is a key metabolic cofactor implicated in the Kreb cycle, pentose phosphate pathway, glycolysis, nucleic acid synthesis, and homocysteine metabolism [100, 102]. Copper, a ligand of the cytochrome c oxidase (complex IV) in the mitochondrial electron transport chain, is necessary for oxidative metabolism [103].
Similar biomolecular pathways affecting mitochondria are hypothesized to be caused by drugs responsible for the development of toxic optic neuropathies [104]. Most but not all drugs observed to induce optic neuropathies, including ethambutol, linezolid, ciprofloxacin, chloramphenicol, macrolides, aminoglycosides, fluoroquinolones, and isoniazid, have a primary antimicrobial function, yet are believed to affect mitochondrial functions. Clinically, they induce clinical features similar to those of other mitochondrial optic neuropathies, e.g. cecocentral scotomas, and act in a dose-dependent manner [91, 92, 104,105,106,107,108].
The antimicrobial action of these antibiotics, except for tuberculosis-targeting drugs and fluoroquinolones, is mediated by bacterial rRNA binding which impairs protein synthesis (Table 1) [92, 104, 106, 108,109,110,111,112,113]. The homology between bacterial and mitochondrial ribosomes has led to a hypothesis that the drug impairs mitochondrial protein synthesis through the same mechanism [114]. Ethambutol, a metal chelator, has been shown to reduce zinc and copper levels in primate models and has been observed to accelerate the development of optic neuropathies in patients with LHON- or DOA-associated mutations [115, 116]. Isoniazid’s mechanism of action remains unelucidated, although it has been shown to enhance levels of reactive oxygen species and cause vitamin B6 deficiency [117,118,119]. Such a finding bolsters the hypothesis that these drugs exert their pathological effects in the mitochondrial electron transport chain by reducing ligand availability [115, 120].
Methanol poisoning is a non-medicinal toxic optic neuropathy associated with MPB degeneration, characterized by optic nerve pallor and OCT thinning of the RNFL and GCL [121,122,123,124,125,126]. While temporal ONH RNFL and GCL thinning are typically associated with methanol toxicity, there have been a few cases in which this was not the case, and RNFL thinning spared the MPB [127, 128]. The molecular mechanism of methanol toxicity likely involves its conversion to formic acid, which can interfere with oxidative metabolism by inhibiting complex IV of the electron transport chain [129].
However, it is crucial to distinguish between the above toxic neuropathies and those associated with alcohol ingestion (ethanol) and tobacco use. Although it is controversial whether these substances are direct causes of optic neuropathies, they can exacerbate the penetrance of genetically predisposed conditions like LHON, evidenced by the increase copy numbers of mitochondrial DNA in these predisposed patients [130, 131]. Cases associated with alcohol and tobacco use differ from classical nutritional and toxic neuropathies, which lack a recognized genetic predisposition [30].
Glaucomatous Optic Neuropathy
Glaucomatous optic neuropathy, or glaucoma, is the leading cause of irreversible, progressive blindness worldwide. The incidence increases with age [132,133,134,135,136], and is responsible for moderate to severe visual impairment in about 4.1 million individuals age 50 years or older worldwide, and blindness in about 3.6 million [135]. The prevalence of primary open-angle glaucoma (POAG) and primary-angle closure glaucoma (PACG) are estimated to be 2.4% and 0.6%, respectively [132, 133]. While POAG is more common among men, PACG is more common among women [132, 133].
Pathophysiologically, glaucoma is categorized by degeneration of retinal ganglion cells (RGCs) and subsequent visual impairment. Although the etiological trigger of the disorder has yet to be established, depending on the region of the world 10%-50% of patients with glaucoma have increased ocular pressure (IOP). Currently, IOP reduction is the only established intervention for slowing glaucomatous progression [137]. However, while increased IOP is a common feature, forms of glaucoma without elevated IOP exhibit similar structural and functional patterns of damage.
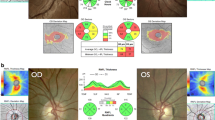
Formerly it was believed that glaucoma predominantly affects RGC axons arising from the peripheral retina, while MPB damage was only seen late in the disease [138]. This understanding evolved starting around 2013, when the widespread use of OCT to measure retinal layers revealed that early glaucoma commonly affects the macula. Hood et al. [139] showed that glaucoma causes atrophy of the GCL and IPL in the inferior macula, projecting to the inferotemporal ONH and sparing the superior MPB. They called this retinal area the macular vulnerability zone (MVZ). Subsequent large cohort studies have corroborated these finding, emphasizing the significance of MVZ damage in glaucoma [140, 141, 142•, 143••].
Recent studies have highlighted deficits in the MPB in early glaucoma. Traditional evaluation methods to assess visual defects in glaucoma, such as Humphrey 24–2 or 30–2 standard automated perimetry (SAP) and current clinical OCT imaging, may under-estimate the extent of macular damage in the early stages of the disease. Enhanced detection in these stages is achievable with 10–2 SAP and with retinal nerve fiber optical texture analysis (ROTA), a novel axonal detection algorithm based on OCT scans [142•, 144]. In a comprehensive cohort study of 204 patients diagnosed with early-stage glaucoma, MPB anomalies were identified by ROTA in 71.6% of the subjects, with a notable subset showing deficits in the inferior MPB. [142•]. Another study using the ROTA technique found MPB abnormalities overlooked by current clinical OCT in about 5% of patients with ocular hypertension [145•].
Decreased visual acuity typically occurs late in progressive glaucoma. There are rare examples of significantly decreased visual acuity at an early stage, although reported cases could include patients where nonglaucomatous optic neuropathy was inadequately excluded. Examples of misattribution of visual loss to glaucoma could include patients with bilateral central scotomas without assessment for LHON or DOA, or unilateral disease assessed with computed tomography but not magnetic resonance imaging focusing on the optic nerve [146]. Such studies failing to completely rule out other optic neuropathies could occur in studies that demonstrated decreased best-corrected visual acuity (BCVA) and color vision deficiency in association with OCT-detected atrophy of the peripapillary RNFL and macular GCL, specifically in regions corresponding to the MPB [32, 147]. That said, there are certainly patients with MPB thinning as a result of the glaucomatous process at the disc, even if the visual acuity is spared [145•, 148•]. Additionally, OCT-A has also shown that vessel density loss correlates with the changes in RNFL, GCL, and IPL thickness in the peripapillary region but not in the macula in patients with structural damage to both areas [149].
Early studies suggested that glaucoma with normal IOP may be more prone to MPB damage than other forms of glaucoma [138]. However, this assertion remains controversial [143••]. Recent studies have linked other retinal characteristics, such as the position of the central vessel trunk (CVT) and the size of the optic disc, with early-stage glaucoma MPB involvement. Positioning of the prelaminar CVT nasally correlates positively with glaucomatous MPB involvement [140, 150]. This distinction in CVT positioning is only notable at the prelaminar segment of the optic nerve as there is no difference between glaucoma-affected eyes and healthy controls at the level of the lamina cribrosa [150]. Furthermore, optic nerve cup morphology is another factor predictive for MPB involvement. Both ONH size and the ratio of the longest to the shortest axis of the ONH positively correlate with paracentral scotomas, macular damage, and nasal shift of the CVT [145•, 148•].
Intriguingly, while the CVT position in glaucoma patients with normal IOP does not significantly differ from that of healthy controls, their lamina cribrosa morphology does. Specifically, the lamina cribrosa exhibits a more pronounced posterior curvature than healthy controls and DOA patients, with the latter two groups showing no significant differences between them [151]. These findings further delineate glaucoma from other optic neuropathies as having unique anatomical characteristics.
Optic Neuritis
Optic neuritis has a disproportionate effect on fibers of the temporal anterior optic nerve. This is particularly manifested by significant dyschromatopsia, loss of contrast sensitivity, and central scotomas [152]. Those features are all consistent with damage to small RGC axons, i.e. the parvocellular system. However, it could also be consistent with a mitochondria-mediated mechanism similar to that seen in LHON and DOA.
Studies exploring reactive oxygen species generation or mitochondrial pathways in animal models of optic neuritis are limited. One study demonstrated that suppression of ROS generation in mitochondria reduced myelin fiber injury in mouse models of optic neuritis and that elevating ROS levels had the opposite effect [153]. Another group demonstrated that ROS generation was a consequence of pro-inflammatory TNFα stimulation. They also showed that treatment with the multiple sclerosis therapy fingolimod in vitro reduced ROS production and neuronal injury [154]. Other animal model studies of optic neuritis demonstrate the importance of SIRT1 in many pathways, among which are antioxidant effects [155, 156]. However, in contrast with MPB-specific diseases, ROS production likely is a consequence of inflammation as opposed to a driving mechanism of pathology.
Although there is insufficient evidence to implicate mitochondria-specific damage in typical inflammatory optic neuritis, the overlap between multiple sclerosis and LHON mutations and other evidence to implicate mitochondria in optic neuritis suggest that this is a worthwhile area for future research [153, 157, 158].
Hypotheses for the Pathogenesis of Degenerative Diseases of the Maculopapillary Bundle
A consistent feature observed in neuropathies selectively affecting the MPB (including hereditary, nutritional, and toxic neuropathies) is the involvement of impaired mitochondrial bioenergetics and metabolism (Table 1). While these etiological factors universally affect mitochondrial functions at the cellular level, the specific pathogenesis underlying MPB-selective RGC degeneration remains unproven. A prevailing hypothesis posits that mitochondrial impairment facilitates the generation of reactive oxygen species (ROS). Elevation of ROS has been noted directly in LHON [48•, 172, 173] and DOA [174, 175]. Indirect evidence from ethambutol toxic neuropathy suggests a similar ROS elevation, based on antioxidant depletion and coenzyme Q supplementation in animal models [176, 177].
The generation of ROS, a byproduct of oxidative metabolism, can trigger apoptotic cell death, especially when the electron transport chain is impaired [178]. Small fiber-selective degeneration in MPB diseases may be attributed to differing bioenergetics across neuron types. In the MPB, the sodium–potassium pump, responsible for restoring the resting potential after neural depolarization, constitutes the primary energy consumer in axons. This ion exchanger is a membrane protein, whereas the mitochondria are cytosolically distributed. Therefore, the ratio of energetic demand to supply can be thought of as the ratio of axonal surface area to volume, which is greatest in small-diameter axons [15•, 179]. Since ROS production is intricately linked to mitochondrial distribution, it follows that greater per-volume oxidative production takes place in smaller-diameter axons.
We have previously suggested that this gap in ROS density creates a vulnerability for the small-diameter P-cell fibers of the MPB to undergo cell death [179]. Yet, the MPB’s typical degeneration doesn’t solely target individual small fibers. Instead, it manifests as a progressively enlarging defect marked by both visual loss and retinal layer degeneration. One plausible explanation lies in the compact arrangement of the small-diameter axonal fibers in the MPB. The demise of even a few of these fibers could release ROS to neighboring axons, potentially triggering a cascade of apoptosis [180].
Using an in-silico model, we illustrated how this axonal vulnerability aligns with clinical and histological findings in LHON [181•]. Given the similarities in mitochondrial metabolism in other MPB disorders and common patterns of neurodegeneration, we propose a similar mechanism in those disorders. One similarity between LHON and DOA is the implications of mitochondrial proliferation dynamics in each disorder. In LHON, asymptomatic mutation carriers have upregulated mitochondrial biogenesis, which may confer a protective effect on the mutations by distributing ROS production more evenly in the optic nerve [49••]. Conversely, many DOA-associated mutations impair mitochondrial fission and are conducive to mitochondrial autophagy [63, 64, 69]. Thus, the underlying mechanisms in these conditions could be deemed functionally synonymous: a decrease in overall mitochondrial count amplifies the oxidative strain on individual mitochondria, potentially triggering the previously mentioned ROS-induced cascade.
Glaucoma is excluded from this classification because, although recent studies have shown an involvement of the MPB even in early disease, neither the clinical findings nor the pathophysiology mirrors the mitochondrial optic neuropathies. Instead, glaucoma affects the peripheral retina concurrently with the MPB in the early stages of the disease. In addition, MPB vulnerability in glaucoma is pronounced in the inferior segment of the MPB. It is distinct from the other MPB disorders discussed above in terms of its etiology [143••] and clinical presentation.
Conclusion
The MPB is affected in a broad spectrum of optic neuropathies. Many of the diseases that are MPB-selective in their early stages, such as LHON, DOA, and metabolic optic neuropathies, implicate mitochondrial metabolism, biogenesis, and motility. Although each has a distinct etiology, all are consistent with mitochondrial mechanisms. The generation of reactive oxygen species (ROS) presents a promising mechanistic nexus linking mitochondrial impairment with the vulnerability and progressive degeneration of small fibers in the MPB. While systemic propagation of reactive oxygen species has been posited in in-silico models, empirical validation though laboratory experimentation has not yet been done.
Recent empirical studies have started to shed light on the MPB involvement in the early stages of glaucoma. However, glaucomatous involvement of the MPB stands in stark contrast to the optic neuropathies described above because of the difference in effects on central vision, particularly visual acuity and color vision.
We advocate for expanded research in several areas relevant to the MPB and disease. First, work is needed to empirically test the ROS propagation hypothesis, through in vitro or in vivo models, by exploring how ROS release affects adjacent axons. Second, it would be beneficial to explore if the death of a subgroup of MPB axons can cause pathological changes in the entire optic nerve other than the distal axons, e.g. mechanisms for secondary degeneration. Third, a deeper exploration into the axon biology of small fibers within the MPB in early glaucoma could help in understanding whether their loss is different from that occurring in non-MPB axons entering the ONH.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Forrester J, Dick A, McMenamin P, Roberts F, Pearlman E. The Eye—4th Edition. 4th ed. Edinburgh UK: Elsevier; 2015.
Standring S. Gray's anatomy e-book: the anatomical basis of clinical practice. 42 ed. Elsevier Health Sciences; 2021.
Fu D, Tong H, Zheng S, Luo L, Gao F, Minar J. Retinal status analysis method based on feature extraction and quantitative grading in OCT images. Biomed Eng Online. 2016;15(1):1–18.
Wässle H, Grünert U, Röhrenbeck J, Boycott BB. Retinal ganglion cell density and cortical magnification factor in the primate. Vision Res. 1990;30(11):1897–911.
Oyster C, Takahashi E, Hurst D. Density, soma size, and regional distribution of rabbit retinal ganglion cells. J Neurosci. 1981;1(12):1331–46. https://doi.org/10.1523/jneurosci.01-12-01331.1981.
Hayreh SS, Vrabec F. The structure of the head of the optic nerve in rhesus monkey. Am J Ophthalmol. 1966;61(1):136–50.
Curcio C, Allen K. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. https://doi.org/10.1002/cne.903000103.
Peng Y-R, Shekhar K, Yan W, Herrmann D, Sappington A, Bryman GS, et al. Molecular Classification and Comparative Taxonomics of Foveal and Peripheral Cells in Primate Retina. Cell. 2019;176(5):1222-37.e22. https://doi.org/10.1016/j.cell.2019.01.004.
Yan W, Peng Y-R, van Zyl T, Regev A, Shekhar K, Juric D, et al. Cell atlas of the human fovea and peripheral retina. Sci Rep. 2020;10(1):9802.
Pavlidis M, Stupp T, Hummeke M, Thanos S. Morphometric examination of human and monkey retinal ganglion cells within the papillomacular area. Retina. 2006;26(4):445–53.
Salazar JJ, Ramírez AI, De Hoz R, Salobrar-Garcia E, Rojas P, Fernández-Albarral JA, et al. Anatomy of the Human Optic Nerve: Structure and Function. IntechOpen; 2019.
Silveira LCL, Saito CA, Lee BB, Kremers J, da Silva FM, Kilavik BE, et al. Morphology and physiology of primate M-and P-cells. Prog Brain Res. 2004;144:21–46.
Kim US, Mahroo OA, Mollon JD, Yu-Wai-Man P. Retinal ganglion cells—diversity of cell types and clinical relevance. Front Neurol. 2021;12:661938.
FitzGibbon T, Taylor S. Mean retinal ganglion cell axon diameter varies with location in the human retina. Jpn J Ophthalmol. 2012;56:631–7.
• Pan BX, Ross-Cisneros FN, Carelli V, Rue KS, Salomao SR, Moraes-Filho MN, et al. Mathematically modeling the involvement of axons in Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2012;53(12):7608–17. https://doi.org/10.1167/iovs.12-10452. This study provides histopathological data from LHON patients demonstrating maculopapillary bundle atrophy predominance.
Hoyt WF. Anatomic considerations of arcuate scotomas associated with lesions of the optic nerve and chiasm. A nauta axon degeneration study in the monkey. Bullet Johns Hopkins Hospital. 1962;111:57–71.
Hoyt WF. The Course of Parapapillary Temporal Retinal Axons Through the Anterior Optic Nerve. Arch Ophthalmol. 1963;69(4):503. https://doi.org/10.1001/archopht.1963.00960040509014.
Hoyt W, Kommerell G. Fundus oculi in homonymous hemianopia. Klin Monatsbl Augenheilkd. 1973;162(4):456–64.
Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107(10):1809–15.
Jansonius NM, Schiefer J, Nevalainen J, Paetzold J, Schiefer U. A mathematical model for describing the retinal nerve fiber bundle trajectories in the human eye: average course, variability, and influence of refraction, optic disc size and optic disc position. Exp Eye Res. 2012;105:70–8.
Jansonius NM, Nevalainen J, Selig B, Zangwill LM, Sample PA, Budde WM, et al. A mathematical description of nerve fiber bundle trajectories and their variability in the human retina. Vision Res. 2009;49(17):2157–63. https://doi.org/10.1016/j.visres.2009.04.029.
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81.
Swanson EA, Izatt JA, Hee MR, Huang D, Lin C, Schuman J, et al. In vivo retinal imaging by optical coherence tomography. Opt Lett. 1993;18(21):1864–6.
Bellusci C, Savini G, Carbonelli M, Carelli V, Sadun AA, Barboni P. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch Clin Exp Ophthalmol. 2008;246:641–7.
Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59(6):963–9.
Medeiros FA, Moura FC, Vessani RM, Susanna R Jr. Axonal loss after traumatic optic neuropathy documented by optical coherence tomography. Am J Ophthalmol. 2003;135(3):406–8.
Zangwill LM, Williams J, Berry CC, Knauer S, Weinreb RN. A comparison of optical coherence tomography and retinal nerve fiber layer photography for detection of nerve fiber layer damage in glaucoma. Ophthalmology. 2000;107(7):1309–15.
Choi SS, Zawadzki RJ, Keltner JL, Werner JS. Changes in cellular structures revealed by ultra-high resolution retinal imaging in optic neuropathies. Invest Ophthalmol Vis Sci. 2008;49(5):2103–19.
Roda M, di Geronimo N, Pellegrini M, Schiavi C. Nutritional optic neuropathies: state of the art and emerging evidences. Nutrients. 2020;12(9):2653.
Baj J, Forma A, Kobak J, Tyczyńska M, Dudek I, Maani A, et al. Toxic and nutritional optic neuropathies—an updated mini-review. Int J Environ Res Public Health. 2022;19(5):3092.
Ewering C, Haşal N, Alten F, Clemens CR, Eter N, Oberwahrenbrock T, et al. Temporal retinal nerve fibre layer thinning in cluster headache patients detected by optical coherence tomography. Cephalalgia. 2015;35(11):946–58.
Yum HR, Park H-YL, Park CK. Characteristics of Normal-tension Glaucoma Patients with Temporal Retinal Nerve Fibre Defects. Scientific Reports. 2020;10(1). https://doi.org/10.1038/s41598-020-63486-7.
Dattilo M, Newman NJ, Biousse V. Acute retinal arterial ischemia. Annals of Eye Science. 2018;3:28. https://doi.org/10.21037/aes.2018.05.04.
Yu-Wai-Man P, Votruba M, Burté F, La Morgia C, Barboni P, Carelli V. A neurodegenerative perspective on mitochondrial optic neuropathies. Acta Neuropathol. 2016;132(6):789–806. https://doi.org/10.1007/s00401-016-1625-2.
Yu-Wai-Man P, Chinnery PF. Leber hereditary optic neuropathy. Mitochondrial Case Studies. Elsevier; 2016. p. 55–64.
Kirches E. LHON: Mitochondrial Mutations and More. Curr Genomics. 2011;12(1):44–54. https://doi.org/10.2174/138920211794520150.
Lenaers G, Hamel CP, Delettre C, Amati-Bonneau P, Procaccio V, Bonneau D, et al. Dominant optic atrophy. Orphanet J Rare Dis. 2012;7(1):46. https://doi.org/10.1186/1750-1172-7-46.
Gowri P, Kumar SM, Vanniarajan A, Bharanidharan D, Sundaresan P. A hospital-based five-year prospective study on the prevalence of Leber’s hereditary optic neuropathy with genetic confirmation. Mol Vis. 2020;26:789.
Mascialino B, Leinonen M, Meier T. Meta-analysis of the prevalence of Leber hereditary optic neuropathy mtDNA mutations in Europe. Eur J Ophthalmol. 2012;22(3):461–5.
Hage R, Vignal-Clermont C. Leber hereditary optic neuropathy: review of treatment and management. Front Neurol. 2021;12:651639.
• Castillo L, Berrozpe‐Villabona C, Miserachs‐García S, Haulani H, Gómez‐Gutiérrez C, Díaz‐García RS, et al. Quantitative assessment of macular and circumpapillary retinal vessel density across all stages of Leber hereditary optic neuropathy using swept source optical coherence tomography angiography. Acta Ophthalmologica. 2022;100(8). https://doi.org/10.1111/aos.15169. Using OCTA, the authors determined that vessel density changes in the macula precede the optic nerve head changes, despite the latter correlating better with a loss of visual acuity.
•• Carbonelli M, La Morgia C, Romagnoli M, Amore G, D’Agati P, Valentino ML, et al. Capturing the pattern of transition from carrier to affected in Leber hereditary optic neuropathy. Am J Ophthalmol. 2022;241:71–9. Using OCT, this study found that in LHON patients the clinical point of transition to the onset of visual loss occurs with the onset of temporal optic disc thinning, following a thickening during the asymptomatic period.
•• Wang D, Liu H-L, Du Y-Y, Yuan J, Li X, Tian Z, et al. Characterisation of thickness changes in the peripapillary retinal nerve fibre layer in patients with Leber’s hereditary optic neuropathy. Br J Ophthalmol. 2021;105(8):1166–71. https://doi.org/10.1136/bjophthalmol-2020-316573. This study quantified the progressive ganglion cell changes in the pRNFL that LHON patients with the m.11778G>A mutation experience.
Quigley C, Stephenson KAJ, Kenna P, Cassidy L. Optic Nerve Structural and Functional Changes in LHON-Affected and Asymptomatic Maternal Relatives: Association with H and HV Mitochondrial Haplogroups. Int J Mol Sci. 2023;24(2):1068. https://doi.org/10.3390/ijms24021068.
Khan NA, Govindaraj P, Soumittra N, Srilekha S, Ambika S, Vanniarajan A, et al. Haplogroup heterogeneity of LHON patients carrying the m. 14484T> C mutation in India. Investig Ophthalmol Visual Sci. 2013;54(6):3999–4005.
Hudson G, Carelli V, Spruijt L, Gerards M, Mowbray C, Achilli A, et al. Clinical expression of Leber hereditary optic neuropathy is affected by the mitochondrial DNA–haplogroup background. Am J Human Gen. 2007;81(2):228–33.
Brown MD, Starikovskaya E, Derbeneva O, Hosseini S, Allen JC, Mikhailovskaya IE, et al. The role of mtDNA background in disease expression: a new primary LHON mutation associated with Western Eurasian haplogroup. J Human genetics. 2002;110:130–8.
• Yang T-C, Yarmishyn AA, Yang Y-P, Lu P-C, Chou S-J, Wang M-L, et al. Mitochondrial transport mediates survival of retinal ganglion cells in affected LHON patients. Hum Mol Genet. 2020;29(9):1454–64. This study used induced pluripotent stem cells to evaluate differences in oxidative stress, mitochondrial transport and apoptosis between symptomatic LHON patients and mutation carriers.
•• Giordano C, Iommarini L, Giordano L, Maresca A, Pisano A, Valentino ML, et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber’s hereditary optic neuropathy. Brain. 2014;137(2):335–53. https://doi.org/10.1093/brain/awt343. LHON patients have less mitochondrial mass and fewer DNA copy numbers than LHON mutation cariers, suggesting a protective effect of mitochondrial biogenesis.
Kline LB, Glaser JS. Dominant optic atrophy: the clinical profile. Arch Ophthalmol. 1979;97(9):1680–6.
Votruba M, Moore AT, Bhattacharya SS. Clinical features, molecular genetics, and pathophysiology of dominant optic atrophy. J Med Genet. 1998;35(10):793–800. https://doi.org/10.1136/jmg.35.10.793.
Yu-Wai-Man P, Griffiths PG, Burke A, Sellar PW, Clarke MP, Gnanaraj L, et al. The Prevalence and Natural History of Dominant Optic Atrophy Due to OPA1 Mutations. Ophthalmology. 2010;117(8):1538-46.e1. https://doi.org/10.1016/j.ophtha.2009.12.038.
Yu-Wai-Man P, Chinnery PF. Dominant Optic Atrophy: Novel OPA1 Mutations and Revised Prevalence Estimates. Ophthalmology. 2013;120(8):1712-e1. https://doi.org/10.1016/j.ophtha.2013.04.022.
Hwang TJ, Karanjia R, Moraes-Filho MN, Gale J, Tran JS, Chu ER, et al. Natural history of conversion of Leber’s hereditary optic neuropathy: a prospective case series. Ophthalmology. 2017;124(6):843–50.
Pretegiani E, Rosini F, Rufa A, Gallus G, Cardaioli E, Da Pozzo P, et al. Genotype-phenotype and OCT correlations in Autosomal Dominant Optic Atrophy related to OPA1 gene mutations: Report of 13 Italian families. J Neurol Sci. 2017;382:29–35.
Corajevic N, Larsen M, Rönnbäck C. Thickness mapping of individual retinal layers and sectors by Spectralis SD-OCT in Autosomal Dominant Optic Atrophy. Acta Ophthalmol. 2018;96(3):251–6. https://doi.org/10.1111/aos.13588.
Yu-Wai-Man P, Bailie M, Atawan A, Chinnery PF, Griffiths PG. Pattern of retinal ganglion cell loss in dominant optic atrophy due to OPA1 mutations. Eye. 2011;25(5):596–602. https://doi.org/10.1038/eye.2011.2.
Balducci N, Ciardella A, Gattegna R, Zhou Q, Cascavilla ML, La Morgia C, et al. Optical coherence tomography angiography of the peripapillary retina and optic nerve head in dominant optic atrophy. Mitochondrion. 2017;36:60–5.
• Cesareo M, Giannini C, Di Marino M, Aloe G, Martucci A, Aiello F, et al. Optical coherence tomography angiography in the multimodal assessment of the retinal posterior pole in autosomal dominant optic atrophy. Acta Ophthalmologica. 2022;100(3). https://doi.org/10.1111/aos.14972. Patients with OPA1 gene mutations have significantly reduced retinal vessel density, which strongly correlates with decreased macular GCL thinning and light sensitivity.
•• Asanad S, Tian JJ, Frousiakis S, Jiang JP, Kogachi K, Felix CM, et al. Optical Coherence Tomography of the Retinal Ganglion Cell Complex in Leber’s Hereditary Optic Neuropathy and Dominant Optic Atrophy. Curr Eye Res. 2019;44(6):638–44. https://doi.org/10.1080/02713683.2019.1567792. This study compared the patterns of optic disk RNFL and macular GCL atrophy in LHON and DOA patients, finding that patients with either disorder exhibit MPB thinning but differ slightly in the exact regions and magnitude of atrophy.
Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26(2):211–5.
Cohn AC, Toomes C, Potter C, Towns KV, Hewitt AW, Inglehearn CF, et al. Autosomal dominant optic atrophy: penetrance and expressivity in patients with OPA1 mutations. Am J Ophthalmol. 2007;143(4):656–62.
Cipolat S, De Brito OM, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci. 2004;101(45):15927–32. https://doi.org/10.1073/pnas.0407043101.
Delettre C, Lenaers G, Griffoin J-M, Gigarel N, Lorenzo C, Belenguer P, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26(2):207–10. https://doi.org/10.1038/79936.
Weisschuh N, Mazzola P, Heinrich T, Haack T, Wissinger B, Tonagel F, et al. First submicroscopic inversion of the OPA1 gene identified in dominant optic atrophy – a case report. BMC Medical Genetics. 2020;21(1). https://doi.org/10.1186/s12881-020-01166-z.
Alavi MV, Fuhrmann N. Dominant optic atrophy, OPA1, and mitochondrial quality control: understanding mitochondrial network dynamics. Mol Neurodegener. 2013;8(1):32. https://doi.org/10.1186/1750-1326-8-32.
Züchner S, De Jonghe P, Jordanova A, Claeys KG, Guergueltcheva V, Cherninkova S, et al. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol. 2006;59(2):276–81.
Gerber S, Charif M, Chevrollier A, Chaumette T, Angebault C, Kane MS, et al. Mutations in DNM1L, as in OPA1, result in dominant optic atrophy despite opposite effects on mitochondrial fusion and fission. Brain. 2017;140(10):2586–96.
Huna-Baron R, Yahalom G, Anikster Y, Ben Zeev B, Hoffmann C, Hassin-Baer S, et al. Neuro-Ophthalmic Phenotype of OPA3. J Neuroophthalmol. 2022;42(1):e147–52.
Charif M, Chevrollier A, Gueguen N, Bris C, Goudenège D, Desquiret-Dumas V, et al. Mutations in the m-AAA proteases AFG3L2 and SPG7 are causing isolated dominant optic atrophy. Neurology Genetics. 2020;6(3):e428. https://doi.org/10.1212/nxg.0000000000000428.
Kuht HJ, Thomas KA, Hisaund M, Maconachie GD, Thomas MG. Ocular Manifestations of PNPT1-Related Neuropathy. J Neuroophthalmol. 2021;41(3):e293–6.
Jurkute N, Leu C, Pogoda HM, Arno G, Robson AG, Nürnberg G, et al. SSBP1 mutations in dominant optic atrophy with variable retinal degeneration. Ann Neurol. 2019;86(3):368–83.
Hung SS, Van Bergen NJ, Jackson S, Liang H, Mackey DA, Hernández D, et al. Study of mitochondrial respiratory defects on reprogramming to human induced pluripotent stem cells. Aging (Albany NY). 2016;8(5):945.
•• Baracca A, Solaini G, Sgarbi G, Lenaz G, Baruzzi A, Schapira AH, et al. Severe impairment of complex I–driven adenosine triphosphate synthesis in Leber hereditary optic neuropathy cybrids. Archives Neurol. 2005;62(5):730–6. This study explored the bioenergetic consequences of LHON mutations by comparing cellular ATP content in cybrid cell lines.
• Sladen PE, Perdigão PRL, Salsbury G, Novoselova T, Van Der Spuy J, Chapple JP, et al. CRISPR-Cas9 correction of OPA1 c.1334G>A: p.R445H restores mitochondrial homeostasis in dominant optic atrophy patient-derived iPSCs. Molecular Therapy - Nucleic Acids. 2021;26:432–43. https://doi.org/10.1016/j.omtn.2021.08.015. CRISPR-Cas9 correction of OPA1 mutations in patient-derived induced pluripotent stem cells restores mitochodrial DNA levels and reduces apoptosis.
Sladen PE, Jovanovic K, Guarascio R, Ottaviani D, Salsbury G, Novoselova T, et al. Modelling autosomal dominant optic atrophy associated with OPA1 variants in iPSC-derived retinal ganglion cells. Hum Mol Genet. 2022;31(20):3478–93.
Zaninello M, Palikaras K, Sotiriou A, Tavernarakis N, Scorrano L. Sustained intracellular calcium rise mediates neuronal mitophagy in models of autosomal dominant optic atrophy. Cell Death Differ. 2022;29(1):167–77.
Zaninello M, Palikaras K, Naon D, Iwata K, Herkenne S, Quintana-Cabrera R, et al. Inhibition of autophagy curtails visual loss in a model of autosomal dominant optic atrophy. Nature Communications. 2020;11(1). https://doi.org/10.1038/s41467-020-17821-1.
Carelli V, La Morgia C, Valentino ML, Rizzo G, Carbonelli M, De Negri AM, et al. Idebenone treatment in Leber’s hereditary optic neuropathy. Brain. 2011;134(9):e188-e.
Klopstock T, Yu-Wai-Man P, Dimitriadis K, Rouleau J, Heck S, Bailie M, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134(9):2677–86.
Maroz A, Anderson RF, Smith RA, Murphy MP. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: implications for in vivo antioxidant activity. Free Radical Biol Med. 2009;46(1):105–9.
Romagnoli M, La Morgia C, Carbonelli M, Di Vito L, Amore G, Zenesini C, et al. Idebenone increases chance of stabilization/recovery of visual acuity in <i>OPA1</i> -dominant optic atrophy. Ann Clin Transl Neurol. 2020;7(4):590–4. https://doi.org/10.1002/acn3.51026.
Zoumalan CI, Agarwal M, Sadun AA. Optical coherence tomography can measure axonal loss in patients with ethambutol-induced optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2005;243:410–6.
González-Quevedo A, Santiesteban-Freixas R, Eells JT, Lima L, Sadun AA. Cuban epidemic neuropathy: insights into the toxic–nutritional hypothesis through international collaboration. MEDICC Rev. 2018;20:27–31.
Mehta S, Das M, Laxmeshwar C, Jonckheere S, Thi SS, Isaakidis P. Linezolid-associated optic neuropathy in drug-resistant tuberculosis patients in Mumbai, India. PLoS ONE. 2016;11(9):e0162138.
Godel V, Nemet P, Lazar M. Chloramphenicol optic neuropathy. Arch Ophthalmol. 1980;98(8):1417–21.
Konnakkodan SM, Solomon CB, Prabhu PB, Kumar AA. Optic nerve head-retinal nerve fiber layer analysis with spectral-domain optical coherence tomography of ethambutol-induced ocular toxicity in patients on a daily regime of anti-tubercular therapy. Kerala J Ophthalmol. 2021;33(3):291–8.
Jin KW, Lee JY, Rhiu S, Choi DG. Longitudinal evaluation of visual function and structure for detection of subclinical Ethambutol-induced optic neuropathy. PLoS ONE. 2019;14(4):e0215297.
Saijo T, Hayashi K, Yamada H, Wakakura M. Linezolid-induced optic neuropathy. Am J Ophthalmol. 2005;139(6):1114–6.
Menon V, Jain D, Saxena R, Sood R. Prospective evaluation of visual function for early detection of ethambutol toxicity. Br J Ophthalmol. 2009;93(9):1251–4.
Sen S, Mandal S, Banerjee M, Gk R, Saxena A, Aalok SP, et al. Ethambutol-induced optic neuropathy: Functional and structural changes in the retina and optic nerve. Seminars in Ophthalmology. 2022;37(6):730–9. https://doi.org/10.1080/08820538.2022.2085517.
Kovač L, Volk M, Šuštar Habjan M, Hawlina M. Oxidative Stress in Antibiotic Toxic Optic Neuropathy Mimicking Acute LHON in a Patient with Exacerbation of Cystic Fibrosis. Stresses. 2023;3(1):387–96.
Vieira LMC, Silva NFA, dos Santos AMD, dos Anjos RS, Pinto LAPA, Vicente AR, et al. Retinal ganglion cell layer analysis by optical coherence tomography in toxic and nutritional optic neuropathy. J Neuroophthalmol. 2015;35(3):242–5.
O’Neill EK, Mankad K, Bowman R, Thompson DA. Electrophysiological assessment of nutritional optic neuropathy: a case report. Doc Ophthalmol. 2023;146(2):181–9.
Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. 2017;96(6):384–9.
Chan W, Almasieh M, Catrinescu M-M, Levin LA. Cobalamin-associated superoxide scavenging in neuronal cells is a potential mechanism for vitamin B12–deprivation optic neuropathy. Am J Pathol. 2018;188(1):160–72.
Richard E, Jorge-Finnigan A, Garcia-Villoria J, Merinero B, Desviat LR, Gort L, et al. Genetic and cellular studies of oxidative stress in methylmalonic aciduria (MMA) cobalamin deficiency type C (cblC) with homocystinuria (MMACHC). Hum Mutat. 2009;30(11):1558–66.
Suarez-Moreira E, Yun J, Birch CS, Williams JH, McCaddon A, Brasch NE. Vitamin B12 and redox homeostasis: cob (II) alamin reacts with superoxide at rates approaching superoxide dismutase (SOD). J Am Chem Soc. 2009;131(42):15078–9.
Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75–81.
Calderón-Ospina CA, Nava-Mesa MO. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther. 2020;26(1):5–13.
Aleyasin A, Ghazanfari M, Houshmand M. Leber hereditary optic neuropathy: do folate pathway gene alterations influence the expression of mitochondrial DNA mutation? Iran J Public Health. 2010;39(3):53.
Ota Y, Capizzano AA, Moritani T, Naganawa S, Kurokawa R, Srinivasan A. Comprehensive review of Wernicke encephalopathy: pathophysiology, clinical symptoms and imaging findings. Jpn J Radiol. 2020;38:809–20.
Lazarchick J. Update on anemia and neutropenia in copper deficiency. Curr Opin Hematol. 2012;19(1):58–60.
Yu JJ, Lee DH, Gallagher SP, Kenney MC, Boisvert CJ. Mitochondrial impairment in antibiotic induced toxic optic neuropathies. Curr Eye Res. 2018;43(10):1199–204.
Samarakoon N, Harrisberg B, Ell J. Ciprofloxacin-induced toxic optic neuropathy. Clin Exp Ophthalmol. 2007;35(1):102–4.
Saldaña NG, Trujillo DMG, Pertierra AMB, Pineda AIM, Olguín HJ. Linezolid-associated optic neuropathy in a pediatric patient with Mycobacterium nonchromogenicum: A case report. Medicine. 2017;96(50). https://doi.org/10.1097/MD.0000000000009200.
Wong S, Silva F, Acheson J, Plant G. An old friend revisited: chloramphenicol optic neuropathy. JRSM short reports. 2013;4(3):1–3.
Kulkarni H, Keskar V, Bavdekar S, Gabhale Y. Bilateral optic neuritis due to isoniazid (INH). Indian Pediatr. 2010;47:533–5.
Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis. 2001;7(2):337.
Bozdogan B, Appelbaum PC. Oxazolidinones: activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents. 2004;23(2):113–9.
Zhao H, Li R, Wang Q, Yan Q, Deng J-H, Han D, et al. Maternally inherited aminoglycoside-induced and nonsyndromic deafness is associated with the novel C1494T mutation in the mitochondrial 12S rRNA gene in a large Chinese family. Am J Human Gen. 2004;74(1):139–52.
Wenzel CQ, Daniels C, Keates RA, Brewer D, Lam JS. Evidence that WbpD is an N-acetyltransferase belonging to the hexapeptide acyltransferase superfamily and an important protein for O-antigen biosynthesis in Pseudomonas aeruginosa PAO1. Mol Microbiol. 2005;57(5):1288–303.
Robbie GJ, Zhao L, Mondick J, Losonsky G, Roskos LK. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob Agents Chemother. 2012;56(9):4927–36.
Blaha GM, Polikanov YS, Steitz TA. Elements of ribosomal drug resistance and specificity. Curr Opin Struct Biol. 2012;22(6):750–8. https://doi.org/10.1016/j.sbi.2012.07.016.
Guillet V, Chevrollier A, Cassereau J, Letournel F, Gueguen N, Richard L, et al. Ethambutol-induced optic neuropathy linked to OPA1 mutation and mitochondrial toxicity. Mitochondrion. 2010;10(2):115–24.
Fonkem E, Skordilis MA, Binkley EM, Raymer DS, Epstein A, Arnold WD, et al. Ethambutol toxicity exacerbating the phenotype of CMT2A2. Muscle Nerve. 2013;48(1):140–4.
Pasáková I, Gladziszová M, Charvátová J, Stariat J, Klimeš J, Kovaříková P. Use of different stationary phases for separation of isoniazid, its metabolites and vitamin B6 forms. J Sep Sci. 2011;34(12):1357–65.
Verma AK, Yadav A, Singh SV, Mishra P, Rath SK. Isoniazid induces apoptosis: Role of oxidative stress and inhibition of nuclear translocation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Life Sci. 2018;199:23–33.
Jena L, Waghmare P, Kashikar S, Kumar S, Harinath BC. Computational approach to understanding the mechanism of action of isoniazid, an anti-TB drug. Int J Mycobacteriology. 2014;3(4):276–82.
Kozak SF, Inderlied CB, Hsu HY, Heller KB, Sadun AA. The role of copper on ethambutol’s antimicrobial action and implications for ethambutol-induced optic neuropathy. Diagn Microbiol Infect Dis. 1998;30(2):83–7.
Anandarajah HR, Walsh RD. Unilateral Optic Neuropathy Resulting From Methanol Poisoning. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 2023. https://doi.org/10.1097/WNO.0000000000001986.
Figuerola B, Mendoza A, Roca M, Lacorzana J. Severe visual loss by inhalation of methanol. Romanian J Ophthalmol. 2021;65(2):176.
Saad SG, Fouad YA, Nowara M, Saad S. Methanol intoxication presenting with bilateral optic neuritis and paracentral acute middle maculopathy. Cureus. 2022;14(1). https://doi.org/10.7759/cureus.21587.
Sun Q, Sun M, Zhang Y, Wang S, Bai W, Wei S, et al. Clinical Characteristics of Methanol-Induced Optic Neuropathy: Correlation between Aetiology and Clinical Findings. Journal of Ophthalmology. 2022;2022. https://doi.org/10.1155/2022/4671671.
Nurieva O, Diblik P, Kuthan P, Sklenka P, Meliska M, Bydzovsky J, et al. Progressive chronic retinal axonal loss following acute methanol-induced optic neuropathy: four-year prospective cohort study. Am J Ophthalmol. 2018;191:100–15.
Galvez-Ruiz A, Elkhamary SM, Asghar N, Bosley TM. Cupping of the optic disk after methanol poisoning. Br J Ophthalmol. 2015;99(9):1220–3.
Klein KA, Warren AK, Baumal CR, Hedges TR. Optical coherence tomography findings in methanol toxicity. Int J Retina Vitreous. 2017;3(1):1–6.
Rajendra S, Shah VM, Manayath GJ, Kumar K. Multimodal Imaging Features of Subacute Methanol-Induced Bilateral Optic Neuropathy. Journal of Neuro-Ophthalmology. 2022:10.1097.
Liesivuori J, Savolainen H. Methanol and formic acid toxicity: biochemical mechanisms. Pharmacol Toxicol. 1991;69(3):157–63.
Kirkman MA, Yu-Wai-Man P, Korsten A, Leonhardt M, Dimitriadis K, De Coo IF, et al. Gene–environment interactions in Leber hereditary optic neuropathy. Brain. 2009;132(9):2317–26.
Giordano L, Deceglie S, d’Adamo P, Valentino M, La Morgia C, Fracasso F, et al. Cigarette toxicity triggers Leber’s hereditary optic neuropathy by affecting mtDNA copy number, oxidative phosphorylation and ROS detoxification pathways. Cell death & disease. 2015;6(12):e2021.
Zhang N, Wang J, Chen B, Li Y, Jiang B. Prevalence of primary angle closure glaucoma in the last 20 years: a meta-analysis and systematic review. Front Med. 2021;7:624179.
Zhang N, Wang J, Li Y, Jiang B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Sci Rep. 2021;11(1):13762.
Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–90.
Steinmetz JD, Bourne RR, Briant PS, Flaxman SR, Taylor HR, Jonas JB, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144–60.
Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–93.
Sultan MB, Mansberger SL, Lee PP. Understanding the importance of IOP variables in glaucoma: a systematic review. Survey Ophthalmol. 2009;54(6):643–62.
Chihara E, Tanihara H. Parameters associated with papillomacular bundle defects in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1992;230(6):511–7. https://doi.org/10.1007/bf00181770.
Hood DC, Raza AS, De Moraes CGV, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1–21. https://doi.org/10.1016/j.preteyeres.2012.08.003.
Rao A, Mukherjee S, Padhy D. Optic nerve head characteristics in eyes with papillomacular bundle defects in glaucoma. Int Ophthalmol. 2015;35:819–26.
Lee WJ, Park KH, Seong M. Vulnerability zone of glaucoma progression in combined wide-field optical coherence tomography event-based progression analysis. Invest Ophthalmol Visual Sci. 2020;61(5):56.
• Leung CK, Guo PY, Lam AK. Retinal nerve fiber layer optical texture analysis: involvement of the papillomacular bundle and papillofoveal bundle in early glaucoma. Ophthalmology. 2022;129(9):1043–55. This paper found evidence of both maculopapillary and papillofoveal bundle deficits in early glaucoma.
••Hood DC, Slobodnick A, Raza AS, de Moraes CG, Teng CC, Ritch R. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Invest Ophthalmol Visual Sci. 2014;55(2):632–49. This study used OCT to demonstrate that the MPB is a zone of vulnerability in early glaucoma.
De Moraes CG, Sun A, Jarukasetphon R, Rajshekhar R, Shi L, Blumberg DM, et al. Association of Macular Visual Field Measurements With Glaucoma Staging Systems. JAMA Ophthalmol. 2019;137(2):139. https://doi.org/10.1001/jamaophthalmol.2018.5398.
•Su CK-Y, Guo PY, Chan PPM, Lam AK-N, Leung CKS. Retinal Nerve Fiber Layer Optical Texture Analysis: Detecting Axonal Fiber Bundle Defects in Patients with Ocular Hypertension. Ophthalmology. 2023. This study estimated the proportion of patients with ocular hypertension that exhibit RNFL defects not detected by traditional OCT but detectable by retinal optical texture analysis.
Pickett JE, Terry SA, O’Connor PS, O’Hara M. Early loss of central visual acuity in glaucoma. Ophthalmology. 1985;92(7):891–6.
Takahashi N, Omodaka K, Pak K, Kikawa T, Kobayashi W, Akiba M, et al. Evaluation of papillomacular nerve fiber bundle thickness in glaucoma patients with visual acuity disturbance. Curr Eye Res. 2020;45(7):847–53.
• Kim M, Hong E, Lee EJ. Optic Disc Morphology and Paracentral Scotoma in Patients with Open-Angle Glaucoma and Myopia. J Clin Med. 2023;12(9):3295. This study fount that in open-angle glaucoma patients a more nasal positioning of the central retinal vessel trunk and greater distance to the optic nerve head is significantly associated with the presence of a paracentral scotoma.
Triolo G, Rabiolo A, Shemonski ND, Fard A, Di Matteo F, Sacconi R, et al. Optical coherence tomography angiography macular and peripapillary vessel perfusion density in healthy subjects, glaucoma suspects, and glaucoma patients. Invest Ophthalmol Vis Sci. 2017;58(13):5713–22.
Wang B, Lucy KA, Schuman JS, Ishikawa H, Bilonick RA, Sigal IA, et al. Location of the central retinal vessel trunk in the laminar and prelaminar tissue of healthy and glaucomatous eyes. Sci Rep. 2017;7(1):9930.
Kim G-N, Kim J-A, Kim M-J, Lee EJ, Hwang J-M, Kim T-W. Comparison of lamina cribrosa morphology in normal tension glaucoma and autosomal-dominant optic atrophy. Invest Ophthalmol Visual Sci. 2020;61(5):9.
Bennett JL. Optic neuritis. Continuum: lifelong learning in neurology. 2019;25(5):1236–64. https://doi.org/10.1212/CON.0000000000000768.
Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J. Suppression of mitochondrial oxidative stress provides long-term neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007;48(2):681–91.
Candadai AA, Liu F, Verma A, Adil MS, Alfarhan M, Fagan SC, et al. Neuroprotective Effects of Fingolimod in a Cellular Model of Optic Neuritis. Cells. 2021;10(11):2938. https://doi.org/10.3390/cells10112938.
Song Y, Wang M, Zhao S, Tian Y, Zhang C. Matrine promotes mitochondrial biosynthesis and reduces oxidative stress in experimental optic neuritis. Frontiers in Pharmacology. 2022;13. https://doi.org/10.3389/fphar.2022.936632.
Khan RS, Fonseca-Kelly Z, Callinan C, Zuo L, Sachdeva MM, Shindler KS. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Frontiers in Cellular Neuroscience. 2012;6. https://doi.org/10.3389/fncel.2012.00063.
Del Negro I, Pauletto G, Verriello L, Spadea L, Salati C, Ius T, et al. Uncovering the Genetics and Physiology behind Optic Neuritis. Genes. 2023;14(12):2192.
Riordan-Eva P, Sanders M, Govan G, Sweeney M, Costa JD, Harding A. The clinical features of Leber’s hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118(2):319–37.
Rabenstein A, Catarino CB, Rampeltshammer V, Schindler D, Gallenmüller C, Priglinger C, et al. Smoking and alcohol, health-related quality of life and psychiatric comorbidities in Leber’s Hereditary Optic Neuropathy mutation carriers: a prospective cohort study. Orphanet J Rare Dis. 2021;16:1–12.
Vestergaard N, Rosenberg T, Torp-Pedersen C, Vorum H, Andersen CU, Aasbjerg K. Increased mortality and comorbidity associated with Leber’s hereditary optic neuropathy: a nationwide cohort study. Invest Ophthalmol Vis Sci. 2017;58(11):4586–92.
Chen AT, Brady L, Bulman DE, Sundaram AN, Rodriguez AR, Margolin E, et al. An evaluation of genetic causes and environmental risks for bilateral optic atrophy. PLoS ONE. 2019;14(11):e0225656.
Yu-Wai-Man P, Griffiths PG, Gorman G, Lourenco C, Wright A, Auer-Grumbach M, et al. Multi-system neurological disease is common in patients with OPA1 mutations. Brain. 2010;133(3):771–86.
Chavala SH, Kosmorsky GS, Lee MK, Lee MS. Optic neuropathy in vitamin B12 deficiency. Eur J Intern Med. 2005;16(6):447–8.
Chiang E-PI, Bagley PJ, Selhub J, Nadeau M, Roubenoff R. Abnormal vitamin B6 status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114(4):283–7.
Masalha R, Rudoy I, Volkov I, Yusuf N, Wirguin I, Herishanu YO. Symptomatic dietary vitamin B12 deficiency in a nonvegetarian population. Am J Med. 2002;112(5):413–6.
Sechi G, Sechi E, Fois C, Kumar N. Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr Rev. 2016;74(5):281–300.
Hvas A-M, Juul S, Bech P, Nexø E. Vitamin B6 level is associated with symptoms of depression. Psychother Psychosom. 2004;73(6):340–3.
Rana D, Patel S, Roy T, Bailey JW, Bailey JW. A case report: ethambutol causes a rare adverse effect of peripheral neuropathy. Cureus. 2022;14(4). https://doi.org/10.7759/cureus.23782.
Lin H-C, Chien C-W, Hu C-C, Ho J-D. Comparison of comorbid conditions between open-angle glaucoma patients and a control cohort: a case-control study. Ophthalmology. 2010;117(11):2088–95.
Dascalu AM, Stana D, Nicolae VA, Cirstoveanu C, Vancea G, Serban D, et al. Association between vascular comorbidity and glaucoma progression: A four-year observational study. Exp Ther Med. 2021;21(3):1.
Ritland J, Egge K, Lydersen S, Juul R, Semb S. Exfoliative glaucoma and primary open-angle glaucoma: associations with death causes and comorbidity. Acta Ophthalmol Scand. 2004;82(4):401–4.
Falabella M, Forte E, Magnifico MC, Santini P, Arese M, Giuffrè A, et al. Evidence for Detrimental Cross Interactions between Reactive Oxygen and Nitrogen Species in Leber’s Hereditary Optic Neuropathy Cells. Oxid Med Cell Longev. 2016;2016:3187560. https://doi.org/10.1155/2016/3187560.
Hoegger MJ, Lieven CJ, Levin LA. Differential production of superoxide by neuronal mitochondria. BMC Neurosci. 2008;9:1–14.
Millet AM, Bertholet AM, Daloyau M, Reynier P, Galinier A, Devin A, et al. Loss of functional OPA 1 unbalances redox state: implications in dominant optic atrophy pathogenesis. Ann Clin Transl Neurol. 2016;3(6):408–21.
Tang S, Le PK, Tse S, Wallace DC, Huang T. Heterozygous mutation of Opa1 in Drosophila shortens lifespan mediated through increased reactive oxygen species production. PLoS ONE. 2009;4(2):e4492.
Irma J, Kartika A, Rini M, Setiohadji B, Salim J. A Protective Role of Coenzyme Q10 in Ethambutol-Induced Retinal Ganglion Cell Toxicity: A Randomised Controlled Trial in Mice. Neuro-Ophthalmology. 2022;46(5):298–303.
Rasool M, Malik A, Manan A, Aziz K, Mahmood A, Zaheer S, et al. Determination of potential role of antioxidative status and circulating biochemical markers in the pathogenesis of ethambutol induced toxic optic neuropathy among diabetic and non-diabetic patients. Saudi Journal of Biological Sciences. 2015;22(6):739–43.
Simon H-U, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–8.
Levin LA. Superoxide Generation Explains Common Features of Optic Neuropathies Associated With Cecocentral Scotomas. J Neuroophthalmol. 2015;35(2):152–60. https://doi.org/10.1097/wno.0000000000000250.
Levin LA. Mechanisms of retinal ganglion specific-cell death in leber hereditary optic neuropathy. Trans Am Ophthalmol Soc. 2007;105:379–91.
• Lambiri DW, Levin LA. Modeling Reactive Oxygen Species-Induced Axonal Loss in Leber Hereditary Optic Neuropathy. Biomolecules. 2022;12(10):1411. https://doi.org/10.3390/biom12101411. This study used an in-silico approach to model reactive oxygen species propogation and axonal degeneration, reproducing outcomes in LHON pathology.
Author information
Authors and Affiliations
Contributions
DWL and LAL wrote the main manuscript text and prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lambiri, D.W., Levin, L.A. Maculopapillary Bundle Degeneration in Optic Neuropathies. Curr Neurol Neurosci Rep 24, 203–218 (2024). https://doi.org/10.1007/s11910-024-01343-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-024-01343-0